Phytochemical screening and HPTLC finger printing analysis of Citrullus lanatus (Thunb.) seed
Sumam Varghese, R. Narmadha, D. Gomathi, M. Kalaiselvi, K. Devaki
Department of Biochemistry, Karpagam University, Coimbatore-641 021, India
Phytochemical screening and HPTLC finger printing analysis of Citrullus lanatus (Thunb.) seed
Sumam Varghese, R. Narmadha, D. Gomathi, M. Kalaiselvi, K. Devaki*
Department of Biochemistry, Karpagam University, Coimbatore-641 021, India
Secondary metabolites
Citrullus lanatus
Sequential extraction
HPTLC
Objective: To find out the secondary metabolites present in the various extracts of Citrullus lanatus (C. lanatus) (Thunb.) and mineral content present in the plant material. Methods: The powdered plant material was extracted using different solvents. Phytochemical screening and HPTLC fingerprinting analysis were then carried out. Result: The ethanolic seed extract of C. lanatus showed the presence of majority of secondary metabolites when compared to other solvent system. The quantitative analysis of the plant material also revealed the presence of various amount of carbohydrates, phenols, flavonoids, proteins, fibre, phosphorus and irons. The HPTLC fingerprinting analysis was carried for flavonid and phenolic compounds by using CAMAG LINOMAT 5 instrument which revealed the presence of flavonoid and phenolic compound especially quercetin in the ethanolic seed extract of C. lanatus. Conclusions: The results scientifically validate the use of C. lanatus in the traditional medicine and it can be used to treat various disorders caused by free radical and chemical substances due to presence of secondary metabolites.
1. Introduction
Plant based drugs have been used world wide in traditional medicines for treatment of various diseases. World plant biodiversity is the largest source of herbal medicine and still about 60%-80% world population rely on plant based medicines which are being used since the ancient ages as traditional health care system. India is the largest producer of medicinal herbs and appropriately called the Botanical garden of the world. It is now clear that the medicinal value of these plants lies in the bioactive phytochemical constituents that produce definite physiological effects on human body. These natural compounds formed the base of modern drugs as we use today[1-3].
Citrullus lanatus (C. lanatus) can be used for smoothes, sorbets or granite depending on the texture whether smooth or coarse. Watermelon (C. lanatus) family Cucurbitaceae is an excellent source of vitaminA, B & C necessary for energy production. The fruit is also diuretic, being effective in the treatment of dropsy and renal stones. The rind of the fruit is prescribed in cases of alcoholic poisoning and diabetes. The root is purgative and in large dose is said to be emetic. The seed is demulcent, diuretic, pectoral and tonic. It is sometimes used in the treatment of the urinary passages bed wetting. The seed is also a good vermifuge and has a hypotensive action. Fatty oil in the seed, as well as aqueous or alcoholic extracts, paralyze tapeworms and roundworms. In Northern Sudan it is often used for burns, swellings, rheumatism, gout and as laxative[4].
Studies have shown that fruits and vegetables contain vital nutrients, an appreciable quantity of vitamins, fibre, antioxidants, phytochemicals and it was also noted that a daily consumption of at least 5 to 10 servings of a wide variety of fruits and vegetables is an appropriate strategy for significantly reducing the risk of chronic diseases and to meet nutrient requirement for optimum health[5].
These fruits are consumed, fresh, canned or processed and its consumption results in the production of vast amount of agricultural waste from their seeds and rind. Despite the numerous nutritionalbenefits from fruits only a small portion of plant material is utilized directly for human consumption, the remaining part may be converted into nutrient for either animal feed or into fertilizer. Disposal of these agricultural wastes can have a serious environmental impact which is becoming harder to solve. Much effort will therefore be needed to exploit the nutritional and industrial potential of by-products waste and the other under-utilized agricultural products. The nutritional value and anti-nutrient content of many fruits, seeds and their rind has not be given much attention such that most times these parts of fruit are discarded even with their hidden nutrients.
The seeds and rind which are often the waste part of the fruits have not generally received much attention with a view to being used or recycled rather than discarded. Interestingly the seeds of some fruits have higher vitamins, fibres, minerals and other essential nutrients activity than the pulp fractions[6]. It is therefore necessary to evaluate the nutritional and anti-nutrients contents of these waste materials.
Hence the present investigation was done to study the phytochemical screening of C. lanatus seed extract in different solvents like petroleum ether, chloroform, ethyl acetate, ethanol, water and to perform qualitative and quantitative analysis of some secondary metabolites, minerals to ascertain ethnomedicinal usage of this seed.
