Method Development and Validation for Determining 1,3-Butadiene in Human Blood by Gas Chromatography-Mass Spectrometry and Head-Space Gas Chromatography
ZHANG Su-jing,SHEN Bao-hua,ZHUO Xian-yi
(1.College of Pharmaceutical Science,Soochow University,Suzhou 215123,China;2.Shanghai Key Laboratory of Forensic Medicine,Institute of Forensic Science,Ministry of Justice,P.R.China,Shanghai 200063,China)
As a colourless volatile gas,1,3-butadiene (BD)is a toxic organic compound which dissolves in most organic solvents,such as benzene.BD is directly obtained by cracking naphtha and light oil.BD is an important chemical raw material,which is widely used not only in the production of synthetic rubber,such as styrene butadiene rubber,polybutadiene rubber,nitrile rubber,and polychloroprene rubber,but also in the production of thermoplastic resins[1-3].Thanks to its volatility,inhalation through the respiratory system is almosttheonly intake pathway of BD.Acute exposure to high concentrations of BD leads to respiratory irritation and narcotic effects on the central nervous system.
BD is a rodent carcinogen,as demonstrated by bioassays[2-4].One study[2]about B6C3F1 mice exposed to BD showed that BD was a potent multiple organ carcinogen(e.g.,lymphocytic lymphoma,neoplastic lesions in the heart,and cancers of lung,forestomach,harderian gland,preputial gland,liver,mammary gland and ovary).The carcinogenicity of BD in humans has been controversial,but the data have indicated that BD is a probable human carcinogen[3].The carcinogenic process of BD is thought to arise from the interactions of the BD metabolites(such as epoxybutane,diepoxybutane and epoxybutanediol)and DNA[5].The epidemiological studies[6-7]have associated an increased risk of leukaemia with occupational exposure to BD.A 25-year longitudinal study[6]found that occupational exposure to BD or a combination of BD and styrene might cause leukaemia in humans.
BD is also present in cigarette smoke and automobile exhaust[8-9].Gas chromatography-mass spectrometry (GC-MS) has been used to analyze BD in smoke and air[8].
Himmelstein et al.[10]used head-space gas chromatography (HS-GC) to measure the blood concentrations of BD and GC-MS to test its metabolites in rats and mice.In this research,we developed andvalidatedamixedmethodofGC-MS and HS-GC for the qualification and quantitation of BD in human blood.Furthermore,the proposed method was applied to an actual caseof a33-year-old crewman who died from exposure to high concentration of BD.His postmortem blood was available for our analysis.
Material and methods
Instruments and reagents
Working solutions
The BD stock solution was 20 wt%in toluene when purchased.BD stock solution of 1.25 g was added to a 25mL volumetric flask to be diluted with ethanolabsolute.Theworking solution wasprepared at a concentration of 10 mg/mL.Using different volumes of the blank blood samples spiked with 10mg/mL of the BD,10μg/mL,50μg/mL,100μg/mL,200μg/mL,300μg/mL,400μg/mL,500μg/mL of BD were obtained.
T-butyl alcohol was diluted with deionised water to obtain a 40μg/mL as internal standard solution.
Sample preparation
For GC-MS analysis,the control sample(BD stock solution,10μL) or the case sample (0.1mL)were transferred to a 5mL sample vial with deionised water (0.5 mL).The vial,was then covered with a silicon rubber pad and aluminium cap and heated for 10min in a water bath at 60℃.
For HS-GC analysis,the spiked sample(working solutions,0.1 mL) and 40.0 μg/mL t-butyl alcohol(internal standard solution,0.50 mL) were transferred to a 5 mL sample vial.The vial,was then covered with a silicon rubber pad and aluminium cap and shaken.
