Pancreatic duct disruption and nonoperative management: the SEALANTS approach
Baltimore, USA
Pancreatic duct disruption and nonoperative management: the SEALANTS approach
Alain Abdo, Niraj Jani and Steven C Cunningham
Baltimore, USA
Pancreatic-duct disruption (PDD) can be diff i cult to manage, with diverse etiologies and sequelae in a heterogeneous population. Common etiologies include pancreatitis, iatrogenic injury, and trauma. Sequelae of PDD include pseudocyst, pancreatic ascites, pancreatic pleural effusion, and fi stulae. Although there are some principles, such as pancreas rest and nutritional support, which are generally agreed upon, no universal consensus exists regarding which specif i c treatment strategies are best, and at which pace.
Available treatment modalities are diverse and numerous, but available data supporting them are often either conf l icting or low-quality. Therefore, pancreatologists have different points of view regarding optimal management. Areas of controversy where thoughtful providers disagree include the following: immediate octreotide vs no or selective octreotide use; early aggressive percutaneous drainage vs limited conservative drainage; immediate nil-per-os (NPO) status with total parenteral nutrition (TPN) vs oral diet as tolerated; mandatory antacid (typically proton-pumpinhibitor [PPI]) therapy vs PPI therapy only as otherwise indicated (e.g., for peptic ulcer disease); early endoscopic pancreatic duct stent vs no stent or its selective use.
Similarly great diversity exists in the literature regarding the time required for the PDD to seal. In a recent review of the literature, performed as part of the Dutch Pancreatitis Study Group's study of endoscopic pancreatic-duct stenting versus conservative treatment for pancreatic fi stulas due to necrotizing pancreatitis, the range was wide, from 2 to 122 days following placement of the stent.[1]Most of the more recent (2005 or later) and larger (35 or greater patients) studies, however, in that review are on the longer end of the spectrum (71-122 days).[1,2]
In an effort in improving upon such long times required for PDD to seal, an aggressive, "shotgun" approach was adopted, as described below, and termed SEALANTS: somatostatin analogues (SAs), external drainage, alternative nutrition, antacids, NPO status, TPN, and stenting of the pancreatic duct. Although there is nothing novel about these approaches, they are often employed in a slowly progressive stepwise fashion, as opposed to the more aggressive, "shotgun" SEALANTS approach (Fig. 1).
Review of SEALANTS: treatments for PDD
SAs
Somatostatin, as the "body stop" etymology suggests, has broad inhibitory effects within the body. For example, in the brain it inhibits release of growth hormone andthyroid-stimulating hormone. In the gastrointestinal (GI) tract, actions include inhibition of the release of glucagon, insulin, secretin, and cholecystokinin, as well as inhibition of pancreatic exocrine secretions. In 1989, Williams et al studied the SA octreotide (half life >100 minutes versus 3 minutes for somatostatin) in patients with known pancreatic fi stula and showed a mean decrease in pancreatic fl uid output of 75%. These GI functions are therefore the basis for the use of SA in preventing and treating pancreatic fi stula.
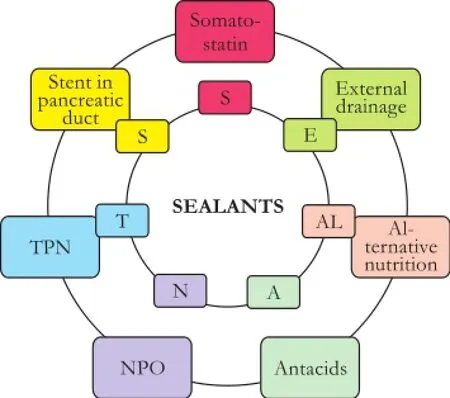
Fig. 1.The "shotgun" SEALANTS approach.
Many randomized controlled trials (RCTs) have been performed to study the use of octreotide as prophylaxis against postpancreatectomy pancreatic fi stula. This diverse literature (17 trials including 2143 patients) has been recently analyzed by the Cochrane Collaboration[3,4]revealing a lower incidence of pancreatic fi stula in the SA group (RR: 0.64; 95% CI: 0.53 to 0.78).
Regarding treatment of established pancreatic fi stula following PDD, the literature is even more diverse and diff i cult to interpret. A systematic review of 10 RCTs (301 patients) performed by Li-Ling and Irving in 2001[5]revealed evidence suggesting a role for SA in treating pancreatic fi stula: two of three trials evaluating time to closure showed signif i cantly reduced time in the treatment group.[6-8]A decade later, Gans et al[9]performed an updated systematic review and meta-analysis of SA for the treatment of pancreatic fi stula. Although SA treatment was not associated with more closures (i.e., a higher rate of closure), the time to closure in those patients destined to enjoy nonoperative closure of their PDD was shorter in fi ve of seven studies reporting closure times, although this difference was signif i cant in only one trial.
