Risk factors for the occurrence of insulinoma: a case-control study
Beijing, China
Risk factors for the occurrence of insulinoma: a case-control study
Han-Xiang Zhan, Lin Cong, Yu-Pei Zhao, Tai-Ping Zhang and Ge Chen
Beijing, China
BACKGROUND:The etiology of insulinoma is poorly understood. Few studies investigated the possible roles of environmental factors and lifestyle in the pathogenesis of insulinoma. The aim of this study is to identify risk factors associated with occurrence of insulinoma in the Chinese population.
METHODS:This study consisted of 196 patients with insulinoma and 233 controls. Demographic information of the patients and controls and risk factors of the disease were analyzed. Univariate and unconditional multivariable logistic regression analyses were made to estimate odds ratios (ORs) and possible risk factors.
RESULTS:Approximately 68.88% (135/196) of the patients were from rural areas in contrast to 10.30% (24/233) of the controls (P<0.0001). This difference was conf i rmed by the multivariate analysis (OR=4.950; 95% CI: 2.928-8.370). Family history of pancreatic endocrine tumor (OR=16.754; 95% CI: 2.125-132.057) and other cancers (OR=2.360; 95% CI: 1.052-5.291) was also related to a high-risk population of insulinoma.
CONCLUSION:Rural residents or people who have a family history of pancreatic endocrine tumor and other cancers are a high-risk population of insulinoma.
(Hepatobiliary Pancreat Dis Int 2013;12:324-328)
insulinoma;risk factors; case-control study; family history
Introduction
Insulinoma is the most common functional endocrine tumor of the pancreas with an incidence of four per million cases per year. Its incidence rate is increasing,[1-3]but its etiology is poorly understood.[4-5]The possible roles of environmental factors and lifestyle in the pathogenesis of insulinoma have been investigated. Capurso et al[6]reported a case-control study which focused on investigating risk factors for all types of sporadic pancreatic endocrine tumors (PETs). The results suggested that family history of any cancer, chronic pancreatitis, and high alcohol intake were risk factors for PET. However, subjects of the study were all PET patients and the number of insulinoma patients was only 17 (10.4%, 17/162), where some possible risk factors may be exaggerated or concealed. Furthermore, this study focused on the Italian population, where the race, environmental factors and life style are different from the Asian population. In this article, we performed a hospital-based case-control study, aiming at identifying risk factors associated with the incidence of insulinoma in the Chinese population.
Methods
Study design and population
This case-control study was conducted at the Peking Union Medical College Hospital in Beijing, China. The study protocol was reviewed and approved by the Institutional Review Board of the hospital.
Included in the study group were 196 patients who had been diagnosed with insulinoma by imaging and conf i rmed histologically between January 2000 and January 2010. Excluded were patients with other PETs such as gastrinoma, glucagonoma or nonfunctioning PETs.
Controls were 233 patients who had undergone operations at our department during the same period. Excluded were patients with (1) benign or malignanttumor; (2) autoimmune or chronic diseases such as Crohn disease, ulcerative colitis, chronic lymphocytic thyroiditis, autoimmune pancreatitis, etc; (3) genetic relation with insulinoma, and specif i c referral for evaluation of a possible cancer familial syndrome, such as multiple endocrine neoplasia type 1 (MEN-1), pituitary adenoma, hyperparathyroidism, and VHL syndrome.
Data collection
The medical records of the patients and controls were reviewed, and their demographics and possible risk factors (height, weight, smoking, alcohol use, family history of PETs or other cancers and their personal medical history) were analyzed. Telephone interviews were conducted to know any changes of the mentioned demographics or factors. Risk factors and exposure def i nition were assessed similarly as the study by Capurso et al.[6]
Statistical analysis
All quantitative data were expressed as mean±standard deviation. Categorical variables were summarized using proportions. Independent samplettest was used to compare quantitative variables. The Chi-square test and Fisher's exact test were used to compare categorical variables. Of the variables tested in univariate analysis, only those withPvalues <0.10 were included for multivariate analysis. Logistic regression was performed for a multivariate analysis to determine the independent risk factors for the occurrence of insulinoma. Epidata 3.02 and SPSS version 16.0 were used for data entry and all statistical analyses. A value ofP<0.05 was considered statistically signif i cant.
Results
Patient characteristics
In the study group of 196 patients with insulinoma, 81 were male and 115 female (1:1.42), with a mean age of 43.8±14.9 years (13-74). Of these patients, 97.96% (192/196) presented with a typical Whipple triad, and the median duration of symptoms before diagnosis was 47.4 months (1-384). Twelve patients (6.12%, 12/196) had MEN-1 related insulinoma and six patients were conf i rmed histologically as having malignant insulinoma. The ethnicity of most of the patients was Han nationality, and 68.88% (135/196) of the patients were from rural areas (Table 1).