Conditions of GC-MS and HS-GC
GC-MS was carried out using an Agilent 7890N-5975C GC-MS.Chromatographic conditions:HP-5MS capillary column (30m×0.25mm,0.25μm);the column temperature was programmed from an initial temperature of 40℃,maintained for 4min,to 280℃at 25℃/min;the helium (purity≥99.999%)was used as carrier gas with a flow rate of 1mL/min.A 0.4mL of headspace gas was injected.Mass spectrum conditions:the detector was operated in electron ionization (EI) with 70 eV of electron energy;the ion source and quadrupole temperatures were 230℃and 150℃;the interface temperature was 280℃;a full scan mode was selected.
GC was performed in a HS-GC system comprised ofan Agilent7695 head-spaceautomatic sampler and an Agilent 7890 gas chromatograph with two flame ionisation detectors (FID1A,FID2B).The head-space automatic sampler operating conditions:oven temperature of 65℃;nitrogen-pressurised vial with a 0.1 min pressurisation time;0.1 min sample-loop fill time;and 0.05 min loop equilibration time.GC conditions:silica capillary DB-ALC1 and DB-ALC2 column as chromatographic columns(30m×0.32mm,1.8μm and 30m×0.32mm,1.2μm,respectively) were both operated at 40℃ for 3min,then rose to 180℃ at a rate of 25℃/min and held for 5 min.The nitrogen(purity≥99.999%)was used as carrier gas with a flow rate of 8mL/min.
当前,QQ、微信、微博等社交软件已经成为大学生进行日常交流的主要媒介,这些新媒体所创造的交流方式,使传统的信息沟通方式黯然失色。在新媒体所创造的虚拟交流空间中,学生可以挣脱面对面交流的腼腆及生涩,让一些不善于直接语言沟通的学生逐渐建立起自信。但凡事都具有利弊,因新媒体社会软件的虚拟性,学生就更容易抛弃道德的约束,失去自身的社会责任感,若学生过于依赖社交软件,会影响学生在现实中的交流,产生沟通障碍。
Method validation
The selectivity of the method was investigated by analyzing 20 blank blood samples from different sources.The calibration curve was obtained using blank blood samples spiked with analytes containing 50 μg/mL,100 μg/mL,200 μg/mL,300 μg/mL,400 μg/mL,500 μg/mL of BD.The limit of detection (LOD)was defined as the spiked concentration at which the analyte signal was at least 3 times the baseline (S/N≥3),and the limit of quantification(LOQ) was defined as the spiked concentration at which the analyte signal was at least 10 times the baseline (S/N≥10).The LOQ (50 μg/mL) was also the lowest concentration in the calibration curve that could be quantified with acceptable precision(≤20%) and accuracy (80%-120%).The precision expressed as the relative standard deviation (RSD)was established atlow and high concentrations(100 μg/mL and 400 μg/mL).The intra-day precision was determined by the analysis of 6 spiked blood samples at each concentration level on the same day,and the inter-day precision was assayed for 6 replicates on each for 4 days.The accuracy was validated by assaying low and high concentrations,and expressed as the percentage of the determined concentration in relation to the spiked concentrations.
Stability assays
The stability of BD in blood was assessed using 50 μg/mL,100 μg/mL and 400 μg/mL samples,each having 5 replicates,over the course of 3 freezethaw cycles(-20℃ for 21h and room temperature for 3 h)and a 24 h room temperature period.The stability of BD in the processed samples under autosampler conditions as pretreated samples was examined over a 12h period.
For freeze-thaw stability,the measured values should be between 80%and 120%of the spiked concentrations.Asto the room temperatureand pretreated samples,BD in blood is considered stable if the measured values should range from 85%to 115%at the end of the study period[11].
Results and discussion
Preparation of BD working solution
We experienced some difficulties in the preparation of BD working solution.The purchased BD stock solution was in toluene;however,BD and toluene barely dissolve in water.We attempted to use 1%and 3%ethanol in deionised water as a solvent to prepare the BD working solution.However,BD and toluene failed to form a homogeneous solution with 1%ethanol in deionised water.As for 3%ethanol in deionised water,the highest concentration was 100 μg/mL,which was insufficient for the current study.Thus,we abandoned the use of toluene as a solvent due to its deleterious health effects[12-13].The working solution was ultimately prepared with ethanol absolute which was spiked to blank blood for making the calibration curve.We found no interferences of ethanol absolute,toluene and endogenous substance in the experiment,and these concentrations of ethanol and toluene did not pollute the instrument.