Given clear experimental and clinical data that SA is effective, the lack of signif i cant safety concerns of this native hormone, and the low cost (a 50-mcg dose costs <$5 in our hospital), SA clearly plays a lead role in the resolution of PDD.
External drainage
External drainage achieved via percutaneous, image-guided approach has played a major role in the successful nonoperative treatment of severe PDD. In the recent Dutch Pancreatitis Study Group's prospective series of 639 patients with necrotizing pancreatitis, external catheter drainage was the most frequent fi rst intervention (63% of cases), and additional pancreatic debridement was not required in 35% of those patients.[10]A recent systematic review of eleven studies, including 384 patients with necrotizing pancreatitis undergoing external drainage concluded that external drainage alone was def i nitive, without the need for operative debridement in 56% of patients, not accounting for bias in the mostly low-level studies.[11]When necrosectomy is required, an external catheter provides a necessary window that allows minimally invasive necrosectomy via the increasingly popular step-up approach. This approach, in which the fi rst intervention is external catheter drainage, followed, only if needed, by videoscopic-assisted retroperitoneal debridement (VARD), followed in turn by, only if needed, open debridement, has been compared to standard open necrosectomy in the PANTER (pancreatitis, necrosectomy versus step up approach) study,[12]with fewer complications in the step-up group.
Not only does external catheter drainage allow access to the retroperitoneum for VARD in cases of necrotizing pancreatitis, but in cases of PDD of other etiologies, the catheter allows decompression of a potentially infected pancreatic fl uid collection to a controlled, measurable fi stula, the measure of which guides therapy and provides a treatment endpoint. External catheter drainage may, of course, also be used in conjunction with the often complementary internal drainage techniques.
Alternative nutrition all the way to the mid-jejunum
Because normal, per-os gastric feeding is a profound stimulus for pancreatic secretion, it may prevent timely closure of PDD. Yet, enteral nutrition has several advantages over parenteral nutrition, including better immune function, fewer infections, better glycemic control, and fewer complications associated with central venous access, some of which benef i ts that have been shown in a meta-analysis of 6 RCTs in patients with acute pancreatitis.[13]To avoid pancreatic stimulation associated with gastric feeding, therefore, common practice has been to feed postpyloric into the duodenum. However, multiple studies often overlooked by this common practice have shown that only mid- to distaljejunal feeds, and not proximal jejunal or duodenal feeds, avoid pancreatic stimulation and the consequent increase in pancreatic exocrine secretion (although such distal feeds may not always be practical).[14-16]
Antacids
The rationale for routine immediate use of PPIs in cases of PDD is multifold: given that gastric acid is one of the many physiologic stimuli of pancreatic exocrine function, antacid therapy is intuitively indicated. However, there are several other, more subtle, potential benef i ts of PPIs in cases of PDD. First, there is recent evidence from animal models of pancreatitis to suggest that PPIs are effective in attenuating experimentallyinduced pancreatitis in rats, mediated, perhaps, through reduced expression of inf l ammatory and adhesive proteins and decreased platelet and leukocyte activation.[17]Second, PPIs may inhibit pancreatic secretion by direct action on the pancreatic ducts and acini. Third, for those patients who require NPO status, which is known to be associated with increased intestinal permeability and the consequent risks of GI immune dysregulation and infectious complications, PPIs may decrease intestinal permeability while patients are NPO. In a clinical study of pancreatic-insuff i cient cystic fi brosis patients, PPI therapy was associated with correction of intestinal permeability.[18]Finally, for those patients who are tolerating an enteral diet, but are exocrine-insuff i cient and on enzyme replacement therapy, antacid therapy may further improve fat malabsorption.[19]Data on direct and indirect effects of H1- and H2-blockers, are inconsistent, and therefore PPIs are the preferred antacid agents.
NPO and TPN
Although enteral nutrition has been accepted as the preferred route of nutritional support in acute pancreatitis, patients with PDD as a late and persistent sequelae of acute pancreatitis may have largely recovered from the acute episode save for the pancreatic leak. These patients, as those with other etiologies of PDD, such as the patient in Fig. 2, are less acutely ill with pancreatitis, and therefore more able to tolerate NPO status and TPN to really achieve the maximal decrease in pancreatic exocrine secretion possible to seal their PDD. In such patients, TPN can safely maintain adequate nutrition and nitrogen balance during the several weeks that may be required to achieve resolution of PDD. Because even sham feeding increases gastric and pancreatic secretion, patients are advised and carefully counseled regarding oral intake, and a balance is struck between patient comfort and pancreas rest, with most patients being very happy taking sips and chips of water for mouth comfort, while maintaining a relatively strict NPO status.
