Chemomodulatory effects of Ichnocarpus frutescens R. Br against 4-vinylcyclohexane induced ovarian cancer in swiss albino mice
Thangarajan Starlin, Perumal Sathiyanathan, Chinthamony Arul Raj, Paramasivam Ragavendran, Balasubramanian Vidya, Martin Sunitha, Velliyur Kanniappan Gopalakrishnan,*
1Department of Bioinformatics, Karpagam University, Coimbatore, Tamilnadu, India, 641 021
2Department of Biochemistry, Karpagam University, Coimbatore, Tamilnadu, India, 641 021
3Department of Chemistry, Vel Tech College, Avadi, Chennai, Tamilnadu, India, 600062
Chemomodulatory effects of Ichnocarpus frutescens R. Br against 4-vinylcyclohexane induced ovarian cancer in swiss albino mice
Thangarajan Starlin1, Perumal Sathiyanathan2, Chinthamony Arul Raj2, Paramasivam Ragavendran2, Balasubramanian Vidya2, Martin Sunitha3, Velliyur Kanniappan Gopalakrishnan1,2*
1Department of Bioinformatics, Karpagam University, Coimbatore, Tamilnadu, India, 641 021
2Department of Biochemistry, Karpagam University, Coimbatore, Tamilnadu, India, 641 021
3Department of Chemistry, Vel Tech College, Avadi, Chennai, Tamilnadu, India, 600062
4-vinylcyclohexane
Antioxidants
Ethanolic extract
Ichnocarpus frutescens
Objective: To evaluate the protective effect of Ichnocarpus frutescens (I. frutescens) R. Br against 4-vinylcyclohexane induced ovarian cancer in Swiss albino mice. Method: The ovarian cancer was induced by 4-vinylcyclohexane which was given to 28-day-old mice in corn oil, intra peritoneally, at 2.7 mmol/kg body weight/day for 30 d. After that serum and tissue were isolated. Enzymatic antioxidants, non-enzymatic antioxidants, lipid peroxidation assessed in liver and ovary homogenate. Metabolites, marker enzymes were analyzed in serum. Tissue was examined histopathology examination. Result: The levels of urea, creatinine, marker enzymes and lipid peroxidation were significantly increased in ovarian cancer induced mice when compared to control group whereas, levels of uric acid, enzymatic and non-enzymatic antioxidants were decreased in cancer induced mice when compared with control mice. From these parameters were brought back to near normal in ethanolic extract of I. frutescens treated animals. The above results were further confirmed by histopathological examination which shows marked edema of lamina propria occurred in cancer induced animals whereas no alterations in ethanolic extract of I. frutescens and cisplatin treated groups. Conclusion: This present study was evaluated that the ethanolic extracts of I. frutescens have effective anticancer activity against 4-vinylcyclohexane induced ovarian cancer.
1. Introduction
Cancer is a complex genetic disease that is caused primarily by environmental factors. Ovarian cancer (OC) is the most lethal malignancy of the female reproductive system and the fifth leading cause of cancer death in women. Ninety percent of Ovarian Cancer is thought to arise from the epithelium and its inclusion cysts due to multiple genetic changes[1]. Animal models of ovarian cancer are crucial for understanding the pathogenesis of the disease and for testing new treatment strategies[2]. Ovarian cancer causes more deaths than any other gynecologic cancer, but it accounts for only about 3percent of all cancers in women. 4-vinylcyclohexene causes wide destruction of small preantral (primordial and primary) follicles, leading to premature ovarian failure in mice and rats. It is an occupational chemical released during the manufacture of rubber tires, plasticizers and pesticides.
Ichnocarpus Frutescens (I. Frutescens) R. Br. (Apocyanaceae) is an evergreen, laticiferous, wood creeper with rusty red appearance, found throughout India. It is commonly known as black creeper and ‘dudhilata’. It is a species of flowering plant in the dogbane family known by the common name black creeper. It is generally called as a “blood purifier. The roots are reported to possess demulcent, tonic, diaphoretic and diuretic properties used in fever, dyspepsia and skin troubles[3] and also found to possess analgesic and anti-inflammatory activity. It has been reported that natural antioxidants in fruits and vegetables were inversely related with the risk of many chronic diseases, such as cardiovasculardiseases and cancer[4]. Hence the interest towards natural sources has become invasively increased by screening the plant for phytochemicals and non-toxicity. The present study is aimed to evaluate the protective effect of I. frutescens against 4-vinylcyclohexene induced ovarian cancer in swiss albino mice.
