Anti-inflammatory activity of leaves of Jatropha gossypifolia L. by hrbc membrane stabilization method
Yerramsetty Nagaharika, Valluri kalyani, Shaik Rasheed, Ramadosskarthikeyan*
1Department of Pharmacognosy, Vignan Pharmacy College, Vadlamudi-522213, A.P., India
2Department of Pharmacology, School of Pharmaceutical Sciences and Technology, JNTU Kakinada - 533003, A.P., India
Anti-inflammatory activity of leaves of Jatropha gossypifolia L. by hrbc membrane stabilization method
Yerramsetty Nagaharika1, Valluri kalyani1, Shaik Rasheed2, Ramadosskarthikeyan1*
1Department of Pharmacognosy, Vignan Pharmacy College, Vadlamudi-522213, A.P., India
2Department of Pharmacology, School of Pharmaceutical Sciences and Technology, JNTU Kakinada - 533003, A.P., India
Jatropha gossypifolia L.
Leaves
Aqueous extract
Alcoholic extract
HRBC membrane stabilization
Object: To evaluate the anti inflammatory activity of leaves extracts of Jatropha gossypifolia (J. gossypifolia) L. Methods: The plant J. gossypifolia L. (Eurphorbiaceae) is known as belly ache bush. The plant originated from Brazil and it is now cultivated in tropical countries throughout the world. The roots, stems, leaves, seeds and fruits of the plant have been widely used in traditional folk medicine in many parts of West Africa. The young stem of the plant is used as tooth brush as well as to clean tongue in the treatment thrush. The tuber of the plant grinded into a paste is also locally used in the treatment of hemorrhoids. The present study aimed to evaluate the anti inflammatory activity of aqueous and alcoholic extract of J. gossypifolia leaves by in vitro HRBC membrane stabilization method. Results: The in vitro method showed significant anti inflammatory property of different extracts tested. Conclusion: The aqueous extract at a concentration of 200 μg/mL showed significant activity when compared with the standard drug Diclofenac sodium.
1. Introduction
Inflammation is a reaction of living tissues towards injury and it comprises systemic and local responses[1]. Inspite of our dependence on local medicine and the tremendous advances in synthetic drugs, a large number of the world populations (80% of people) cannot afford the products of the western pharmaceutical industry and have to rely upon the use of traditional medicines, which are mainly derived from plant material. The fact is well recognized by the WHO which has recently complied an inventory of medicinal plants listing over 20 000 species. The family Euphorbiaceous consists of several important medicinal plants with wide range of pharmacological, biological activities and interesting phytochemical constituents. The main action of anti inflammatory agents is the inhibitionof cyclooxygenase enzymes which are responsible for the conversion of arachidonic acid to prostaglandins. Since human red blood cell (HRBC) membranes are similar to these lysosomal membrane components, the prevention of Hypotonicity induced HRBC membrane lysis was taken as a measure in estimating the anti inflammatory property of various extracts of Jatropha gossypifolia (J. gossypifolia) L. Thus human blood cell membrane stabilization (HRBC method[2]) has been used as a method in estimating the anti inflammatory property. In certain parts the leaf of this plant was traditionally used in the treatment of hemorrhoids. The present study aimed to authenticate the traditional anti inflammatory activity of this species by in vitro anti inflammatory screening.
2. Materials and methods
2.1. Preparation of extracts
The plant material was collected from the plant J. gossypifolia L. which are collected during the monthof December at Potharlanka near Repalle, Guntur (Dist) of Andhra Pradesh. Then it was authentified by Dr. SM. Khasim, professor, Department of Botany and Microbiology, Acharya Nagarjuna University, Nagarjuna nagar, Guntur. The shade dried leaves were powdered and extracted with soxhlet apparatus using ethanol (yield 5.8%) and distilled water (yield 4.2%) separately. Both the extracts were evaporated to dryness. The samples were prepared by suspending the residues in hot water and used for anti–inflammatory study.
2.2. Chemicals and instruments
All chemicals used in the study were of analytical grade. Dextrose, Sodium citrate, Citric acid, Sodium chloride, Sodium hydroxide and Dihydrogen phosphate was purchased from SD fine chemicals, Mumbai. Reference standard Diclofenac was obtained from Cipla Ltd, Bangalore. Shimadzu 1701 UV-Visible spectrophotometer was used for the estimation of anti inflammatory activity.
