Growth,Metabolism and Physiological Response of the Sea Cucumber,Apostichopus japonicus Selenka During Periods of Inactivity
DU Rongbin,ZANG Yuanqi,TIAN Xiangli,and DONG Shuanglin
1) School of Oceanography,Yantai University,Yantai 264005,P.R.China
2) The Key Laboratory of Mariculture, Ministry of Education of China,Ocean University of China,Qingdao 266003,P.R.China
3) Provincial Marine Fisheries Research Institute of Shandong,Yantai 264006,P.R.China
1 Introduction
Sea cucumber (Apostichopus japonicusSelemka) is the most important commercial holothurian species in China(Liao,1980,1997; Wang,2008b,2009).As a temperate species,A.japonicusinhabits Asian coast from 35˚N to 44˚N,and survives at 0–30℃ (Yuet al.,1999; Chen,2004;Gaoet al.,2011).Temperature is a principal environmental factor determining the growth ofA.japonicusand the optimal temperature range is between 15℃ and 18℃(Donget al.,2005).Under extremely high temperature,the animals can enter a state of dormancy (i.e.,aestivation)for survival (Yanget al.,2006; Jiet al.,2008).In north China Sea,the duration of aestivation ofA.japonicuscan last over 110 days (Liuet al.,1996).Typical changes in the behavior and physiology ofA.japanicusunder aestivation include cessation of feed,reduction of activities and depression of metabolism (Tanikawaet al.,1955; Yuet al.,1999; Yanget al.,2005; Yanget al.,2006).Previous studies have shown that aestivation is temperature-dependent threshold temperature for aestivation ranges from 20℃ to 24.5℃ at different locations and body weights (Choe,1963; Sui,1989; Liuet al.,1996; Liet al.,1996).Aestivating animals can be collected by artificial induction through continuous temperature elevation (Jiet al.,2008; Yanget al.,2005) or from culture ponds during summer when the water temperature is much higher than the threshold for aestivation (Wanget al.,2009).Starvation is unavoidable forA.japanicusin marine culture practice,and is usually caused by the shortage of food in the culture ponds.Although starvation is different from aestivation,these two states show similar physiological characteristics including the organism’s alimentary tract depression (Liet al.,1996) and no food intake.However,knowledge is limiting regarding the difference in physiological stress response ofA.japanicusto aestivation (caused by artificial or natural temperature elevation) and starvation.
Cellular stress response including enhancement of enzymatic activities,synthesis of molecular chaperones and activation of DNA repair system and proteases plays an important role in resisting environmental stress (Sørensenet al.,2003).Acidic phosphatase (ACP),a typical lysosomal enzyme involved in digesting and killing micro-bial pathogens,is sensitive to temperature variation (Liuet al.,2004).A marked increase of ACP activity was found in haemolymph ofC.farrerimaintained at 30℃ for two weeks (Liuet al.,2004).Alkaline phosphatase (AKP)is a metalloenzyme which catalyzes the nonspecific hydrolysis of phosphate monoesters (Zhang,2004).When exposed to a variety of environmental stresses,lysosomal enzymes as well as ACP and AKP participate in the degradation of foreign proteins,lipids and carbohydrates(Ottaviani,1984; Pipeet al.,1993; Xue and Renault,2000).Superoxide dimutase (SOD) and catalase (CAT)are two primary antioxidases directly involved in eliminating reactive oxygen species (ROS) such as superoxide anion (O2−),hydrogen peroxide (H2O2),hydroxyl radical(OH) and singlet oxygen (Roch,1999),thus are qualified as the main markers of the antioxidant defense(Wilhelm-Filhoet al.,1993,2001; Leiniö and Lehtonen,2005; Changet al.,2010; Guet al.,2011).InApostichopus japonicus,the SOD and CAT activities show significant increase when the ambient temperature changes daily in a range of ± 4℃ (Donget al.,2008a).
Heat shock protein 70 (Hsp70) is qualified as a direct index of the heat shock response.It is widely established that Hsps can be induced under certain physical,chemical and biological stresses (Feder and Hofmann,1999; Menget al.,2009).A strong correlation has been reported between the heat shock response and the thermal tolerance of animals (Parsell and Lindquist,1993; Feder and Hofmann,1999; Tomanek,2002).Previous studies showed that the high Hsp70 content could result in growth retardation (Federet al.,1992; Krebs and Feder,1997; Viantet al.,2003),mainly because of the high energy consumption during the synthesis of Hsp 70 (Feder and Hofmann,1999;Somero,2002).Hsp 70 plays an important role in resisting thermal stress in sea cucumberA.japonicus(Dong and Dong,2008; Donget al.,2008b).
In present study,the growth,oxygen consumption rate(OCR),enzyme activity and Hsp 70 content ofA.japonicuswere studied during different inactive periods including starvation at constant 17℃,experimental aestivation at constant 24℃,and natural aestivation at outdoor seawater temperatures ranging from 24.5℃ to 28.9℃.The results will provide an insight into the physiological adaptation ofA.japonicusto the environmental stresses.
2 Material and Methods
2.1 Experimental Animals and Acclimation
Sea cucumber,A.japonicas,was collected in June 2009 from the coastal culture pond located in Jimo,Qingdao,Shandong,China.The animals were cultured in a 1000-L fiberglass tank filled with filtered seawater for 15 d to fully ensure the thermal and environmental adaptation.During acclimation,the seawater was renewed daily with temperature maintained at 17℃± 0.5℃ and salinity between 28 and 30.The animals were fed to satiation once a day at 18:00 with a formulated diet.Sea cucumber in natural aestivation was collected from the same pond in July 2009 and acclimated at an outdoor seawater temperature under experimental conditions to ensure the aestivation state.Other acclimation conditions were controlled as the same for experimental aestivation.The body size of animals averaged 34.54 g ± 0.65 g.The experiments were performed from July 2 to August 6,2009.