Optimization of HS-GC temperature program
The boiling point of BD and toluene were-4.5℃and 110.6℃.The temperature programs listed were investigated (Table 1).

Table 1 Temperature programs
With the same sample preparation,the linear coefficient of 0.98 was obtained with temperature program 1.In temperature program 2,temperature and time were added.The final temperature program was described in the previous section.Increasing temperature and time were in order to reduce the impurities interfering with the next sample (peaks in Fig.1).Good linearity with the linear coefficient>0.99 and reproducibility were obtained with temperature program 2 according to the good results of method validation.
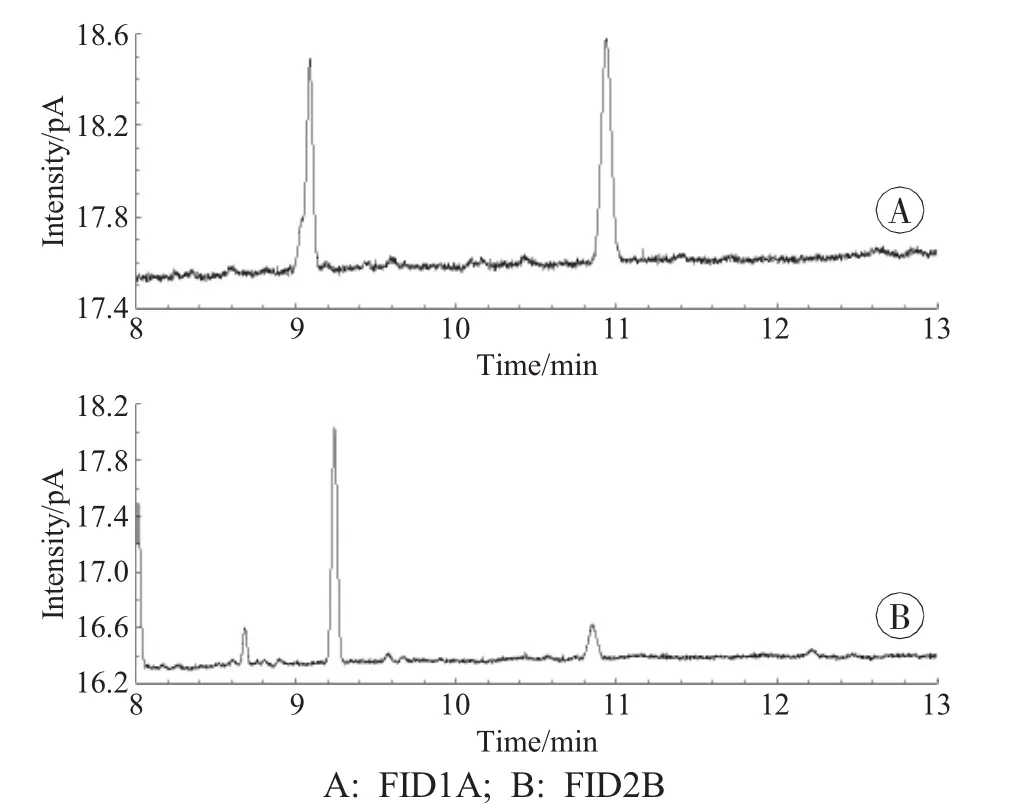
Fig.1 Impurities peaks in temperature program 2
Results of qualitative method
BD was confirmed by both GC-MS and HSGC.For GC-MS,identification of compounds was achieved by comparing the mass spectra and retention time of the chromatographic peak with those of authentic samples.The total ion chromatogram(TIC) (m/z 20-100) and the mass spectra of BD were shown to have the same fragment ions of m/z 54,39,27 in BD control sample (stock solution,10μL)and case sample,with the retention time of BD 1.685 min (Figs.2-4).