Stent of the pancreatic duct
Endoscopic transpapillary stenting (ETS) of the pancreatic duct in cases of PDD provides a path of least resistance to the fl ow of pancreatic exocrine secretions. Endoscopic retrograde pancreatography and ultrasound, when indicated, may be performed at the same setting to assess ductal anatomy, place internal drains, etc. Although no RCT exists to assess the role of ETS in cases of PDD, several single- and multi-institutional series have been published. ETS has been successfully used over the past several decades, as reported in small series, for various manifestations of PDD, including pancreatic ascites, loculated pancreatic fl uid collections, pancreaticocutaneous fi stula, pancreaticoenteric fi stula, and pancreatic pleural effusion. An analysis of ETS for PDD found that predictors of successful closure of PDD following ETS included the successful bridging of the PDD with the stent and the duration of time the stent was maintained in place (only the former, bridging, remained a signif i cant predictor on multivariate analysis).[20]
More recently, the Dutch Pancreatitis Study Group evaluated a prospective cohort of patients with acute pancreatitis, all of whom underwent ETS or conservative treatment for PDD, and in addition a literature review of similar studies was performed.[1]Of 731 patients with acute pancreatitis, 19 were treated with ETS and 16 were treated conservatively for PDD (nearly for all pancreatocutaneous fi stula). The PDD was sealed in 16 of 19 patients (84%) in the ETS group comparedwith 8 of 12 (75%) patients in the conservative group (P=0.175).[1]In the ETS group, the PDD was sealed in a median of 71 days versus 120 days in the conservative group (P=0.130).[1]

Fig. 2.Illustrative case. A 53-year-old man with carcinomatosis underwent cytoreduction with splenectomy, failed to thrive postoperatively, with recurrent ascites and pleural effusions initially suspected to be tumor-related. Only after 3 months PDD was suspected and the patient referred.A: CT scan reveals two dominant fl uid collections;B: Both drained percutaneously;C: ETS placed in pancreatic duct.
Anecdotal snapshot experience
In a one-year period (12/2010-12/2011), 7 of 12 patients referred with PDD were deemed to be appropriate for the described "shotgun" SEALANTS approach to nonoperative management. The 7 patients had a mean or median time from initial drain placement to PDD closure within 40 days (range 24-48). Two of these seven patients required operation (Roux-en-Y cystojejunostomy) for recurrence of symptoms several months after initial resolution of PDD, possibly due to disconnected duct syndrome. Of the remaining fi ve not deemed to be candidates for SEALANTS, two did not require any initial drainage or ETS and recovered with supportive care alone. Two patients had massive necrosis and disconnected ducts treated with delayed transgastric necrosectomy and have fully recovered, and one died of severe aspiration pneumonia and adult respiratory distress syndrome.
Conclusion
Although PDD can be diff i cult to manage and to study largely because of diverse etiologies and sequelae that occur in a very heterogeneous population, several studies have shown that several interventions, such as SAs, aggressive, image-guided external drainage, alternative nutritional support, mandatory antacid therapy (proton-pump inhibitors), immediate NPO status and TPN when enteral nutrition is not feasible, and stenting of the pancreatic duct, here termed the SEALANTS approach, are likely benef i cial in these diff i cult patients. Furthermore, anecdotal experience suggests that, the "shotgun" approach of the SEALANTS measures might successfully affect resolution of otherwise severe, refractory PDD more rapidly than a slower step-wise progression of similar standard interventions. Although the sample size is too small to meaningfully compare to historical controls, this anecdotal experience suggests the possibility of a future trial of SEALANTS as a care bundle versus routine care in the treatment of PDD.
Contributors:CSC proposed the study. AA, JN and CSC performed the work and wrote the fi rst draft. All authors contributed to the design and interpretation of the study and to further drafts. CSC is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Bakker OJ, van Baal MC, van Santvoort HC, Besselink MG, Poley JW, Heisterkamp J, et al. Endoscopic transpapillary stenting or conservative treatment for pancreatic fi stulas in necrotizing pancreatitis: multicenter series and literature review. Ann Surg 2011;253:961-967.
2 Halttunen J, Weckman L, Kemppainen E, Kylänpää ML. The endoscopic management of pancreatic fi stulas. Surg Endosc 2005;19:559-562.
3 Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database Syst Rev 2010:CD008370.