2.2 Experimental Design and Management
2.2.1 Experimental Design
Four experimental groups of sea cucumber were designed,i.e.,starvation at constant 17℃,experimental aestivation at constant 24℃,natural aestivation at an outdoor seawater temperature,and feeding group at constant 17℃ as control.For starvation and control groups,105 individuals were cultured at constant 17℃ with satiate feed supply.For experimental aestivation,105 sea cucumbers were acclimated to 24℃ by increasing the temperature 1℃ per day.The sea cucumber was fed with normal rations until the cessation of feed intake,which indicated that the individuals entered experimental aestivation.Then,105 sea cucumber of the natural aestivation group were collected from the outdoor ponds and cultured at an outdoor seawater temperature fluctuating from 24.5℃ to 28.9℃,and considered to be in their natural inactive environmental state and,thus,would reflect their dormant physiological characteristics.During the trial,the outdoor seawater temperature used for natural aestivation treatment was detected daily with a high-precision thermometer (Fig.1).The outdoor seawater temperature fluctuated between 24.5℃ at Day 0 (July 2,2009) and 28.9℃ at Day 36 (August 6,2009).

Fig.1 Outdoor seawater temperature during the natural aestivation treatment of sea cucumber.
The sea cucumber was maintained in glass aquaria (55 cm×30 cm×35 cm) containing 45 L water,5 individuals each.For each treatment,the growth,OCR and physiological parameters were determined for 25,40 and 40 individuals,respectively.
2.2.2 Experimental Management
Seawater used in the experiment was filtered by composite sand filter,and 50% of the water each aquarium was exchanged daily to ensure the good water quality.Aeration was provided continuously to maintain the dissolved oxygen level above 6 mg L−1.
During the experiment,the seawater was maintained at a content of ammonia less than 0.025 mg L−1,an acidity of pH7.8 to 8.2 and a salinity of 28–30.A simulated natural photoperiod (14 h light: 10 h dark) was used throughout the period of experiment.
2.3 Growth Measurement
For growth experiment,the animals were weighted every 6 d from day 0 to day 36.Before weight,the animals were wrapped by sterile gauze for 15 min to dry their body (Tanet al.,2008).
2.4 Oxygen Consumption Rate Determination
The OCR was determined on day 0,3,6,9,12,18,26,and 36 for the fasting and feeding groups of sea cucumber.Each measurement was performed on five individuals and five blank controls (17℃,24℃ and temperature ranging from 24.5 to 28.9℃).The animals were put in a 1 L conical flask individually for 4 h.Then,the final level of dissolved oxygen in each chamber was determined using the Winkler method (Strickland and Parsons,1968; Jiet al.,2008).The OCR was calculated using the following equation (Omori and Ikeda,1984):
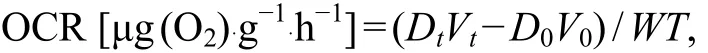
whereTis time duration (h);Dtis the change in the oxygen content (μg O2L−1) before and after test in 1 L bottle;D0is the change in the oxygen content (μg O2L−1) before and after test in the blank bottle;Vtis the volume of the test bottle (L);V0is the volume of the blank bottle (L);Wis the wet weight of the sea cucumber (g).
2.5 Tissue Preparation and Physiological Parameters Assays
Muscle and coelomic fluid samples were collected on day 0,3,6,9,12,18,26 and 36 for the fasting and feeding groups.Muscle was cut off from the backside of the body,rinsed with water and then blot-dried.Coelomic fluid (3.5–5.0 mL) was immediately withdrawn with a syringe.All the samples were frozen in Eppendorf tubes in liquid nitrogen and stored at (−80℃) until use.
At each sampling interval,muscle samples were thawed,weighed and homogenized in 5 volumes of icecold physiological saline using a manual glass homogenizer.The homogenates were then centrifuged at 10000 ×gat 4℃for 20 min.Samples of frozen coelomic fl uid were thawed and the cell contents were released with an ultrasonic cell disruptor.For assay of enzyme activities,the whole coelomic fluid samples were centrifuged at 376 ×gat 4℃for 10 min.The supernatant of muscle and coelomic fluid samples was then transferred into clean centrifuge vials and stored at 4℃ until use (less than 12 h).
分析:前面的Nine Peaches本身就是对design的说明,因此无需再用design。另外从表一可以看出,凡是带图案的瓷瓶或其他属体,以英语为母语的博物馆则只保留了图案部分,是没有再缀design一词的。这说明故宫部分文物名称的翻译不符合英文表述习惯,语义重复,中式特点明显。
The protein content of the homogenates was measured following the method of Spector (1978),using bovine serum albumin as the standard.All assays for enzyme activities were carried out in duplicate with a UV 2102PC spectrophotometer (Unico,Shanghai,China).
The ACP and AKP activities were analyzed according to Barrett (1972) using a commercial kit (Nanjing Jiancheng Biotech Company,China).The concentration of phenol was measured spectrophotometrically at 520 nm after incubation at 37℃ for 30 min (for ACP),or 15 min(for AKP).ACP and AKP activities were defined as the amount of phenol (mol) produced per milligram protein for muscle and per milliliter.
The SOD activity was determined according to Ji(1991) with an assay kit (Nanjing Jiancheng Biotech Company,China).The assay reaction contained 65 μmol phosphate buffer,pH7.8,1 μmol hydrochloric hydroxylamine,0.75 μmol xanthine and 2.3 × 10−3IU xanthine dismutase.Fifty microliters of the supernatant given no blank reaction were incubated in the system at 37℃ for 40 min,and terminated with 2 mL 3.3 g L−1p-aminobenzene sulfonic acid and 10 g L−1naphthylamine.A SOD unit was defined as the amount of enzyme that inhibits the superoxide-induced oxidation (monitored at 550 nm)by 50%.
The CAT activity was measured according to Góth(1991) with an assay kit (Nanjing Jiancheng Biotech Company,China).The base system (4.60 μmol phosphate buffers,pH 7.4,6.5 × 10−3μmol H2O2) was incubated at 37℃for 1 min,and the reaction was terminated immediately by 32.4 μmol ammonium molybdate.A CAT unit was defined as catalyzing the use of 1 μmol H2O2per second.
The expression of hsp70 was measured using a commercially available Hsp 70 enzyme-linked immunosorbent assay (ELISA) kit (R&D,Minneapolis,MN,USA)with anti-Hsp70 antibody (H5147,Sigma,USA,Donget al.,2008a; Jiet al.,2008) according to the manufacturer’s instructions.
2.6 Statistical Analysis
Statistical analysis was performed with the SPSS statistical package 11.0 for windows (SPSS Inc.,Richmond,CA,USA).Values were presented as the means ± standard error.The data of body weight,OCR,enzymatic activities and Hsp70 content were tested for homogeneity of variances,and possible differences were compared among time intervals with one-way ANOVA followed by a Tukey’s HSD multiple comparison test,while those between the treatment and the control were tested by Student’st-test.The Differences were considered significant among treatment groups atP< 0.05.