2. Materials and methods
2.1. Collection of plant material
The seeds of C. lanatus (Thunb.) were collected from Kerala, authenticated by Dr. C.V. Moorthy, Botanical Survey of India, Tamilnadu Agricultural University Campus, Coimbatore (Voucher No.BSI/SRC/5/23/2010-Tech 1728). The seeds were washed with water. They were air dried at room temperature for 10 days in the absence of sunlight and powdered well using a mixer. Then they were weighed and kept in an airtight container.
2.2. Sample extraction
The powdered plant material was subjected to successive solvent extraction using different solvents (petroleum ether, chloroform, ethyl acetate, ethanol and water) in the increasing order of polarity. A total of 50 g of dried plant powder was extracted in 250 mL of various solvent in an orbitory shaker for 72 h. Obtained extract was evaporated to dryness by using a rotary vacuum evaporator at 40-50 °C and stored at 0-4 °C in an air tight container for further use.
2.3. Phytocemical screening of the plant material
Phytochemical screening was done for analyzing the presence of secondary metabolites that are responsible for curing ailments. The phytochemical screening of the plant extract was carried out by following the method of Trease and Evans[7] and Harborne[8].
2.4. Biochemical characterization of the plant material
The total carbohydrate content in plant material was analyzed by the method of Sadasivam and Manickam[9], estimation of protein was done by the method of Lowry et al[10], estimation of total phenol content was done by the method of Singleton and Rossi[11] and flavonoids by the method of Ordon et al[12].
2.5. Preparation of ash solution
The silica crucible was heated up to 600 °C and cooled. A sample of 2.0 g was weighed and taken in the crucible. The crucible was placed in an incubator at 100-110 °C for 2-3 h and cooled in a desiccator. The crucible was then placed in a clay pipe triangle and heated over a low flame till all the minerals were completely charred. The charred mineral was then heated in a muffle furnace for 6 h at 600 °C. The crucible was then cooled in a desiccator and weighed. The ash thus obtained was used for the estimation of minerals like calcium, phosphorous and iron.
The fibre content present in plant material was estimated by the method of Maynard[13], phosphorous content by the method of Fiske and Subbarow[14], calcium was determined by the method of Cornfield and Pollard[15] and the iron content were estimated by the method of Raghuramulu[16].
2.6. HPTLC fingerprinting analysis
For HPTLC fingerprinting analysis, 2 µL of the above test solution and 2 µL of standard solution were loaded as 5 mm band length in the 3 × 10 Silica gel 60F254TLC plate using Hamilton syringe and CAMAG LINOMAT 5 instrument. The samples loaded plate was kept in TLC twin trough developing chamber (after saturated with Solvent vapor) with respective mobile phases (flavonoids and phenols) and the plate was developed in the respective mobile phase up to 90 mm. The developed plate was dried by hot air to evaporate solvents from the plate.
The plate was kept in Photo-documentation chamber (CAMAG REPROSTAR 3) and captured the images at White light, UV 254 nm and UV 366 nm. The developed plate was sprayed with respective spray reagent(flavonoids and phenols) and dried at 100 °C in hot air oven. The plate was photo-documented at daylight and UV 366 nm mode using Photo-documentation (CAMAG REPROSTAR 3) chamber. After derivatization, the plate was fixed in scanner stage (CAMAG TLC SCANNER 3) and scanning was done at 500 nm. The peak table, peak display and peak densitogram were noted.
3. Result
Phytoconstituents are the natural bioactive compounds found in plants. It works with nutrients and fibers to form an integrated part of defense system against various diseases and stress conditions. They are basically divided into two groups like primary and secondary constituents; according to their functions in plant metabolism. Primary constituents consists of common sugars, amino acid, proteins and chlorophyll while secondary constituents consists of alkaloids, terpenoids, saponins, phenolic compounds, flavonoids, tannins and so on[17]. The present study was undertaken to find out the qualitative and quantitative phytochemistry of C. lanatus seedextracts.