4 Koti RS, Gurusamy KS, Fusai G, Davidson BR. Meta-analysis of randomized controlled trials on the effectiveness of somatostatin analogues for pancreatic surgery: a Cochrane review. HPB (Oxford) 2010;12:155-165.
5 Li-Ling J, Irving M. Somatostatin and octreotide in the prevention of postoperative pancreatic complications and the treatment of enterocutaneous pancreatic fi stulas: a systematic review of randomized controlled trials. Br J Surg 2001;88:190-199.
6 Torres AJ, Landa JI, Moreno-Azcoita M, Argüello JM, Silecchia G, Castro J, et al. Somatostatin in the management of gastrointestinal fi stulas. A multicenter trial. Arch Surg 1992;127:97-100.
7 Sancho JJ, di Costanzo J, Nubiola P, Larrad A, Beguiristain A, Roqueta F, et al. Randomized double-blind placebo-controlled trial of early octreotide in patients with postoperative enterocutaneous fi stula. Br J Surg 1995;82:638-641.
8 Hernández-Aranda JC, Gallo-Chico B, Flores-Ramírez LA, Avalos-Huante R, Magos-Vázquez FJ, Ramírez-Barba EJ. Treatment of enterocutaneous fi stula with or without octreotide and parenteral nutrition. Nutr Hosp 1996;11:226-229.
9 Gans SL, van Westreenen HL, Kiewiet JJ, Rauws EA, Gouma DJ, Boermeester MA. Systematic review and meta-analysis of somatostatin analogues for the treatment of pancreatic fi stula. Br J Surg 2012;99:754-760.
10 van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 2011;141:1254-1263.
11 van Baal MC, van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG, et al. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg 2011;98:18-27.
12 van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491-1502.
13 Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ 2004;328:1407.
14 O'Keefe SJ, McClave SA. Feeding the injured pancreas.Gastroenterology 2005;129:1129-1130.
15 Vu MK, van der Veek PP, Frölich M, Souverijn JH, Biemond I, Lamers CB, et al. Does jejunal feeding activate exocrine pancreatic secretion? Eur J Clin Invest 1999;29:1053-1059.
16 Kaushik N, Pietraszewski M, Holst JJ, O'Keefe SJ. Enteral feeding without pancreatic stimulation. Pancreas 2005;31:353-359.
17 Hackert T, Tudor S, Felix K, Dovshanskiy D, Hartwig W, Simon WA, et al. Effects of pantoprazole in experimental acute pancreatitis. Life Sci 2010;87:551-557.
18 Hendriks HJ, van Kreel B, Forget PP. Effects of therapy with lansoprazole on intestinal permeability and inf l ammation in young cystic fi brosis patients. J Pediatr Gastroenterol Nutr 2001;33:260-265.
19 DiMagno EP. Gastric acid suppression and treatment of severe exocrine pancreatic insuff i ciency. Best Pract Res Clin Gastroenterol 2001;15:477-486.
20 Telford JJ, Farrell JJ, Saltzman JR, Shields SJ, Banks PA, Lichtenstein DR, et al. Pancreatic stent placement for duct disruption. Gastrointest Endosc 2002;56:18-24.
Received December 19, 2012
Accepted after revision February 16, 2013
AuthorAff i liations:Department of Surgery, Saint Agnes Hospital, Baltimore, MD, USA (Abdo A and Cunningham SC); Department of Medicine, Greater Baltimore Medical Center, Baltimore, MD, USA (Jani N)
Steven C Cunningham, MD, Co-Director of Pancreatic and Hepatobiliary Surgery, Saint Agnes Hospital, 900 Caton Avenue, Mailbox #207, Baltimore, MD 21229, USA (Tel: 410-368-2748; Fax: 410-951-4007; Email: steven.cunningham@stagnes.org)
This work was presented as an oral poster at the 2012 AHPBA meeting.
© 2013, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(13)60039-9
 Hepatobiliary & Pancreatic Diseases International2013年3期
Hepatobiliary & Pancreatic Diseases International2013年3期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Double-blind randomized sham controlled trial of intraperitoneal bupivacaine during emergency laparoscopic cholecystectomy
- Risk factors for the occurrence of insulinoma: a case-control study
- Survival outcomes of right-lobe living donor liver transplantation for patients with high Model for End-stage Liver Disease scores
- Propofol inhibits the adhesion of hepatocellular carcinoma cells by upregulating microRNA-199a and downregulating MMP-9 expression
- Inferior vena cava obstruction and collateral circulation as unusual manifestations of hepatobiliary cystadenocarcinoma
- Pancreatic Castleman disease treated with laparoscopic distal pancreatectomy