3 Results
3.1 Growth
During the 36-day trial,the body weight of sea cucumber in the feeding group increased by 38.46% in 36 d,while those in the starvation,experimental aestivation and natural aestivation groups decreased significantly (P<0.05) by 12.11%,18.81%,12.26%,respectively (Fig.2).
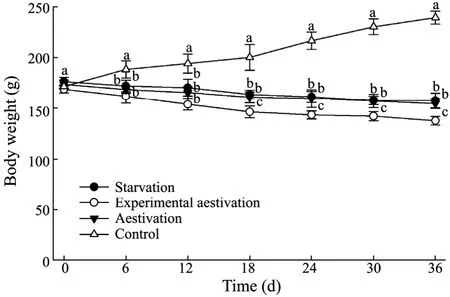
Fig.2 Body weight of A.japonicus on day 0,6,12,18,24,30,and 36 in the feeding,starvation,experimental aestivation,and natural aestivation treatments.Bar represents the standard error of five replicates.Different letters mean significant difference among the data at the same sampling time.
3.2 Oxygen Consumption Rate
During the experimental period,the OCR in starved animals decreased significantly (P< 0.05),and remained at a significantly lower level than the fed ones (Fig.3).The OCR of animals in the experimental aestivation treatment decreased from a higher level with no significant differences found in the control from the day 18 (P>0.05).Comparatively,the OCR in the natural aestivation animals decreased within the first 8 d,and then increased from day 9.

Fig.3 Oxygen consumption rate of A.japonicus in the treatments.Bar and letters represent as those in Fig.2.
3.3 Enzymatic Activities
Enzymatic activities in the body wall and coelomic fluid ofA.japonicuswere detected on day 0,3,6,9,12,18,26,and 36.No significant changes were found in the activities of hydrolases and antioxidases in the feeding group throughout the experimental period (Figs.3–8,P>0.05).However,the animals in the starvation treatment had gradually decreased activities of hydrolases and antioxidases with time,significantly lower than the control (P> 0.05).Generally,temporal patterns of activities for the two antioxidases were different between the experimental and natural aestivation.
3.3.1 Hydrolases
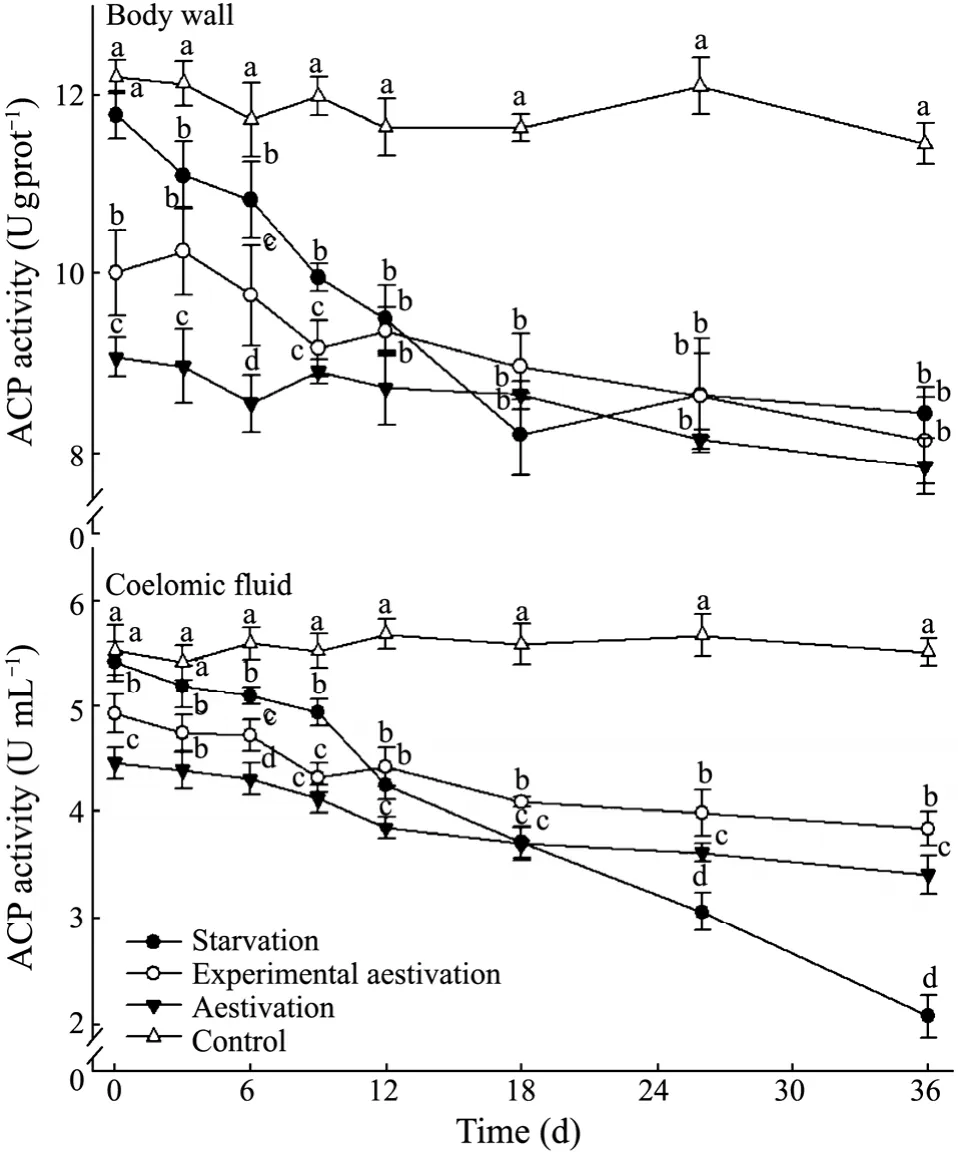
Fig.4 Acidic phosphatase activity in the body wall and coelomic fluid of A.japonicus in the treatments.Bar and letters represent as those in Fig.2.

Fig.5 Alkaline phosphatase activity in body wall and coelomic fluid of A.japonicus in the treatments.Bar and letters represent as those in Fig.2.