2. Materials and methods
2.1. Collection of plant material
The whole plant of I. frutescens was collected from in and around Coimbatore district. The plant material was identified by Dr. G.V.S. Murthy, Botanical Survey of India, Tamilnadu Agriculture University Campus, Coimbatore, Tamilnadu and the voucher number is (BSi/ SRC/5/23/2001-12/Tech-1326). The whole plant was washed well with water. They were air dried at 25 ℃ for 5 d in the absence of sunlight and powered well using a mixer. This powdered material is used for the study.
2.2. Preparation of plant extract
50 g of the powdered plant material I. frutescens is weighed and extracted with 250 mL of ethanol for 72 h in soxhlet apparatus, concentrated and used for further studies.
2.3. Experimental animals
Female mice weighing about 25-35 g were obtained from the animal house of Karpagam University, Coimbatore and were used for the study. Mice were housed in polycarbonate cages in a room with a 12 h day-night cycle, at constant temperature of 22 ℃ and humidity of 45%-64%. During the experimental study mice were fed on pellets (Gulmohur rat feed, Lipton India, Bangalore) with free access to tap water. The animal study was approved by IAEC.
2.4. Experimental protocol
The animals were divided into five groups. Group 1 served as control animals, Group 2 received ovarian cancer induced by 4-vinyl cyclohexane were mixed with corn oil by intraperitoneally administration, at 2.7 mmol/ kg bw/d for 30 d. Group 3 served as carcinogenic mice treated with plant extract at a concentration of 400 mg/kg body weight, Group 4 served as carcinogenic mice treated with standard drug, cisplatin. Group 5 received control animals treated with plant extract only at a dose of 400 mg/kg bw.
After 30 d all the animals were killed, blood was collected under jugular vein, serum were separated by centrifugation which is used for the estimations of urea[5], uric acid[6], creatinine[7], AST and ALT[8], ALP[9]. The liver and ovary was excised rinsed in ice-cold normal saline solution followed by cold 0.1 M Tris-HCl (pH 7.4), blotted, dried and weighed. A 10% w/v homogenate was prepared in 0.1 M Tris-HCl buffer and used for the estimations of Protein[10], Superoxide Dismutase[11], Catalase[12], Glutathione Peroxidase[13], Glutathione-S-Transferase[14], Total Reduced Glutathione[15], Vitamin C[16], Vitamin E[17], and lipid peroxidation (LPO)[18]. Sections of liver and ovaries were fixed with 10% formalin, embedded in paraffin sectioned at 5 μm thick and stained with haematoxylin and eosin for histological analysis.
2.5. Statistical analysis
Results are expressed as the mean ± SD. Statistical significance was evaluated by One Way Analysis of Variance (ANOVA) using SPSS version (10.0) and the individual comparisons were obtained by the Duncan multiple range test (DMRT)[19]. A value of P<0.05 was considered to indicate a significant difference between groups.
3. Results
The ethanolic extract of the plant I. frutescens was subjected to mice to evaluate the anti-ovarian cancer activity and the histopathological analysis was carried out. Activities of biochemical profiles and liver marker enzymes in serum were shown in Table 1. Data pertaining to the levels of urea, creatinine and liver marker enzymesare significant elevated in the ovarian cancer induced group compared to normal control group however the level of uric acid was depleted in group 2 mice. All these parameters were restored to near normal levels in group 3, 4 treated animals when compared to group 2. There is no change in the levels of those parameters in group 5 when compared to group 1.