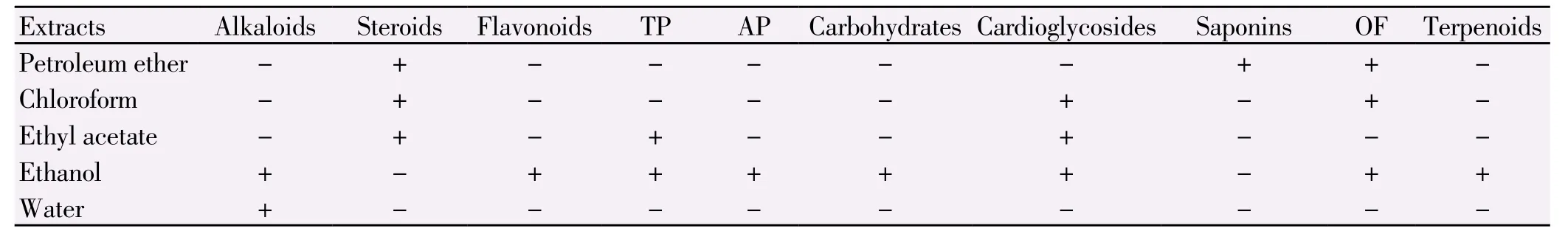
Table 1Phytochemical screening of seeds.
The phytochemical screening test has been conducted after the successive solvent extraction. The results of the phytochemical screening are given in Table 1. The phytochemical screening of C. lanatus seeds showed the presence of nine phytochemical constituents like alkaloids, flavanoids, tannins, aminoacids, carbohydrates, cardioglycosides, terpenoids, oils and fats in the ethanolic extract of plant material when compared with other solvents. Hence, further studies were carried out by using the ethanolic extract of plant material.
These results were supported by Johnson et al[18] who reported that saponins, alkaloids, tannins, phytates, oxalate, phenols, HCN and flavonoids known phytochemicals were present in the fruit, seed and rind of C. lanatus.
3.1. Biochemical charecterization and mineral content analysis of C. lanatus
Estimation of carbohydrates, phenols, flavanoids, crude fibre, phosphorous, calcium and iron are shown in Table 2. The total carbohydrate content of the ethanolic extract of C. lanatus was found to be 16.4 mg/g. The total phenolic content of the ethanolic extract of C. lanatus was found to be 40.5 mg/g of tannic acid equivalent (GAE) and flavanoid content was found to be 154.26 mg/g of quercetien equivalent.
The present study showed the presence of more amounts of flavonoid contents in the ethanolic extract of seed material when compared with all biochemical compounds. The results agreed with the findings of FAO/WHO, Joint FAO/WHO food standards programmes, 1991[19] and Johnson et al.[18] who reported that natural colouring in plant based food with antioxidant, anti-inflammatory and diuretic effect is due to flavonoid as is observed in the pulp and seed of C. lanatus.
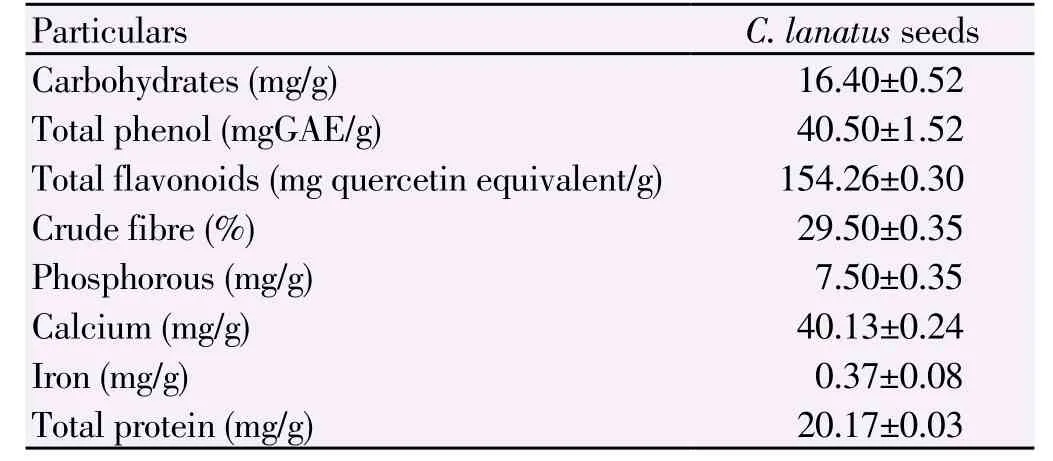
Table 2Biochemical characterization and mineral content analysis of C. lanatus seeds.
C. lanatus comprising 50% oil and 35% protein, the seeds have both nutritional and cosmetic importance. The seeds contain vitamin C and B2, minerals, riboflavin, fat, carbohydrates and protein[20]. Total crude fibre was found to be 29.5 mg/g.
The phosphorous, calcium and iron content of ethanolic extract of C. lanatus was found to be 7.5, 40.13 and 0.33 mg/g respectively. Our results were comparable with those of Ojieh et al[21] who reportedthat the seed contained crude protein, crude fibre and total carbohydrate.