As shown in Figs.4 and 5,the activities of ACP and AKP in the starvation treatment decreased continuously in both body wall and coelomic fluid,significantly lower than those in the feeding group after day 6 (P< 0.05).In the experimental aestivation and natural aestivation treatments,ACP and AKP activities of sea cucumber showed similar variation tendency with time,both decreasing gradually with no significant differences between the two treatments (P> 0.05).ACP and AKP activities of sea cucumber in the experimental aestivation and natural aestivation treatment were significantly lower than those in the feeding treatment at the end of the experiment (P< 0.05).
3.3.2 Antioxidases
The activities of CAT and SOD in both body wall and coelomic fluid of sea cucumber in the starvation treatment decreased continuously,significantly lower than those in the feeding group from day 9 (P< 0.05,Figs.6 and 7).In the natural aestivation treatment,CAT and SOD activities were initially detected at a low level,and then were found to increase throughout the trial,while those in experimental aestivation group decreased in both body wall and coelomic fluid.CAT and SOD activities for sea cumber in the natural aestivation group were significantly higher than those in starvation and experimental aestivation group at the end (P< 0.05).
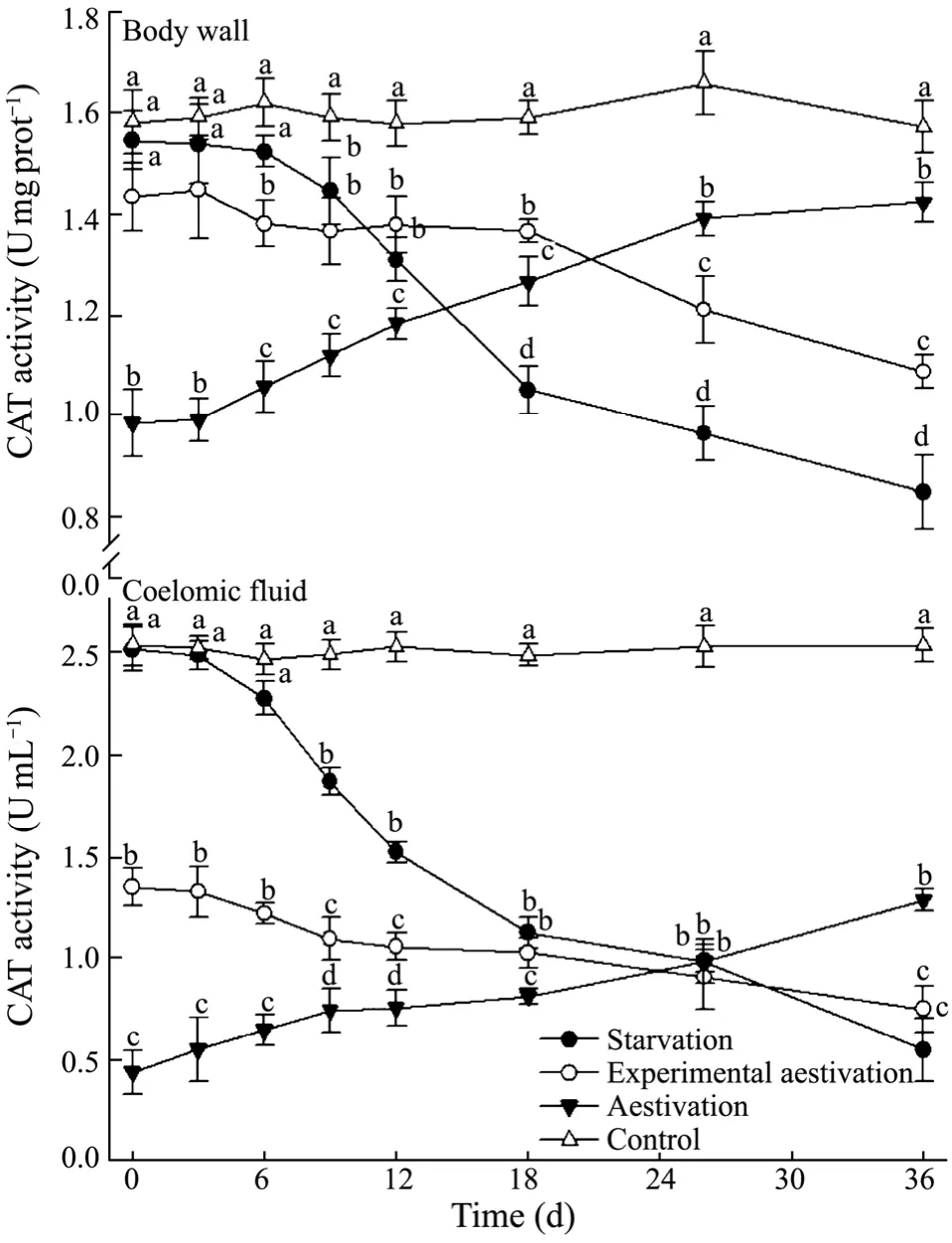
Fig.6 Catalase activity in the body wall and coelomic fluid of A.japonicus in the treatments.Bar and letters represent as those in Fig.2.