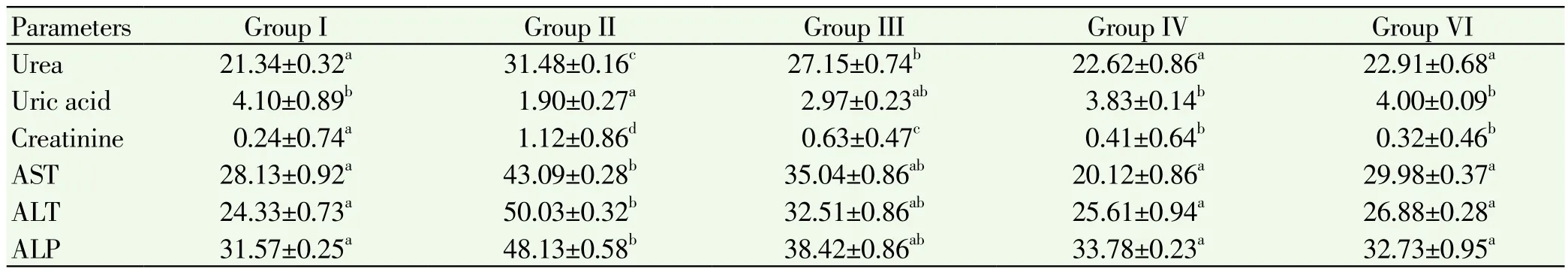
Table 1.Estimations of urea, uric acid, creatinine, ALT, AST, ALP in serum of control and experimental mice.
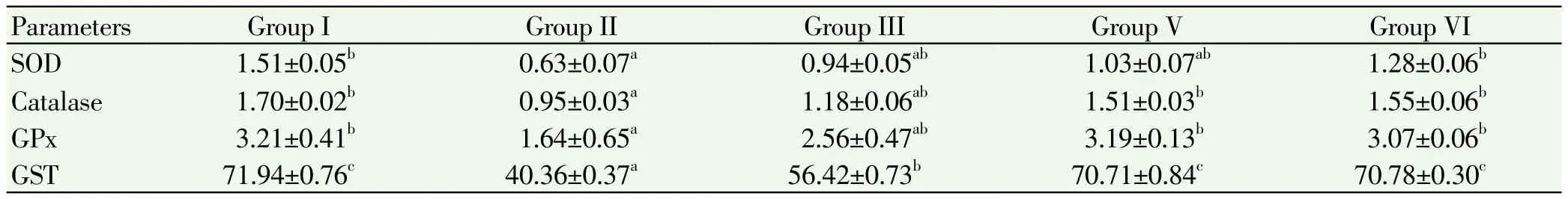
Table 2.Estimation of SOD, catalase, glutathione peroxidase, glutathione s-transferase in liver of control and experimental mice.
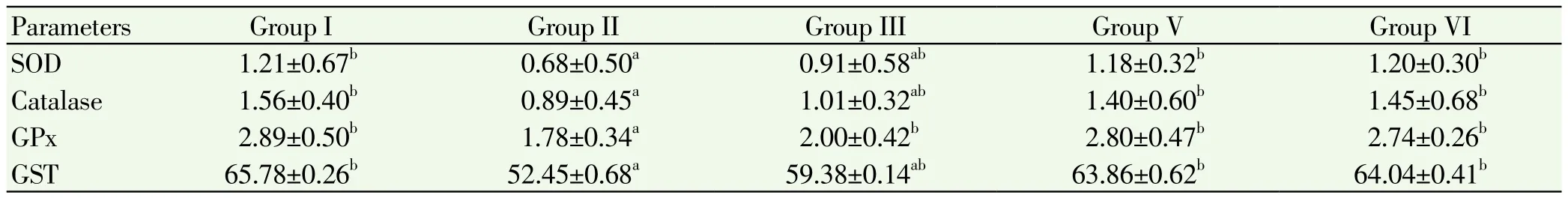
Table 3.Estimation of SOD, catalase, glutathione peroxidase, glutathione s-transferase in ovary of control and experimental mice.