Risk factors for occurrence of insulinoma
Body mass index (BMI)
BMI was higher in the study group than in the control group (27.42±4.54 vs 23.59±3.21,P<0.0001), but it was not shown in the multivariate analysis in spite of the signif i cant difference in the univariate analysis (Table 2).
Rural residents
Approximately 68.88% (135/196) of the patients in the study group were from rural areas in contrast to 10.30% (24/233) in the control group (P<0.0001). This fi nding was conf i rmed by multivariate analysis, with an odds ratio (OR) of 4.950, and a 95% CI (conf i dence interval) of 2.928-8.370.
Cigarette smoking
The proportion of cigarette smokers was 26.02% (51/196) in the study group and 18.03% (42/233) inthe control group (P=0.045) (Table 3), but it was not associated with a greater OR of insulinoma shown by multivariate analysis. The smokers were divided into subgroups according to smoking index. Univariate and multivariate analysis showed that smoking index >10 packs per year was correlated with insulinoma (OR=2.229; 95% CI: 1.094-4.539) (Table 4). Smokers with >20 packs per year were not associated with an increased risk of insulinoma (P=0.275).
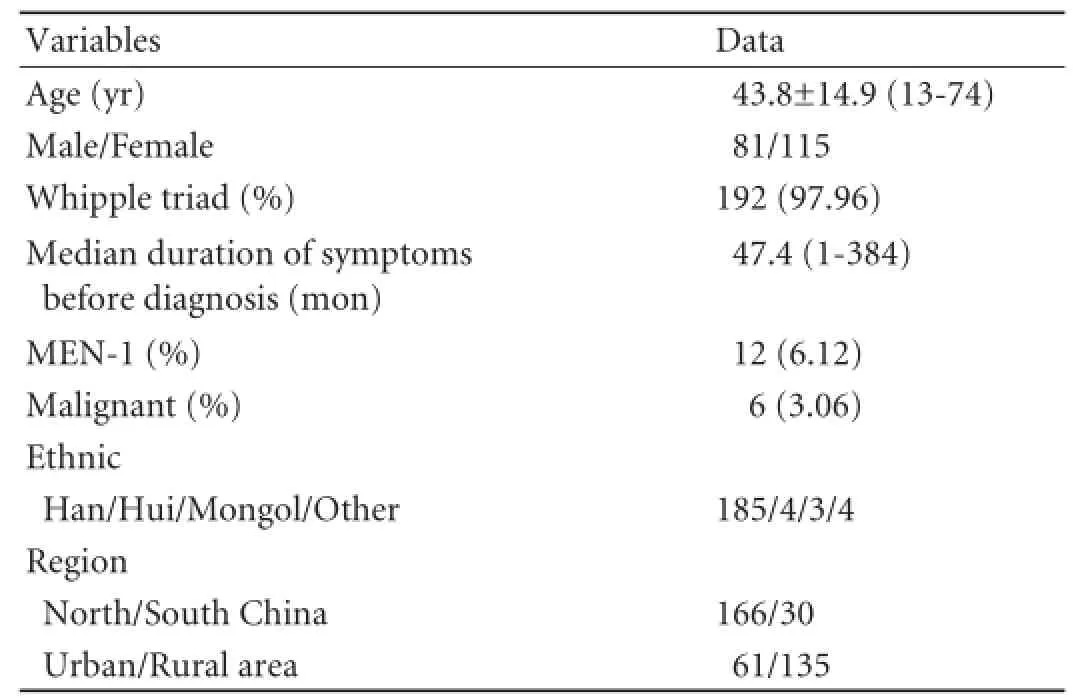
Table 1.Clinical features of 196 patients with insulinoma in a Chinese population
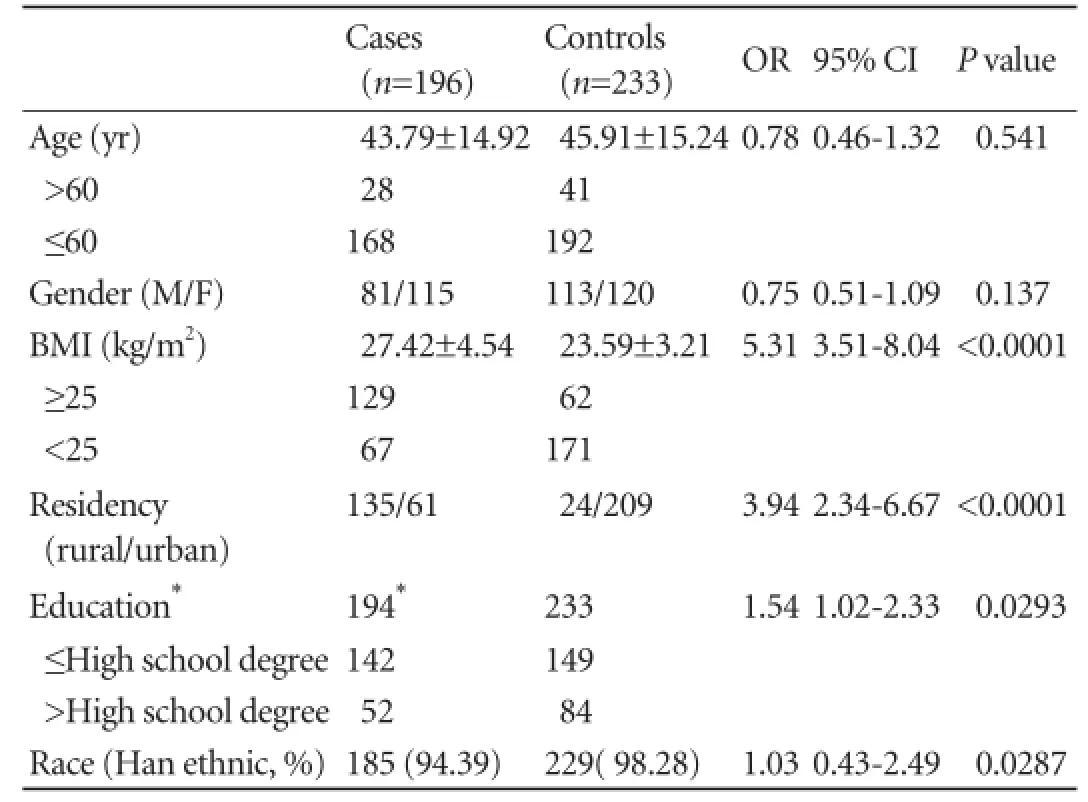
Table 2.Demographic information of cases and controls

Table 3.Possible risk factors of occurrence of insulinoma in case and control groups

Table 4.Risk factors for insulinoma at the multivariate logistic regression analysis
Alcohol consumption
Alcohol consumption was not signif i cantly different between the patients with insulinoma and controls (P=0.142). The proportion of heavy drinker was higher in the study group (10.20% vs 4.72%,P=0.029), but multivariate analysis revealed that it was not associated with an increased risk of the tumor.
Family history of PETs