3.4 Hsp70
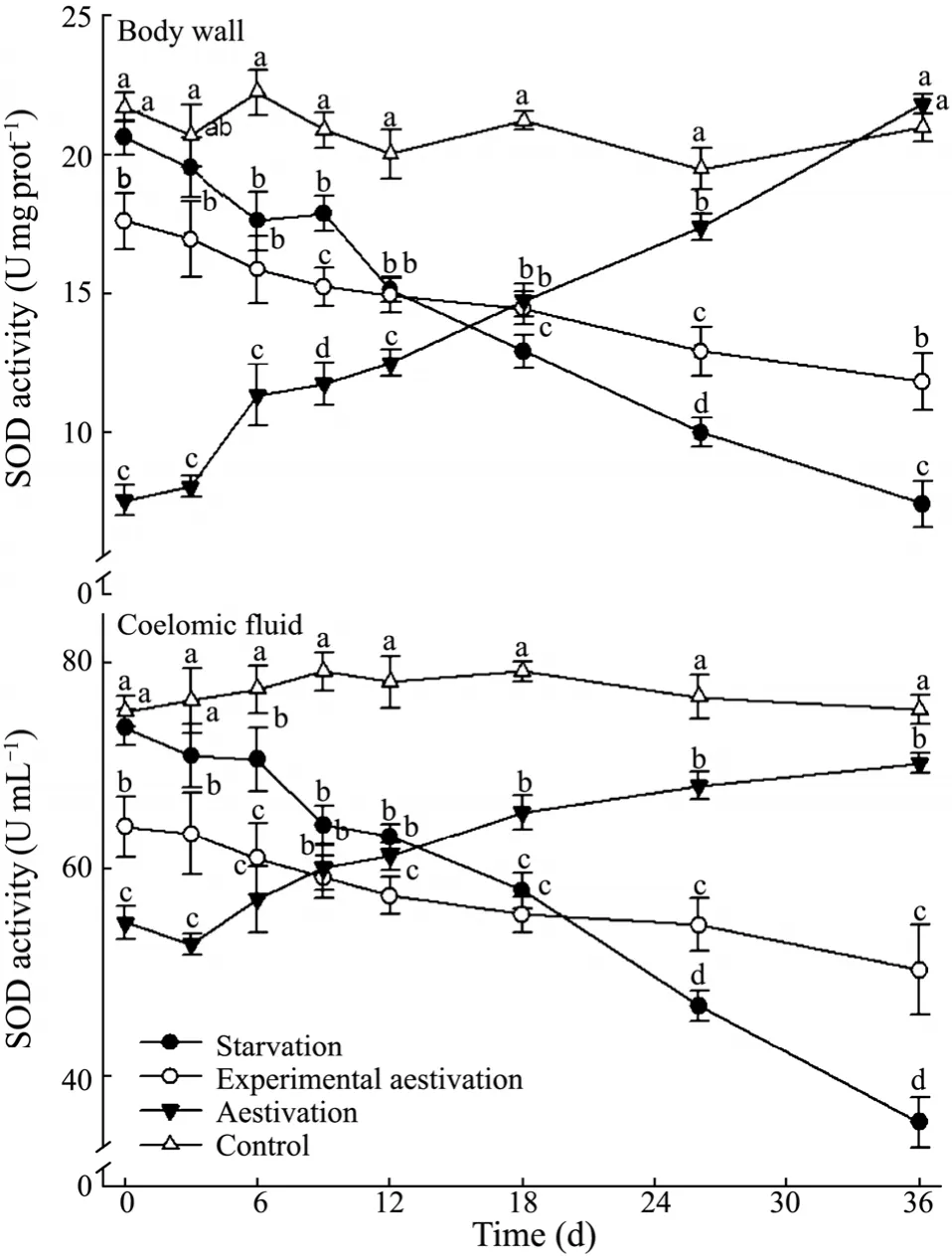
Fig.7 Superoxide dismutase activity in the body wall and coelomic fluid of A.japonicus in the treatments.Bar and letters represent as those in Fig.2.

Fig.8 Hsp70 content in the body wall and coelomic fluid of A.japonicus in the treatments.Bar and letters represent as those in Fig.2.
Hsp70 content in the body wall and coelomic fluid of sea cucumber kept relatively stable in the feeding treatment,while that in the starvation treatment decreased gradually with time and was significantly lower than that of the feeding treatment after day 6 (P< 0.05,Fig.8).Generally,temporal patterns of Hsp70 content were different between the experiment aestivation and natural aestivation treatments.For animals in the experiment aestivation,Hsp70 content in coelomic fluid initially in-creased,peaked on day 9,and then decreased till day 36,while that in the body wall first increased,peaked on day 3,and then showed a downward trend till the end of the trial.In contrast,Hsp70 content in the natural aestivation group showed an upward trend,significantly higher than those in other treatments from day 12 (P< 0.05).
4 Discussion
Water temperature is one of the most important environmental factors that affect the growth and metabolism processes of aquatic animals.A.japonicusgrows in water with a temperature range from 10 to 20℃ (Sui,1990; Yu and Song,1999; Chen,2004; Yanget al.,2005; Donget al.,2006),and the optimum temperature is between 15 and 18℃.Aestivation is recognized as a behavioral and physiological state that ensures the survival of animals through fluctuations of environmental conditions,such as temperature,water quality and/or food threat (Yang,2005,2006).Previous reports have suggested thatA.japonicusgradually stops feeding and starts dormancy when seawater temperature is above 20℃ (Choe,1963; Liet al.,1996; Liuet al.,1996; Chenet al.,2004).In present study,17℃ was chosen as the optimum temperature for sea cucumber in the control and starvation treatments,while 24℃ was chosen as the threshold temperature for animals in the experimental aestivation.The results indicated that sea cucumber kept growing,whereas the enzyme activities and Hsp70 content remained at relatively stable levels in the feeding group.However,the body weight for sea cucumber in the starvation,experimental and natural aestivation treatments decreased gradually.These indicated that during the period of the experiment,the sea cucumber only consumed endogenous substance (i.e.,the energy accumulated in the body) due to lack of food intake.Furthermore,changes in the metabolism,enzymatic activities and Hsp70 content were observed in the sea cucumber reared at 24℃ and outdoor ambient seawater temperature.
Aestivation significantly influences the metabolism ofA.japonicus.A marked depression of OCR is commonly found in the initialization period of aestivation,as shown in this study and previous research by Yanget al.(2006).However,we observed different trends in the OCR of animals in various treatments.A gradual depression of body weight and OCR in sea cucumber for the experimental aestivation treatment was detected during the whole experimental period,suggesting that the initialization of the dormant state induced by elevated temperature was a long,gradual process inA.japonicus.Comparatively,the OCR of animals in an aestivation state was quite different.Despite the decrease within the first 10 days,the OCR increased gradually afterwards during the experiment.It should be noticed that the aestivation state ofA.japonicusin the experimental aestivation treatment was induced by a high constant temperature (24℃) rather than the simulated natural fluctuating thermal regime for the natural aestivation treatment (24.5℃ to 28.9℃) (Fig.1).This was consistent with the findings by several previous studies (Yanget al.,2006,148.5 g,25℃; Yuanet al.,2007,149.9 g,25℃ and 30℃).The experimental constant temperature and natural fluctuating thermal regimes had different impacts on the OCR ofA.japonicusduring the initialization period of aestivation and in aestivation state.Further research is needed to demonstrate the mechanism of aestivation inA.japonicus.