2.3. Acute oral toxicity
The acute toxicity for the JGE was conducted in rats with body weight 200–250 g as per the prescribed guidelines. Ten animals of either sex were used in each group of extract. Their weights were recorded before beginning the study. They were administered with bolus dose of the JGE (500, 1 000, 1 500, 2 000 mg/kg) per oral and observed over 14 d for mortality and physical or behavioral changes[3]. The study was approved by IAEC of Vignan Pharmacy College, Vadlamudi. (1499/PO/a/11/CPCSEA).
2.4. In vitro anti – inflammatory activity
The anti inflammatory activity of leaf extract of Jatropha gossypifolia Linn was determined by HRBC membrane stabilization method. Blood was collected from healthy volunteers. The collected blood was mixed with equal volume of (2% dextrose, 0.8% sodium citrate, 0.05% citric acid & 0.42% sodium chloride in water). The blood was centrifuged at 300 rpm and packed cells were washed with isosaline (0.85%, pH 7.2) & 10% v/v suspension was made with isosaline.
The assay mixture contained the drug (concentration as mentioned in Table 1). 1 mL phosphate buffer (0.15 M, pH 7.4), 2 mL of hyposaline (0.36%) % 0.5 mL of HRBC suspension. Diclofenac was used as the reference drug. Instead of hyposaline, 2 mL of distilled water was used as control. All the assay mixtures were collected at 37 ℃for 30 min and centrifuged. The hemoglobin content in the supernatant solution was estimated using colorimeter at 560 nm. The percentage heamolysis was calculated by assuming the heamolysis produced in the presence of distilled water as 100%. The percentage of HRBC membrane stabilization or protection was calculated using the following formula[4-8].
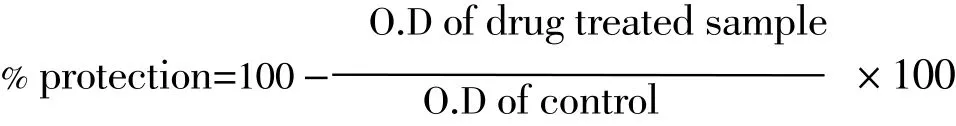
2.5. Statistical analysis
Statistical analysis was done using one way ANNOVA followed by Dunnets test. P values greater than > 0.05 were considered as significantC
3. Results
3.1. Acute oral toxicity study
The extracts of J. gossypifolia L. did not show any sign of toxicity up to 2 000 mg/mL body weight and hence it was considered to be safe.
3.2. In vitro anti inflammatory activity
J. gossypifolia L. ethanolic and aqueous extracts at different concentrations (100, 200 μg/mL) showed significant stabilization towards HRBC membrane. The percentage protection of aqueous extract at concentration 200 μg/mL was higher than that of concentrations. However the percentage protection was found to be increased at higher concentrations. The results were tabulated in Table 1.
Table 1Protection of heamolysis from HRBC membrane stabilization method.
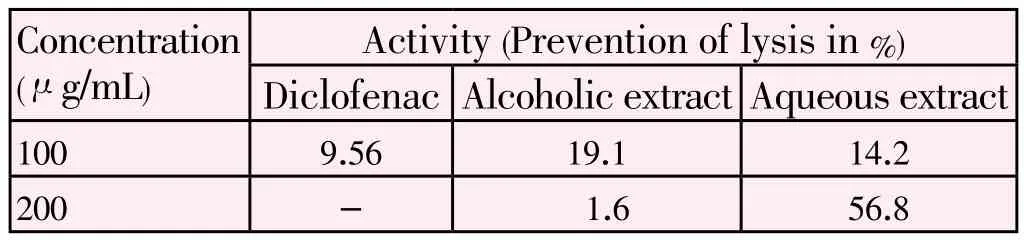
Table 1Protection of heamolysis from HRBC membrane stabilization method.
Aqueous extract is extremely significant, Alcoholic extract is significant.