3.2. HPTLC fingerprinting profile of ethanolic seed extract of C. lanatus
HPTLC profile of plant extracts was generated in solvent systems of different polarities in order to ascertain the total number of chemical moieties which will also help in designing the method of isolation and characterization of bioactive compounds. Phenolic compounds are considered as secondary metabolites that are synthesized by plants during normal development[22].
They are also found in many medicinal plants, and herbal medicines containing these compounds have often been used in pharmacy. Flavonoids and phenolic acids have analgesic, antiallergic, anticancer, antidiabetic, antihepatotoxic, antiinflammatory, antiosteoporotic, antioxidant, antispasmotic and antivascular effects[23-26] while tannins have antidiarrhoeal, antioxidant and antiseptic properties[27].
HPTLC phenolic profile of ethanolic extract of C. lanatus seed was recorded in Figure 1 and Table 3. Blue, brown color zone was detected in UV after derivatization in the chromatogram confirms the presence of polyphenols. The extract was run along with the standard polyphenols compounds. The result showed the presence of Quercetin (polyphenol) with in peak 5 with an Rf value of 0.72 in the ethanolic seed extract of C. lanatus.
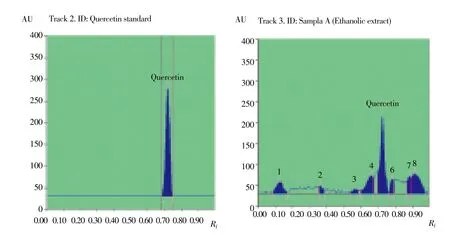
Figure 1. Densitogram display for phenols of seed ethanolic extract of C. lanatus.

Table 3Peak table with Rfvalues, height and area of phenols.
Flavanoid HPTLC profiles of ethanolic seed extract of C. lanatus was represented in Figure 2 and Table 4. Yellow coloured fluorescent zone observed in the chromatogram after derivatization confirms the presence of flavanoid in the given standard and in the sample.
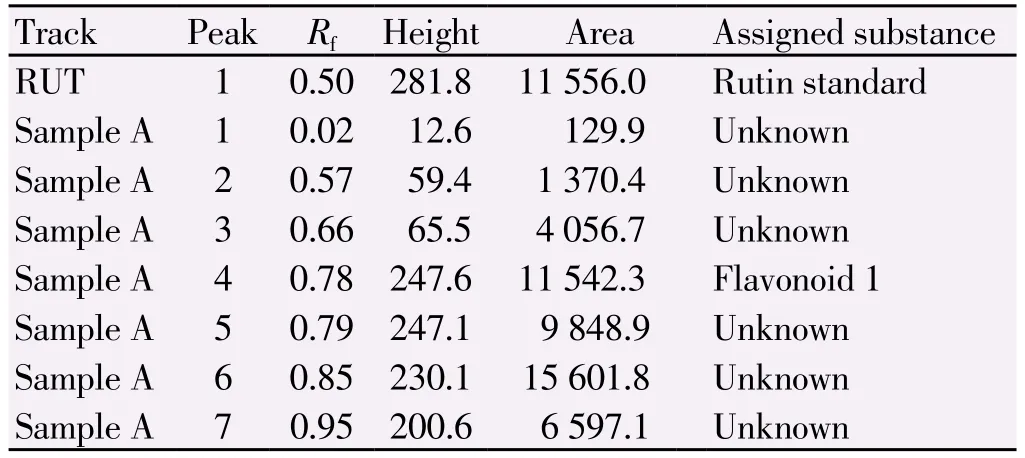
Table 4Peak table with Rfvalues, height and area of flavonoids.
4. Discussion
The results indicated that the seed contain an appreciable amount of bioactive compounds. Medically the presence of these phytochemicals especially the phenols and flavonoids explains the use of C. lanatus in ethnomedicine for the management of various ailments.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We, the authors, are thankful to our Chancellor, Advisor, Vice Chancellor and Registrar of Karpagam University for providing facilities and encouragement.
[1] Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian nedicinal plants. Afr J Biotech 2005; 4(7): 685-688.
[2] Akinmo-laudn AC, Ibukun EO, Afor E, Obuotor EM, Farombi EO. Phytochemical constituents and antioxidant activity of extracts from leaves of O. gratissimum. Sci Res Essay 2007; 2: 163-166.
[3] Rout SP, Choudhary KA, Kar DM, Das L, Jain A. Plants in traditional medicinal system-future source of new drugs. Int J Pharm Pharm Sci 2009; 1(1): 1-23.