The activities of major physiological enzymes,including ACP,AKP from hydrolytic system and SOD,CAT from antioxidant system,can be used to evaluating the status of sea cucumber (Wanget al.,2008a; Wanget al.,2008b).In present study,both ACP and AKP activities in the body wall and coelomic fluid ofA.japonicusfor all the three treatments decreased during the 36-day trial compared to those in the control.This indicated that the hydrolytic capability likely declined due to starvation or/and high temperature.However,the antioxidase activities of sea cucumber in the starvation and aestivation treatments showed a different tendency.High temperature initially up-regulated the antioxidase (e.g.,SOD and CAT)activities of animals in the experimental treatment,then kept reducing afterwards when the sea cucumber entered the aestivation state.Comparatively,in the natural aestivation treatment,the activities of SOD in both body wall and coelomic fluid and CAT in body wall were significantly higher than those in the control during the whole experiment.Changes in CAT and SOD activities are usually related to the OCR (Regoliet al.,2004; Sies,1997).The production of reactive oxygen species (ROS) increases when the tissue oxygen consumption enhances.However,the oxidative stress occurs when ROS generation exceeds its removal capacity (Sies,1986).The oxidized or nitrosylated products of ROS attack have been shown to lose body weight and energy metabolism,leading to decreases in the biological activity and other major functional degradation (Vincent,2004).Oxidative damage is counteracted by the antioxidant defense systems.CAT and SOD play important roles in cellular antioxidants and are involved in removing of ROS directly(Hemes-limaet al.,1998; Changet al.,2010).In present study,the changes in CAT and SOD activities in the experimental aestivation treatment indicated that the decrease in OCR was an adaptive strategy of sea cucumber to high temperature in order to reduce the ROS accumulation and related oxidative stress.However,an increasing trend was found in CAT and SOD activities of animals in the natural aestivation treatment throughout the experiment,which was quite different from those in the experimental aestivation treatment.It was likely that the sea cucumber become physiological stressed due to the accelerating temperature,and increases in the SOD and CAT activities helped to remove excess superoxide production.Similarly,Wang reported a decrease in catecholamines and an increase in antioxidants enzymes in the coelomic fluid ofA.japonicusduring aestivation(Wanget al.,2008).This was considered to be a compensatory strategy to the immune activity,as observed for fishes (Randell and Perry,1992).
Hsp70,as a molecular chaperone,assists the folding of stress denatured cellular proteins and keeps these proteins from aggregating in cell (Parsell and Lindquist,1993).Results from this study showed that Hsp70 content remained at a high level before day 6 in the experimental aestivation treatment as well as increased with temperature in the natural aestivation treatment.This indicated that the temperature of 24℃ was beyond the normal temperature limit forA.japonicus,and some proteins lost their structures and activities,leading to the expression of Hsps for refolding proteins and preventing further damages.More interestingly,Hsp70 content of animals in the natural aestivation treatment showed a variation trend similar to that of antioxidases.Niedzwiecki found that Hsp directly released and increased Cu/Zn SOD and CAT enzymatic activities in a different,not necessarily coordinate manner (Niedzwieckiet al.,1992).Therefore,the increasing oxidative stress was likely induced by an increasing ROS,which was possibly responsible for the high Hsp70 content in this study.
According to Choe (1963) and Li and Wang (2007),the whole aestivation process is divided into three stages,i.e.,pre-aestivating stage (July),aestivating stage (August–September) and arousal stage (October) (Wanget al.,2008a).In present study,the animals with an average body size of (34.54 g ± 0.65 g were collected from a pond in different periods,i.e.,July for natural aestivation treatment and June for other treatments.Thus,the sea cucumber used for natural aestivation group was in a pre-aestivation stage.We observed that the enzyme activities differ in different aestivating stages and are not correlated with temperature and salinity,in agreement with a previous study (Wanget al.,2008a).As we carried out a single experiment,our results were limited to one pond in one year.Further study of sea cucumber for prolonged periods at additional locations will strengthen of our conclusions.Whether these individuals have similar responses at different stages of development is worthy of further investigation.
In conclusion,this study demonstrated that during the starvation,the body weight,metabolic rate and enzymatic activity ofA.japonicuschanged due to the stress of fasting.The sea cucumber entered a state of aestivation when at a constant temperature of 24℃.To some extent,the animals in the experimental aestivation were different from those in natural aestivation in terms of OCR,enzymatic activity and Hsp70 content.This suggests that the mechanism of aestivation inA.japonicusis complex and may not be attributed to environmental changes only,such as elevated temperature (Liet al.,1996; Liuet al.,1996).To further understand the changes inA.japonicusin inactivity states,more studies should be conducted on numerous enzymatic parameters and Hsp70 content,as well as the physiological response ofA.japonicasto different environmental conditionals at different stages of development.
Acknowledgements
This research was supported by the Natural Science Funds for Distinguished Young Scientists of Shandong Province (No.JQ201009),the National Great Project of Scientific and Technical Supporting Programs (No.2011-BAD13B03),the National Natural Science Foundation of China (No.30771661),the Public Science and Technology Research Funds Projects of Ocean,State Oceanic Administration of China (No.200905020),and the 111 Project of China Ministry of Education (No.B08049).
Barrett,A.J.,1972.Lysosomal enzymes.In:Lysosomes:A Laboratory Handbook.Dingle,J.T.,ed.,North-Holland,Amsterdam,46-135.
Chang,J.,Zhang,W.B.,Mai,K.S.,and Ma,H.M.,2010.Glucan and glycyrrhizin on non-specific immunity and disease resistance of the sea cucumber (ApostichopusjaponicusSelenka) challenged withVibrio splendidus.Journal of Ocean University of China, 9: 389-394.
Chen,J.,2004.Present status and prospects of sea cucumber industry in China. In:Advances in Sea Cucumber Aquaculture and Management.Lovatelli,A.,et al.,eds.,FAO,Rome,Italy,25-38.
Choe,S.,1963.Study of Sea Cucumber: Morphology,Ecology and Propagation of Sea Cucumber.Kaibundo Publishing House,Tokyo,219pp.
Dong,Y.W.,Dong,S.L.,Tian,X.L.,Zhang,M.Z.,and Wang,F.,2005.Effects of water temperature on growth,respiration and body composition of young sea cucumberApostichopus japonicus.Journal of Fishery Sciences of China,12: 33-38.
Dong,Y.W.,and Dong,S.L.,2008a.Induced thermotolerance and expression of heat shock protein 70 in sea cucumberApostichopus japonicus.Fisheries Science,74: 573-578.
Dong,Y.W.,Dong,S.L.,and Ji,T.T.,2008b.Effect of different thermal regimes on growth and physiological performance of the sea cucumberApostichopus japonicusSelenka.Aquaculture,275: 329-334.