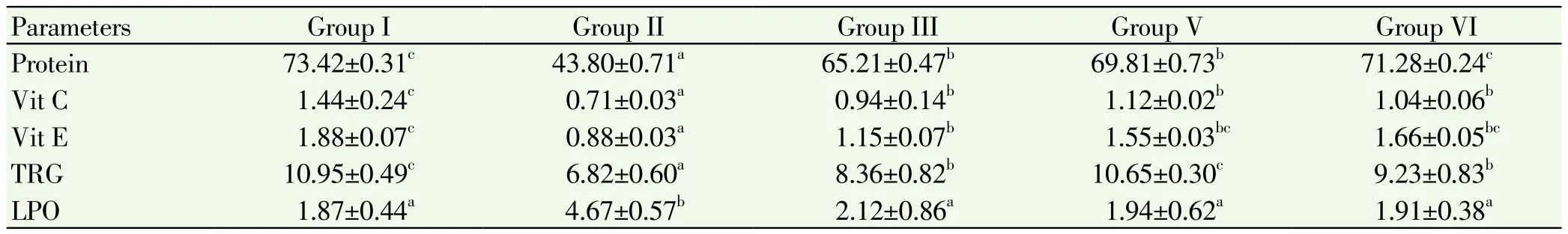
Table 4.Estimation of protein, Vitamin C, Vitamin E, total reduced glutathione in liver of control and experimental mice.
The levels of enzymatic antioxidants like superoxide dismutase, catalase, glutathione peroxidase and glutathione S-transferase in liver and ovary of control and experimental mice were showed in Table 2 and 3. The levels of these enzymes in liver and ovary are significant depleted (P<0.05) in the cancer induced group compared to normal control group. Treatment with ethanolic extract and standard drug administration showed significant increased of those enzymes in group 3, 4 treated animals when compared to group 2. No alterations in the levels of these enzymes in group 5 when compared to group 1.
Levels of non enzymatic antioxidants in liver and ovary of control and experimental animals were showed in Table 4 and 5. The levels of these enzymes in liver and ovary are significant decreased (P<0.05) in the cancer induced group compared to normal control Group. But the levels of LPO showed increased in cancer induced Group. Treatment with plant extract and cisplatin showed significant increased in group 3, 4 animals when compared to group 2. No variation in the levels of these enzymes in group 5 when compared to group 1.
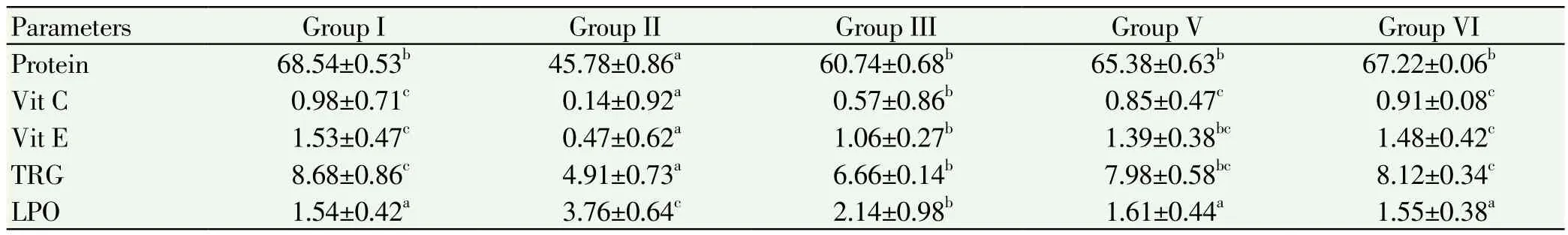
Table 5.Estimation of protein, Vitamin C, Vitamin E, total reduced glutathione in ovary of control and experimental mice.