Activity (Prevention of lysis in%) Diclofenac Alcoholic extract Aqueous extract 100 9.56 19.1 14.2 200 - 1.6 56.8 Concentration (μg/mL)
4. Discussion
In the present study the preliminary phytochemical screening was studied in broad sense to explore its chemical nature, it reveals the presence of considerable amount of alkaloid, steroid, phenolic substances and vitamin C (Ascorbic acid), moderately saponins and carbohydrates, trace amount of glycoside and resins were explored from the phytochemical screening. Anti inflammatory activity of its various extracts wereperformed to explore its bioefficiency. The study was took HRBC membrane stabilization method for screening of activity[9,10]. The results revealed the methanolic extract of J. gossypifolia showed percentage lysis of 19.1% for 100 mg/mL followed by 1.6% for 200 mg/mL of the methanolic extract. Where as aqueous extract showed 14.2% for 100 mg/mL and 56.8% for 200 mg/mL. Hence this study concludes methyanolic extrac of J. gossypifolia produced significant activity (membrane protection) tabulated in Table 1. The anti inflammatory activity may be due to alkaloid or steroid present in the alcoholic and aqueous extract of J. gossypifolia.
Conflict of interest
The authors declare they have no conflict of interests.
Acknowledgements
The authors are thankful to the management of Vignan Pharmacy College, Vadlamudi for rendering necessary facilities to complete this work.
[1] Mohamed Saleem TK, Azeem AK, Dilip C, Sankar C, Prasanth NV, Duraisami R. Anti-inflammatory activity of the leaf extacts of Gendarussa vulgaris Nees. Asian Pac J Trop Biomed 2011; 147-149.
[2] Ejebe DE, Siminialayi IM, Emudainowho JOT, Ofesi U, Morka L. Analgesic and anti inflammatory activities of the ethanol extract of the leaves of Helianthus annus in wistar rats. Asian Pac J Trop Med 2010; 3(5): 341-347.
[3] Anonymus. The Wealth of India, Raw Materials Publication and Information Directorate Vol.I. New Delhi: CSIR; 1992, p. 305-308.
[4] Purshottam Kaushik, AnilKumar Dhiman. Medicinal Plants of Raw Drugs of India. 1999, p. 304.
[5] Kirtikar KR, Basu BD. Indian Medicinal Plants, Vol 2. (CM Basu, Allahabad); 1933, p. 1777-1781.
[6] Government of India. The Ayurvedic pharmacopoeia of India, Part–I, Vol. II. New Delhi: Departmaent of Indian Sysytems of Medicine & Homeopathy; 2001, p. 155-157.
[7] Gandhidasan R, Thamaraichelvan A, Baburaj S. Anti inflammatory action of Lannea coromandelica by HRBC membrane stabilization. Fitoterapia 1991; 62: 81-83.
[8] Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 5th Edn. India: Elsevier; 2005, p. 27-29.
[9] Bruton LL. Goodman and Gilman’s pharmacological basis of therapeutics. 11th Ed. USA: McGrew Hill; 2005, p. 1102-1104.
[10] Di Rosa M, Giroud JP, Willoughby DA. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol 1971; 104: 15–29.
Congratulation Journal of Acute Disease cooperation with Elsevier
Our rapid growing journal, Journal of Acute Disease (ISSN 2221-6189) is proud to announce the cooperation with Elsevier (www.elsevier.com).
With its headquarters based in Amsterdam, Netherlands, Elsevier serves more than 30 million scientists, students, and health and information professionals worldwide. It publishes about 2 000 journals, 19 000 books; with 2 000 new books each year. Its archives contain 7 million past publications. Total yearly downloads amount to 240 million.
2 April 2013
*Corresponding author: Ramadoss Karthikeyan, Assistant Professor, Department of Pharmacognosy, Vignan Pharmacy College, Vadlamudi-522213, A.P., India.
Tel: 09966847127
E-mail: rkcognosy@gmail.com
ARTICLE INFO
Article history:
Received in revised form 29 April 2013
Accepted 30 April 2013
Available online 20 June 2013
 Journal of Acute Disease2013年2期
Journal of Acute Disease2013年2期
- Journal of Acute Disease的其它文章
- An overview of the current methodologies used for evaluation of aphrodisiac agents
- Chemomodulatory effects of Ichnocarpus frutescens R. Br against 4-vinylcyclohexane induced ovarian cancer in swiss albino mice
- Development of bioanalytical parameters for the standardization of Zingiber officinale
- Phytochemical screening and HPTLC finger printing analysis of Citrullus lanatus (Thunb.) seed
- A review on antimicrobial efficacy of some traditional medicinal plants in Tamilnadu
- Medicinal significance, pharmacological activities, and analytical aspects of solasodine: A concise report of current scientific literature