Thirteen patients with insulinoma had a family history of fi rst-degree (9 patients) and second-degree (4) PETs. Twelve patients had insulinoma and 1 patient had nonfunctioning PET. Compared with the study group, none had a family history of PETs in the control group. Multivariate logistic regression analysis showed that the family history of PETs may be correlated with the occurrence of insulinoma (OR=16.754; 95% CI: 2.125-132.057).
Family history of other cancers
Twenty-one patients with insulinoma and 11 controls had a family history of primary hepatocellular carcinoma, colorectal or gastric cancer, cancer of the esophagus, larynx, lung or prostate in addition to PETs. This factor was also correlated as an independent risk factor for the occurrence of insulinoma (OR=2.360; 95% CI: 1.052-5.291).
Discussion
Little is known about the inf l uence of environmental factors and lifestyle on insulioma. We retrieved the databases of insulinoma in China before[2]performing a case-control study to assess the potential inf l uence of environmental factors on the occurrence of insulinoma.
In this study, rural residency was an independent risk factor for the occurrence of insulinoma (OR=4.950). In clinical practice, patients with insulinoma mostly came from rural areas or low-income families, while patients with pancreatic adenocarcinoma were frequently seen in residents of cities. To the present, there are no studies on the inf l uence of residential environment on the incidence of insulinoma. We found differences in natural environment, life style, income and diet between urban and rural areas in China, which seem to be related to the incidence of insulinoma. Hence we presume that dietary factors and natural environment may play a role in the pathogenesis of insulinoma.
Because of the retrospective nature of the study, recruitment bias may be involved. In our center, more PETs patients are treated, whereas patients with other surgical problems from rural areas may be handled in other hospitals. According to the nation-wide population census, rural residents account for 50.3% of the Chinese population, while 49.7% are urban residents. The number of patients with insulinoma from rural areas (n=135) was larger than that from urban areas (n=61).