For HS-GC,as BD control sample obtained,GC retention data were to be used as qualitative analysis in this method.In practice,however,one-column confirmation was unreliable.In the current study,two columns were used to address this challenge[14].A qualitative method with two columns of different polarities can avoid the issue of several substances having the same retention value.To accomplish this aim,we used two different polar columns,DB-ALC1 and DB-ALC2 from Agilent.The results of the retention times of BD and internal standard solution in spiked sample (BD,10 μg/mL) and case sample both were 1.163 min and 2.051 min(FID1A/DBALC1) and 1.059 min and 2.154 min (FID2B/DBALC2),respectively (Figs.5-6).The Agilent columns of DB-ALC1 and DB-ALC2 are to be used for the measurement of blood alcohol,and we used ethanol to prepare our working solution.However,ethanol did not influence the results.Both BD and internal standard solution exhibited good resolution (>1.5)with neighbouring peaks,satisfactory precision and accuracy obtained.
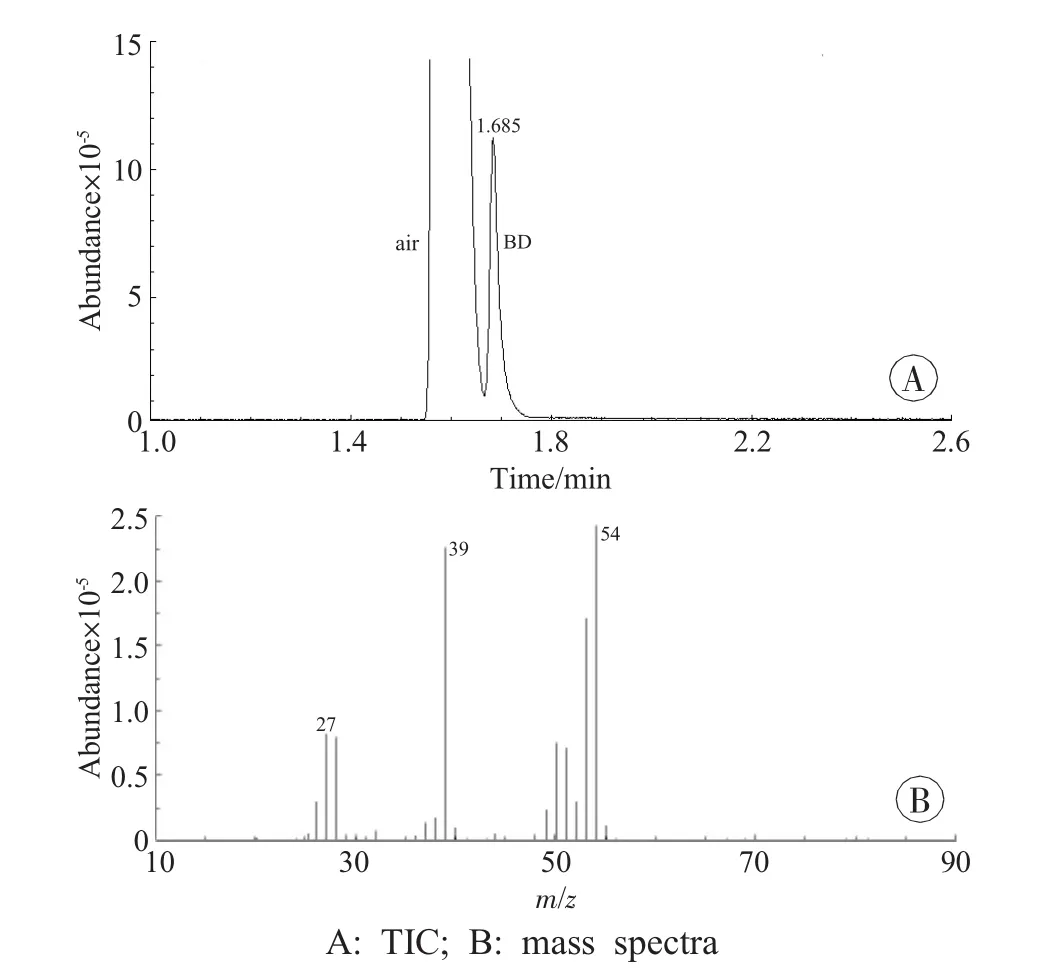
Fig.2 TIC and the mass spectra of BD control sample

Fig.3 TIC and the mass spectra of the case sample

Fig.4 TIC of the blank blood sample

Fig.5 Chromatogram of the spiked sample(BD,10μg/mL)

Fig.6 Chromatogram of the case sample
Validation
Selectivity was examined by analyzing 20 blood samples from different sources.Under the improved conditions,there were no interfering peaks at the retention times of BD and internal standard solution in the blood matrix.The chromatograms of blank blood sample and the spiked sample (BD,10μg/mL)were shown (Figs.5,7).