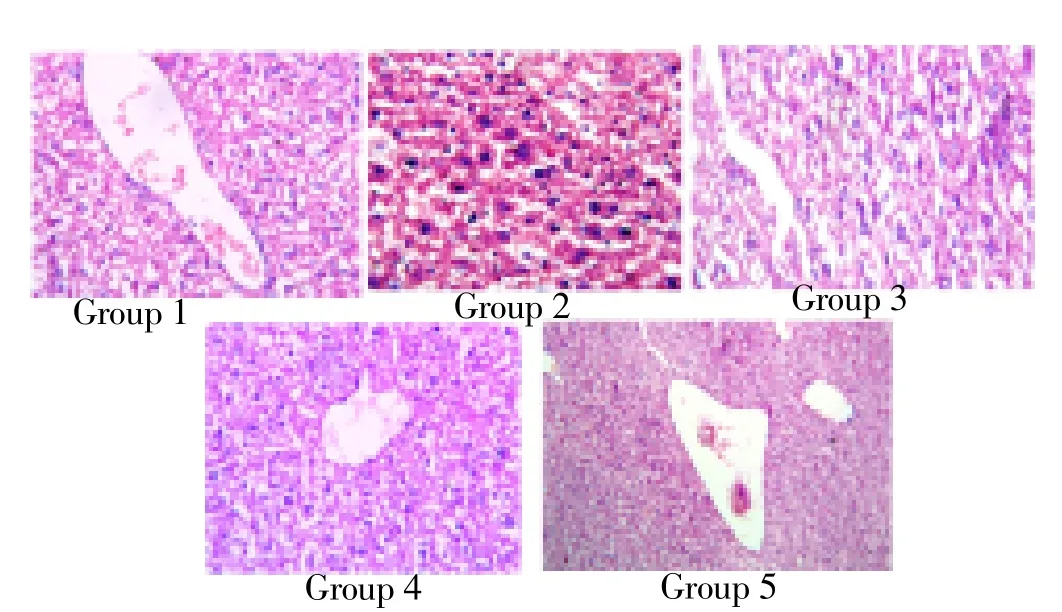
Figure 1. Histopathological analysis of liver.
Figure 1 & 2 shows the histopathological examination revealed alterations in the liver and ovary of 4-vinyl cyclohexane induced mice. There is marked edema of lamina propria in group II animals. In some areas there is dense lymphoid hyperplasia expanding the mucosa and submucosa. The pathomorphological changes observed in 4-vinyl cyclohexane induced intestinal damage become apparently normal after treatment with ethanol extract of I. frutescens (400 mg/kg bw) and with standard cisplatin whereas animals treated with ethanol extract of I. frutescens alone showed normal architecture.
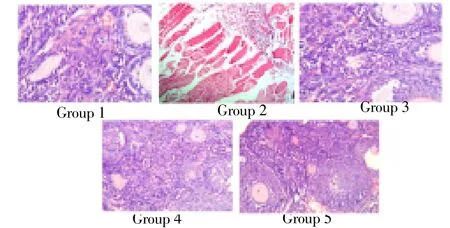
Figure 2. Histopathological analysis of ovary.
4. Discussion
The study entails the protective effect of I. frutescens against 4-vinylcyclohexene induced ovarian cancer. I. frutescens is a medicinal plant to treat against various disorders. This present study is aimed to explore the anti -cancer effect of oral administration of I. frutescens against 4-vinylcyclohexene induced ovarian cancer in rats. Uric acid, the metabolic end product of purine metabolism, has proven to be a selective antioxidant, capable especially of reacting with free radicals and hypochlorous acid[20]. Selvakumar et al[21]who reported the increased levels of creatinine and urea in fibrosarcoma transplanted animals this was reverted to normal on treatment with I. aspalathoides. Elevation of urea might be a consequence of impaired solute transport in the proximal tubules. Rejection of solutes in the proximal tubules would retard water reabsorption and ultimately enhance fluid delivery to the distal nephron. This would increase the driving force for urea reabsorption in the collecting duct[22].
It is well known that the elevation of AST and ALT activity is repeatedly credited to hepatocellular damage[23]. Srigopalram et al[24]reported that a significant increase (P<0.05) in the levels of serum AST and ALT in DEN induced animals was observed and it was significantly decreased by treatment with Cassia fistula leaf extract. The increased activity of liver marker enzymes was brought back to near normal levels by the therapeutic efficacy of the drug.
On induction of carcinogen there is over helming of oxidative stress and in these conditions, the various inherent defense mechanisms (such as the antioxidant defense mechanisms, intracellular concentration of glutathione, superoxide dismutase (SOD) and catalase (CAT) activities become significantly impaired and insufficient[25].
Glutathione Peroxidase-1 (GPx1) is an enzyme playing an important role in the defense against oxidative stress which is associated with many pathological conditions. Thus, changes in the expression of this enzyme in different human tissues and fluids could be an indicator used for oxidative status assessment[26]. Pracheta et al[27]have reported decline in the levels of antioxidants in cancer induced mice which are brought back to near normal level on administration with Euphorbia neriifolia.