BMI is generally considered as a risk factor for theincidence of pancreatic adenocarcinoma.[7-10]In this study, BMI was higher in the study group (27.42±4.54 vs 23.59±3.21,P<0.0001). We consider that the higher BMI may be due to the occurrence of insulinoma, so we did not add this variable in the multivariate analysis.
Although cigarette smoking is a known risk factor for many malignant tumors,[11-14]its role in the pathogenesis of insulinoma was not proved in our study. However, smoking of >10 packs per year was strongly correlated with insulinoma, as revealed by univariate or multivariate analysis (OR=2.229). This fi nding may be due to the small sample size and selection bias.
In this study, alcohol consumption was not associated with the development of insulinoma. The role of alcohol in the development of neuroendocrine tumors has not been extensively studied. Two studies[15,16]from the USA found no relationship between alcohol and insulinoma, but an Italian study indicated a positive relationship.[6]In the Italian study, the percentage of patients with a higher alcohol consumption was signif i cantly lower.[6]The relationship between alcohol and the pathogenesis of insulinoma remains uncertain. A study[17]suggested that large consumption of alcohol may induce oxidative stress and nutritional def i ciency, which may lead to damage of islet cells and promote the development of tumors.
In this study, we also found a relationship between family history of PETs and insulinoma. In the 196 patients with insulinoma, 13 (6.63%) had a family history of PETs, but in the control group none had such a history. Among the 13 patients, 3 had MEN-1 syndrome and 10 had sporadic insulinoma. The incidence of a positive family history of PETs was 25% (3/12) in patients with MEN-1 insulinoma and 5% (10/184) in patients with sporadic insulinoma. The MEN1 gene has been described as a candidate gene in insulinoma.[18,19]It has more inf l uence on MEN-1 related insulinoma than on sporadic insulinoma.[4,20]Our study suggests that the relatives of patients with insulinoma have a higher risk of insulinoma. Family screening of high-risk populations may be helpful in early diagnosis of the tumor. Hence, detection of MEN1 mutation in high-risk populations is necessary.[21-23]
Family history of cancers other than PETs was also a risk factor for insulinoma (OR=2.360).[6,15,16]In an Italian study, 4.3% of patients with PET had a family history of pancreatic adenocarcinoma, compared with 1.2% of controls. The authors of the study believed that pancreatic exocrine and endocrine neoplasm may have a common genetic background. In our study, however, we did not fi nd such relationship.
This study has limitations. Since it is a case-control study performed in a single institution, selection bias is inevitable. To overcome this weakness, all patients and controls were histologically conf i rmed and were selected according to the inclusion and exclusion criteria. For analysis, demographic data and exposure to the possible risk factors were collected from medical records and telephone interviews. No subjects with incomplete information were included in the study.
In conclusion, our fi ndings suggest that residents from rural areas and those with a family history of PETs or other cancers may have a higher risk for the development of insulinoma in China.
Acknowledgement:The authors would like to express their deepest thanks to Drs. Jian-Wei Xu, Yin-Xing Wei, Xuan Meng and Yan-Min Zhang for their cooperation in this study.
Contributors:ZHX, ZYP and ZTP proposed the study. ZHX and CL wrote the fi rst draft, collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. ZYP is the guarantor.
Funding:This study was supported by a grant from the Ministry of Health Key Lab Foundation (KLF2009011).
Ethical approval:The study protocol was reviewed and approved by the Institutional Review Board of the hospital.
Competing interest:No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Nikfarjam M, Warshaw AL, Axelrod L, Deshpande V, Thayer SP, Ferrone CR, et al. Improved contemporary surgical management of insulinomas: a 25-year experience at the Massachusetts General Hospital. Ann Surg 2008;247:165-172.
2 Zhao YP, Zhan HX, Zhang TP, Cong L, Dai MH, Liao Q, et al. Surgical management of patients with insulinomas: Result of 292 cases in a single institution. J Surg Oncol 2011;103:169-174.
3 Tucker ON, Crotty PL, Conlon KC. The management of insulinoma. Br J Surg 2006;93:264-275.
4 Jonkers YM, Ramaekers FC, Speel EJ. Molecular alterations during insulinoma tumorigenesis. Biochim Biophys Acta 2007;1775:313-332.
5 Fendrich V, Waldmann J, Bartsch DK, Langer P. Surgical management of pancreatic endocrine tumors. Nat Rev Clin Oncol 2009;6:419-428.