Fig.7 Chromatogram of the blank blood sample
Good linearity was observed in the range from 50 to 500 μg/mL,the regression functions y=0.689 2 x+0.192 9(r=0.997) for column 1 and y=0.663 6 x+0.1799(r=0.997) for column 2.The LOD and LOQ were 10 μg/mL and 50 μg/mL,respectively.This concentration met our analytical requirements.
The precision (1.83%-6.08%)and accuracy(96.98%-103.81%) were obtained (Table 2).

Table 2 Data of precision and accuracy(%)
Stability
Our results indicated that BD in blood showed s no significant deviations after 3 freeze-thaw cycles,RSDs less than 4.17%,and the accuracy between 91.84%to 111.06%.BD was found to be stable in the blood matrix under auto-sampler conditions for 12 h as pretreated samples,RSDs less than 4.63%,and the accuracy between 85.82%to 108.80%.The effect of freeze-thaw cycles and pretreated samples were shown in Table 3.However,BD in blood was not stable in room temperature for 24h,whose accuracies were all below 80%.This was consistent with the known volatility of BD;therefore we suggest that the specimen must be kept in frozen torage and sent for an immediate analysis.
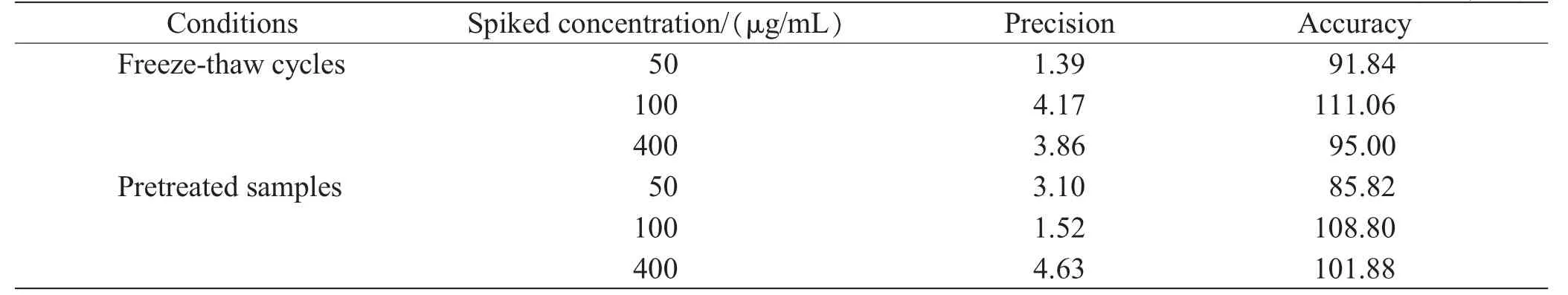
Table 3 Stability of Freeze-thaw and pretreated samples (n=5,%)
Application
A 33-year-old first mate,together with two of his crewmates,were working in the ship’s hold,which once stored BD.Approximately 45 min later,the two crewmates went to the deck because they felt unwell,while the first mate remained in the hold.Two minutes later,the two crewmates failed to contact the first mate.They took him out of the ship’s hold,but he died after failed resuscitation.