Vitamin C or L-ascorbic acid or L-ascorbate is an essential nutrient for humans and certain other animal species. In living organisms ascorbate acts as an antioxidant by protecting the body against oxidative stress. It is also a cofactor in at least eight enzymatic reactions including several collagen synthesis reactions that, when dysfunctional, cause the most severe symptoms of scurvy. In animals these reactions are especially important in wound-healing and in preventing bleeding from capillaries. Vitamin C may reduce carcinogenesis through the stimulation of immune systems, where cytotoxic T lymphocytes, macrophages, and natural killer cells can lyses tumor cells[28]. Vitamin E has very good antioxidant properties which neutralize reactive oxygen molecules which reduces oxidative DNA damage and genetic mutation[29]. Vitamin E can directly scavenge reactive oxygen molecules. Vitamin E is thought to be an important chain-breaking antioxidant, which plays an important role in various stages of carcinogenesis through its contribution to immuno competence, membrane and DNA repair and decreasing oxidative DNA damage[30].
All these observations clearly indicate that the ethanolic extract of I. frutescens showed a significant anticancer activity against 4-vinylcyclohexane induced ovarian cancer. Further studies is to characterize the active principles and to elucidate the mechanism action are in progress.
Conflict of interest
We declare that we have no conflict of interest
Acknowledgements
We, the authors are thankful to our Chancellor, Chief Executive Officer, Vice-Chancellor and Registrar of Karpagam University for providing facilities and encouragement.
[1] Romero I, Bast RC. Mini review: Human ovarian cancer: biology, current management and paths to personalizing therapy. Endocrinology 2012; 153: 1593-1602.
[2] Stewart SL, Querec TD, Ochman AR, Gruver BN, Bao R, Babb JS. Characterization of a carcinogenesis rat model of ovarian preneoplasia and neoplasia. Cancer Res 2009; 64: 8177-8183.
[3] Chaudhary K, Aggarwal B, Singla RK. Ichnocarpus frutescens: A medicinal plant with broad spectrum. Indo Global J Pharm Sci 2012; 2: 63-69.
[4] Singh NK, Singh VP. Phytochemistry and pharmacology of Ichnocarpus frutescens. Chin J Nat Med 2012; 10: 241-246.
[5] Natelson S, Scott M, Beffa C. A rapid method for the estimation of urea in biologic fluids, by means of the reaction between diacetyl and urea. Am J Clin Pathol 1951; 21: 1153-1172.
[6] Caraway WT. Uric acid. In: Selingson D, (Ed.) Standard methods of clinical chemistry. New York: Academic press; 1963, p. 239-247.
[7] Owen JA, Iggo JB, Scandrett FJ, Stemart IP. Determination of creatinine in plamsma or serum, a critical examination. Biochem J 1954; 58: 426-437.
[8] Reitman S, Frankel S. A calorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminase. Am J Clin Pathol 1957; 28: 56-63.
[9] King EJ, Armstrong AR. Estimation of alkaline phosphatase. Can Med Assoc J 1934; 311: 152-156.
[10] Lowry OH, Rosebrough NJ, Farr AL, Randall RJ, Protein measurement with Folin phenol reagent. J Biol Chem 1951; 193; 265-275.
[11] Das S, Vasishat S, Snehlata R, Das N, Srivastava LM, Correlation between total antioxidant status and lipid peroxidation in hypercholesterolemia. Curr Sci 2000; 78: 486-487.
[12] Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972; 47: 389-394.
[13] Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973; 179: 588-590.
[14] Mannervik B, Theisozymes of glutatione transferase. Adv Enzymol Relat Areas Mol Biol 1985; 57: 357-417.
[15] Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochem Biophys Acta 1979; 582: 67-78.
[16] Omaye ST, Turbull, TP, Sauberchich HC. Selected methods for determination of ascorbic acid in cells, tissues and fluids. Methods Enzymol 1979; 6: 3-11.
[17] Rosenberg HR. Chemistry and physiology of the vitamins. New York: Inter science publishers Inc; 1992, p. 452-453.