6 Capurso G, Falconi M, Panzuto F, Rinzivillo M, Boninsegna L, Bettini R, et al. Risk factors for sporadic pancreatic endocrine tumors: a case-control study of prospectively evaluated patients. Am J Gastroenterol 2009;104:3034-3041.
7 Jiao L, Berrington de Gonzalez A, Hartge P, Pfeiffer RM, Park Y, Freedman DM, et al. Body mass index, effect modif i ers, and risk of pancreatic cancer: a pooled study of seven prospective cohorts. Cancer Causes Control 2010;21:1305-1314.
8 Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009;301:2553-2562.
9 Anderson LN, Cotterchio M, Gallinger S. Lifestyle, dietary, and medical history factors associated with pancreatic cancer risk in Ontario, Canada. Cancer Causes Control 2009;20:825-834.
10 Urayama KY, Holcatova I, Janout V, Foretova L, Fabianova E, Adamcakova Z, et al. Body mass index and body size in early adulthood and risk of pancreatic cancer in a central European multicenter case-control study. Int J Cancer 2011; 129:2875-2884.
11 Wang JB, Jiang Y, Wei WQ, Yang GH, Qiao YL, Boffetta P. Estimation of cancer incidence and mortality attributable to smoking in China. Cancer Causes Control 2010;21:959-965.
12 Hecht SS. Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbecks Arch Surg 2006;391:603-613.
13 Newcomb PA, Carbone PP. The health consequences of smoking. Cancer. Med Clin North Am 1992;76:305-331.
14 Gram IT, Lukanova A, Brill I, Braaten T, Lund E, Lundin E, et al. Cigarette smoking and risk of histological subtypes of epithelial ovarian cancer in the EPIC cohort study. Int J Cancer 2012;130:2204-2210.
15 Hassan MM, Phan A, Li D, Dagohoy CG, Leary C, Yao JC. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int J Cancer 2008;123:867-873.
16 Hassan MM, Phan A, Li D, Dagohoy CG, Leary C, Yao JC. Family history of cancer and associated risk of developing neuroendocrine tumors: a case-control study. Cancer Epidemiol Biomarkers Prev 2008;17:959-965.
17 Das SK, Vasudevan DM. Alcohol-induced oxidative stress. Life Sci 2007;81:177-187.
18 Schnepp RW, Chen YX, Wang H, Cash T, Silva A, Diehl JA, et al. Mutation of tumor suppressor gene Men1 acutely enhances proliferation of pancreatic islet cells. Cancer Res 2006;66:5707-5715.
19 Larsson C, Skogseid B, Oberg K, Nakamura Y, Nordenskjöld M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature 1988;332: 85-87.
20 Jonkers YM, Claessen SM, Perren A, Schmid S, Komminoth P, Verhofstad AA, et al. Chromosomal instability predicts metastatic disease in patients with insulinomas. Endocr Relat Cancer 2005;12:435-447.
21 Falchetti A, Marini F, Luzi E, Giusti F, Cavalli L, Cavalli T, et al. Multiple endocrine neoplasia type 1 (MEN1): not only inherited endocrine tumors. Genet Med 2009;11:825-835.
22 Tham E, Grandell U, Lindgren E, Toss G, Skogseid B, Nordenskjöld M. Clinical testing for mutations in the MEN1 gene in Sweden: a report on 200 unrelated cases. J Clin Endocrinol Metab 2007;92:3389-3395.
23 Newey PJ, Thakker RV. Role of multiple endocrine neoplasia type 1 mutational analysis in clinical practice. Endocr Pract 2011;17:8-17.
Received March 29, 2012
Accepted after revision October 19, 2012
AuthorAff i liations:Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing 100730, China (Zhan HX, Cong L, Zhao YP, Zhang TP and Chen G)
Yu-Pei Zhao, MD, Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing 100730, China (Tel: 86-10-65296014; Fax: 86-10-65296014; Email: zhao8028@263.net)
© 2013, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(13)60051-X
 Hepatobiliary & Pancreatic Diseases International2013年3期
Hepatobiliary & Pancreatic Diseases International2013年3期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Clinical features and outcomes of patients with severe acute pancreatitis complicated with gangrenous cholecystitis
- Survival outcomes of right-lobe living donor liver transplantation for patients with high Model for End-stage Liver Disease scores
- Propofol inhibits the adhesion of hepatocellular carcinoma cells by upregulating microRNA-199a and downregulating MMP-9 expression
- Double-blind randomized sham controlled trial of intraperitoneal bupivacaine during emergency laparoscopic cholecystectomy
- Pancreatic Castleman disease treated with laparoscopic distal pancreatectomy
- Pancreatic duct disruption and nonoperative management: the SEALANTS approach