[4] Hassan LEA, Sirat HM, Yagi SMA, Koko WS, Abdelwahab SI. In vitro antimicrobial activities of chloroformic, hexane and ethanolic extracts of Citrullus lanatus var. citroides (Wild melon). J Med Plants Res 2011; 5(8): 1338-1344.
[5] Liu RH. J Nutrit 2004; 4: 1340-3475.
[6] Jayaprakasha GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem 2001; 73: 285-290.
[7] Trease GE, Evans WC. Pharmacology. 11th Ed. London: Bailliere Tindall Ltd.; 1978, p. 60-75.
[8] Harborne JB. Phytochemical methods–A guide to modern techniques of plant analysis. 2nd Ed. London: Chapman and Hall; 1984, p. 9-15.
[9] Sadasivam S, Manickam A. Biochemical methods. 3rd Ed. New Delhi: New age international Limited Publishers; 1996, p. 8-9.
[10] Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem 1951; 193: 265-275.
[11] Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolbdic phosphor tugnstic acid reagents. Am J Enol Vitic 1965; 16: 144-158.
[12] Ordon LE, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem 2006; 97: 452-458.
[13] Maynard AJ. Methods in food analysis: physical, chemical, and instrumental methods of analysis. University of Michigan: Academic Press; 1970.
[14] Fiske, Subbarow. The colorimetric determination of phosphorous. J Biol Chem 1925; 66: 375-400.
[15] Cornfield AH, Pollard AG. Response of tomato plant to calcium deficiency. J Sci Food Agr 1951; 2: 135-136.
[16] Raguramaulu N, Nair MK, Kalyanasundaram S. A manual of laboratory techniques. National Instit Nutri 1983; 8: 84-95.
[17] Koche D, Shirsat R, Imran S, Bhadange DG. Phytochemical screening of eight traditionally used ethnomedicinal plants from Akola district (MS) India. Int J Pharma Bio Sci 2010; 1(4): 253-256.
[18] Johnson JT, Iwang EU, Hemen JT, Odey MO, Efiong EE, Eteng OE. Evaluation of anti-nutrient contents of watermelon Citrullus lanatus. Ann Biol Res 2012; 3(11): 5145-5150.
[19] FAO/WHO, Joint FAO/WHO food standards programmes. In: Cordex alimentarius commission, xii (Suppl. 4). Rome: FAO; 1991.
[20] Lazos ES. Nutritional, fatty acid and oil characteristics of pumpkin and melon seeds. J Food Sci 1986; 51(5): 1382.
[21] Ojieh GC, Oluba OM, Ogunlowo YR, Adebisi KE, Eidangbe GO, Orole RT. Compositional studies of Citrullus lanatus (Egusi melon) seed. Int J Nutr Wellness 2008; 6(1): 1-5.
[22] Naczk M, Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr A 2004; 1054: 95-111.
[23] Benavente-Garcia O, Castillo J, Lorente J, Ortuno A, Del Rio JA. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem 2000; 68: 457-462.
[24] Brac AA, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol 2002; 79: 379-381.
[25] Dicarlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci 1999; 65: 337-353.
[26] Yu J, Walzem RL, Miller EG, Pike LM, Patil BS. Antioxidant activity of citrus limonoids, flavonoids and coumarins. J Agric Food Chem 2005; 23: 2009-2014.
[27] Suleiman MM, Dzenda T, Sani CA. Antidiarrhoeal activity of the methanol stem-bark extract of Annona senegalensis Pers. (Annonaceae). J Ethnopharmacol 2008; 116: 125-130.
19 February 2013
*Corresponding author: Dr. K. Devaki, Assistant Professor, Department of Biochemistry, Karpagam University, Coimbatore, India.
Tel: 091-0422-2645377
Fax: 091-0422-2611043
E-mail: dr.devaki.bc@gmail.com
ARTICLE INFO
Article history:
Received in revised form 25 February 2013
Accepted 15 March 2013
Available online 20 June 2013
 Journal of Acute Disease2013年2期
Journal of Acute Disease2013年2期
- Journal of Acute Disease的其它文章
- An overview of the current methodologies used for evaluation of aphrodisiac agents
- Anti-inflammatory activity of leaves of Jatropha gossypifolia L. by hrbc membrane stabilization method
- Chemomodulatory effects of Ichnocarpus frutescens R. Br against 4-vinylcyclohexane induced ovarian cancer in swiss albino mice
- Development of bioanalytical parameters for the standardization of Zingiber officinale
- A review on antimicrobial efficacy of some traditional medicinal plants in Tamilnadu
- Medicinal significance, pharmacological activities, and analytical aspects of solasodine: A concise report of current scientific literature