[18] Niehius WG, Samuelsson D. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipidperoxidation. Eur J Biochem 1968; 6: 126-130.
[19] Duncan BD. Multiple ranges tests for correlated and heteroscedastic means. Biometrics 1957; 13: 359-364.
[20] Hasugawa T, Kuroda M. A new role of uric acid as antioxidant in human plasma. Jpn J Clin Pathol 1989; 37: 1020-1027.
[21] Selva Kumar S, Ram Krishna Rao M, Balasubramanian MP. Chemopreventive effects of Indigofera aspalathoides on 20-methylcholanthrene induced fibrosarcoma in rats. Int J Cancer Res 2011; 7: 144-151.
[22] Jayakumar T, Sridhar MP, Bharathprasad TR, Ilayaraja M, Govindasamy S, Balasubramaniam MP. Experimental studies of Achyranthes aspera (L) preventing nephrotoxicity induced by lead in albino rats. J Health Sci 2009; 55: 701-708.
[23] Al-Rejaie SS, Aleisa AM, Al-Yahya AA, Bakheet SA, Alsheikh A, Fatani AG, et al. Progression of diethylnitrosamine-induced hepatic carcinogenesis in carnitine-depleted rats. World J Gastroenterol 2009; 21: 1373-1380.
[24] Srigopalram S, Jayraaj IA. Effect of Terminalia chebula retz on den induced hepatocellular carcinogenesis in experimental rats. Int J Pharm Pharm Sci 2012; 4: 440-445.
[25] Szymonik-Lesiuk S, Czechowska G, Stryjecka-Zimmer M, Słomka M, Madro A, Celiński K, et al. Catalase, superoxide dismutase, and glutathione peroxidase a ctivities in various rat tissues after carbon tetrachloride intoxication. J Hepatobiliary Pancreat Surg 2003; 10: 309-315.
[26] Gomez-Espina J, Blanco-Gonzalez E, Montes-Bayon M, Sanz-Medel A. Elemental mass spectrometry for Sedependent glutathione peroxidase determination in red blood cells as oxidative stress biomarker. J Anal At Spectrom 2012; 27: 1949-1954.
[27] Pracheta P, Sharma V, Singh L, Paliwal R, Sharma S, Yada S. Chemoprotective activity of hydro-ethanolic extract of Euphorbia neriifolia Linn leaves against DENA-induced liver carcinogenesis in mice. Biol Med 2011; 3: 36-44.
[28] Hussein HK, Elnaggar MH, Al-Dailamy JM. Protective role of Vitamin C against hepatorenal toxicity of fenvalerate in male rats. Global Adv Res J Environ Sci Toxicol 2012; 1: 60-65.
[29] Frei B. Reactive oxygen species and antioxidant vitamins: Mechanisms of action. Am J Med 1994; 97: 5S-13S.
[30] Kimmick G, Bell RA, Bostick RM. Vitamin E and breast cancer: A review. Nutr Cancer 1997; 27: 109-117.
25 March 2013
*Corresponding author: Dr. V. K. Gopalakrishnan, Professor and Head, Department of Biochemistry and Bioinformatics, Karpagam University, Coimbatore - 641 021, Tamil Nadu, India.
Tel: 091-0422-6453777
Fax: 091-0422-2611043
E-mail: vkgopalakrishnan@gmail.com
ARTICLE INFO
Article history:
Received in revised form 29 March 2013
Accepted 30 March 2013
Available online 20 June 2013
 Journal of Acute Disease2013年2期
Journal of Acute Disease2013年2期
- Journal of Acute Disease的其它文章
- An overview of the current methodologies used for evaluation of aphrodisiac agents
- Anti-inflammatory activity of leaves of Jatropha gossypifolia L. by hrbc membrane stabilization method
- Development of bioanalytical parameters for the standardization of Zingiber officinale
- Phytochemical screening and HPTLC finger printing analysis of Citrullus lanatus (Thunb.) seed
- A review on antimicrobial efficacy of some traditional medicinal plants in Tamilnadu
- Medicinal significance, pharmacological activities, and analytical aspects of solasodine: A concise report of current scientific literature