Dong,Y.W.,Dong,S.L.,and Meng,X.L.,2008c.Effects of thermal and osmotic stress on growth,osmoregulation and Hsp70 in sea cucumber (ApostichopusjaponicusSelenka).Aquaculture,276: 179-186.
Feder,J.H.,Rossi,J.M.,Solomon,J.,Solomon,N.,and Lindquist,S.,1992.The consequences of expressing Hsp70 in Drosophila cells at normal temperatures.Genes and Development,6: 1402-1413.
Feder,M.E.,and Hofmann,G.E.,1999.Heat-shock proteins,molecular chaperones and the stress response: evolutionary and ecological physiology.Annual Review of Physiology,61:243-282.
Gao.Q.F.,Wang.Y.S.,Dong,S.L.,Sun,Z.L.,and Wang,F.,2011.Absorption of different food sources by sea cucumberApostichopus japonicus(Selenka) (Echinodermata: Holothuroidea): Evidence from carbon stable isotope.Aquaculture,319: 272-276.
Gu,M.,Ma,H.M.,Mai,K.S.,Zhang,W.B.,Bai,N.,and Wang,X.J.,2011.Effects of dietary b-glucan,mannan oligosaccharide and their combinations on growth performance,immunity and resistance againstVibrio splendidusof sea cucumber,Apostichopus japonicus.Fish and Shellfish Immunology, 31: 303-309.
Góth,L.,1991.A simple method for determination of serum catalase activity and revision of reference range.Clinica Chimica Acta,196: 143-151.
Ji,J.P.,1991.An ultramicroanalytic and rapid method for de-termination of superoxide dismutase activity.Journal of Nanjing Railway Medical College,10: 27-30.
Ji,T.T.,Dong,Y.W.,and Dong,S.L.,2008.Growth and physiological responses in the seacucumber,Apostichopus japonicusSelenka: Aestivation and temperature.Aquaculture,283:180-187.
Krebs,R.A.,and Feder,M.E.,1997.Deleterious consequences of Hsp70 over-expression inDrosophila melanogasterlarvae.Cell Stress Chaperones,2: 60-71.
Leiniö,S.,and Lehtonen,K.K.,2005.Seasonal variability in biomarkers in the bivalvesMytilus edulisandMacoma balthicafrom the northern Baltic Sea.Comparative Biochemistry and Physiology,140C: 408-421.
Li,F.,Liu,Y.,Song,B.,Sun,H.,Gu,B.,and Zhang,X.,1996.Study on aestivating habit of sea cucumber (Apostichopus japonicusSelenka): 2.The factors relating to aestivation.Journal of Fishery Sciences of China,3: 49-57.
Li,X.,and Wang,X.,2007.The histological observation of alimentary tract and respiratory tree in sea cucumber,Apostichopus japonicusduring aestivation induced in lab.Journal of Dalian Fisheries University,22: 81-85.
Liao,Y.,1980.The aspidochirote holothurians of China with erection of a new genus in echinoderms,present and past.In:Proceeding of European Colloquium on Echinoderm. Jangoux,M.,ed.,A.A.Balkema Publishers,Rotterdam,Netherlands,115-120.
Liao,Y.,1997.Fauna Sinica:Phylum Echinodermata Class Holothuroidea.Science Press,Beijing,148-150.
Liu,S.L.,Jiang,X.L.,Hu,X.K.,Gong,J.,Hwang,H.M.,and Mai,K.S.,2004.Effects of temperature on non-speci fi c immune parameters in two scallop species:Argopecten irradians(Lamarck 1819) andChlamys farreri(Jones & Preston 1904).Aquaculture Research, 35: 678-682.
Liu,Y.,Li,F.,Song,B.,Sun,H.,Zhang,X.,and Gu,B.,1996.Study on aestivating habit of sea cucumberApostichopus japonicus Selenka: 1.Ecological characteristic of aestivation.Journal of Fishery Sciences of China,3: 41-48.
Meng,X.L.,Ji,T.T.,Dong,Y.W.,Wang,Q.L.,and Dong,S.L.,2009.Thermal resistance in sea cucumbers (Apostichopus japonicus) with differing thermal history: The role of Hsp70.Aquaculture,294: 314-318.
Niedzwiecki,A.,Reveillaud,I.,and Fleming,J.E.,1992.Changes in superoxide dismutasc and catalase in aging heat-shockedDrosophila.Free Radical Research and Communication,17: 355-367.
Omori,M.,and Ikeda,T.,1984.Methods in Marine Zooplankton Ecology.John Wiley and Sons Inc,New York,173-209.
Ottaviani,E.,1984.Composition of the serum haemolymph ofPlanorbis corneus(Gastropoda,Pulmonata).Comparative Biochemistry and Physiology,78B: 227-239.
Parsell,D.A.,and Lindquist,S.,1993.The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins.Annual Review of Genetics,27:437-496.
Pipe,R.K.,Porte,C.,and Livingstone,D.R.,1993.Antioxidant enzymes associated with the blood cells and hemolymph of the musselsMytilus edulis.Fish and Shellfish Immunology,3:221-233.
Randell,D.J.,and Perry,S.F.,1992.Catecholamines. In:Fish Physiology:the Cardiovascular System.Vol.XIIB.Hoar,W.S.,and Randall,D.J.,eds., Academic Press,New York,255-300.
Regoli,F.,Cerrano,C.,and Chierici,E.,2004.Seasonal variability of prooxidant pressure and antioxidant adaptation to symbiosis in the Mediterranean demospongePetrosia ficiformis.Marine Ecology Progress Series,275: 129-137.
Roch,P.,1999.Defense mechanisms and disease prevention in farmed marine invertebrate.Aquaculture,172: 125-145.
Sies,H.,1986.Biochemistry of oxidative stress.Angewandte Chemie International Edition,25: 1058-1071.
Sies,H.,1997.Oxidative and antioxidants.Experimental Physiology,82: 291-295.
Sloan,A.N.M.,1984.Echinoderm fi sheries of the world: a review. In:Echinodermata.Proceedings of the Fifth International Echinaoderm Conference.Keegan,B.F.,and O'Connor,B.D.S.,eds., Balkema AA Publishers,Rotterdam,109-124.
Snyder,K.S.,and Nestler,J.R.,1990.Relationships between body temperature,thermal conductance,Q10 and energy metabolism during daily torpor and hibernation in rodents.Journal of Comparative Physiology, 159B: 667-675.