Results of autopsy: (1) Coronary atherosclerotic heart disease:myocardium hypertrophy(400 g of heart weight);coronary atherosclerosis of the left anterior descending branch and circumflex branch(luminal stenosis of Ⅲ-Ⅳ class);fibrous scar scattered in the muscular layer. (2) Small arteriosclerosis of the kidney and spleen. (3) Fatty liver (moderate to severe). (4) Congestion and edema in multiple organs (heart,lung,brain,kidney,etc.).
BD was identified as the predisposing cause of his death.
In this case,BD was once stored in the ship’s hold.We found 242 μg/mL of BD in the first mate’s blood using the approach established in the current study.Because there was little literature on the similar cases,we did not know how much damage this concentration of BD caused to the human body.However,the victim lost the ability to respond to others in a short time,which might suggest that the man went unconscious quickly as a result of acute exposure of a high BD concentration.
Conclusion
The established simple approach was validated to be useful for determining BD in blood samples,as indicated by the results of the case study.It can be concluded that high BD concentrations may affect the respiratory and central nervous system.
Acknowledgements
This current study was funded by the Council of Shanghai Key Laboratory of Forensic Medicine(12DZ2271500).We are grateful to SHEN Yi-wen,Department of Forensic Science,Shanghai Medical College,Fudan University,for her helpful technical support.
[1]Himmelstein MW,Acquavella JF,Recio L,et al.Toxicology and epidemiology of 1,3-butadiene[J].Crit Rev Toxicol,1997,27(1):1-108.
[2]Melnick RL,Huff J,Chou BJ,et al.Carcinogenicity of 1,3-butadiene in C57BL/6×C3H F1mice at low exposure concentrations[J].Cancer Res,1990,50(20):6592-6599.
[3]Jackson MA,Stack HF,Rice JM,et al.A review of the genetic and related effects of 1,3-butadiene in rodents and humans[J].Mutat Res,2000,463(3):181-213.
[4]Seaton MJ,Plopper CG,Bond JA.1,3-Butadiene metabolism by lung airways isolated from mice and rats[J].Toxicology,1996,113(1-3):314-317.
[5]Zhao C,Vodicka P,Srám1 RJ,et al.Human DNA adducts of 1,3-butadiene,an important environmental carcinogen[J].Carcinogenesis,2000,21(1):107-111.
[6]Sathiakumar N,Delzell E,Hovinga M,et al.Mortality from cancer and other causes of death among synthetic rubber workers[J].Occup Environ Med,1998,55(4):230-235.
[7]Cheng H,Sathiakumar N,Graff J,et al.1,3-Butadiene and leukemia among synthetic rubber industry workers:exposure-response relationships[J].Chem Biol Interact,2007,166(1-3):15-24.
[8]Brunnemann KD,Kagan MR,Cox JE,et al.Analysis of 1,3-butadiene and other selected gas-phase components in cigarette mainstream and sidestream smoke by gas chromatography-mass selective detection[J].Carcinogenesis,1990,11(10):1863-1868.
[9]Fowles J,Dybing E.Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke[J].Tob Control,2003,12(4):424-430.
[10]Himmelstein MW,Turner MJ,Asgharian B,et al.Comparison of blood concentrations of 1,3-butadiene and butadiene epoxides in mice and rats exposed to 1,3-butadiene by inhalation[J].Carcinogenesis,1994,15(8):1479-1486.
[11]Peters FT,Drummer OH,Musshoff F.Validation of new methods[J].Forensic Sci Int,2007,165(2-3):216-224.
[12]Sikkema J,de Bont JA,Poolman B.Interactions of cyclic hydrocarbons with biological membranes[J].J Biol Chem,1994,269(11):8022-8028.
[13]Weber FJ,Isken S,de Bont JA.Cis/trans isomerization of fatty acids as a defence mechanism of Pseudomonasputida strainsto toxic concentrationsof toluene[J].Microbiology,1994,140(Pt 8):2013-2017.
[14]Christie WW.Gas chromatography analysis of fatty acid derivatives,in:Gas chromatography and lipids[M].Oliy Press Ltd.,Scotland,1998,chapter 5,section C.