Somero,G.N.,2002.Thermal physiology and vertical zonation of intertidal animals: optima,limits,and costs of living.Integrative and Comparative Biology,42: 780-789.
Spector,T.,1978.Refinement of the coomassie blue method of protein quantitation.Analytical Biochemistry,86: 142-146.
Strickland,J.D.H.,Parsons,T.R.,1968.A practical handbook of seawater analysis.Fisheries Research Board of Canada Bulletin, 167: 1-11.
Sui,X.,1989.The main factors influencing the larval development and survival rate of the sea cucumber.Oceanologia et Limnologia Sinica,20: 314-321.
Sui,X.,1990.Culture and Enhance of Sea Cucumber.China Agriculture Publishing House,Beijing,288pp.
Sørensen,J.G.,Kristensen,T.N.,and Loeschcke,V.,2003.The evolutionary and ecological role of heat shock proteins.Ecology Letters,6: 1025-1037.
Tanikawa,E.,Akiba,M.,and Yoshitani,S.,1955.Studies on the nutritive value of the meat of sea cucumber (Stichopus japonicusSelenka)-II.Seasonal changes of chemical components of the meat ofStichopus japonicus.Bulletin of the Faculty of Fisheries Hokkaido University,5: 341-345.
Tan,Y.K.,Li,X.,and Duan,J.J.,2008.Energy metabolism and biochemical composition ofApostichopus japonicusduring the regeneration of viscera.Journal of Fishery Sciences of China,15 (4): 683-688.
Tomanek,L.,2002.The heat-shock response: its variation,regulation and ecological importance in intertidal gastropods(genusTegula).Integrative and Comparative Biology,42:797-807.
Viant,M.R.,Werner,I.,Rosenblum,E.S.,Gantner,A.S.,Tjeerdema,R.S.,and Johnson,M.L.,2003.Correlation between heat-shock protein induction and reduced metabolic condition in juvenile steelhead trout (Oncorhynchus mykiss)chronically exposed to elevated temperature.Fish Physiology and Biochemistry,29: 159-171.
Vincent,A.M.,Russell,J.W.,Low,P.,and Feldman,E.L.,2004.Oxidative stress in the pathogenesis of diabetic neuropathy.Endocrine Reviews,25: 612-628.
Wang,F.Y.,Yang,H.S.,Gabr,H.R.,and Gao,F.,2008a.Immune condition ofApostichopus japonicusduring aestivation.Aquaculture,285: 238-243.
Wang,F.Y.,Yang,H.S.,Gao,F,and Liu,G.B.,2008b.Effects of acute temperature or salinity stress on the immune response in sea cucumber,Apostichopus japonicus.Comparative Biochemistry and Physiology,Part A,151: 491-498.
Wang,F.Y.,Yang,H.S.,Gao,F.,and Liu,G.B.,2009.Annual changes of immune enzymes in coelome fluid of sea cucum-ber,Apostichopus japonicus.Marine Science,33: 75-80.
Wilhelm-Filho,D.W.,Giulivi,C.,and Boveris,A.,1993.Antioxidant defenses in marine fi sh.I: Teleosts.Comparative Biochemistry and Physiology,106C: 409-413.
Wilhelm-Filho,D.,Tribess,T.,Gáspari,C.,Claudio,F.D.,Torres,M.A.,and Magalhães,A.R.M.,2001.Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna).Aquaculture,203: 149-158.
Xing,J.,Zhan,W.B.,and Zhou,L.,2002.Endoenzymes associated with haemocyte types in the scallop (Chlamys farreri).Fish and Shell fi sh Immunology,13: 271-278.
Xue,Q.,and Renault,T.,2000.Enzymatic activities in European flat oyster,Ostrea edulis,and Pacific oyster,Crassostrea gigas,hemolymph.Journal of Invertebrate Pathology,76: 155-163.
Yang,H.S.,Yuan,X.T.,Zhou,Y.,Mao,Y.,Zhang,T.,and Liu,Y.,2005.Effects of body size and water temperature on food consumption and growth in the sea cucumberApostichopus japonicus(Selenka) with special reference to aestivation.Aquaculture Research,36: 1085-1092.
Yang,H.S.,Zhou,Y.,Zhang,T.,Yuan,X.T.,Li,X.,Liu,Y.,and Zhang,F.,2006.Metabolic characteristics of sea cucumberApostichopus japonicus(Selenka) during aestivation.Journal of Experimental Marine Biology and Ecology,330: 505-510.
Yu,D.X.,and Song,B.X.,1999.Variation of survival rates and growth characteristics of pond cultural juvenileApostichopus japonicus.Journal of Fishery Sciences of China,6: 109-110
Yuan,X.T.,Yang,H.S.,Wang,L.L.,Zhou,Y.,Zhang,T.,and Liu,Y.,2007.Effects of aestivation on the energy budget of sea cucumberApostichopus japonicus(Selenka) (Echinodermata: Holothuroidea).Acta Ecologica Sinica,27 (8): 3155-3161.
Zhang,M.,Wang,L.,Guo,Z.Y.,and Wang,B.J.,2004.Effect of lipopolysaccharide andVibrio anguillarumon the activities of phosphatase,superoxide dismutase and the content of hemocyanin in the serum ofFenneropenaeuschinensis.Marine Science,28: 22-25.
Zhang,W.Z.,Wu,X.Z.,Li,D.F.,and Sun,J.F.,2005.Immunological functions of blood cells in the scallopChlamys farreri.Acta Zoologica Sinica,51: 669-677.
 Journal of Ocean University of China2013年1期
Journal of Ocean University of China2013年1期
- Journal of Ocean University of China的其它文章
- Isolation and Expression Analysis of FTZ-F1 Encoding Gene of Black Rock Fish (Sebastes schlegelii)
- Chemical Characteristics and Anticoagulant Activities of Two Sulfated Polysaccharides from Enteromorpha linza(Chlorophyta)
- Optimization of the Purification Methods for Recovery of Recombinant Growth Hormone from Paralichthys olivaceus
- Seasonal Changes in Food Uptake by the Sea Cucumber Apostichopus japonicus in a Farm Pond: Evidence from C and N Stable Isotopes
- Purification and Characterization of a New Thermostable κ-Carrageenase from the Marine Bacterium Pseudoalteromonas sp. QY203
- What Depth Should Deep-Sea Water be Pumped up from in the South China Sea for Medicinal Research?
