Chemical Characteristics and Anticoagulant Activities of Two Sulfated Polysaccharides from Enteromorpha linza(Chlorophyta)
QI Xiaohui,MAO Wenjun,CHEN Yin,CHEN Yanli,ZHAO Chunqi,LI Na,and WANG Chunyan
Key Laboratory of Marine Drugs, Ministry of Education,Institute of Marine Drug and Food,Ocean University of China,Qingdao 266003, P.R.China
1 Introduction
Marine algae are considered to be an abundant source of new anticoagulant polysaccharides (Athukoralaet al.,2007; Liet al.,2012).A variety of anticoagulant and antithrombotic-active sulfated polysaccharides isolated from red and brown algae have been characterized,including various sulfated galactans and sulfated fucans among the most abundant non-mammalian sulfated polysaccharides in nature (Fonsecaet al.,2008; Pereiraet al.,1999,2005;Xieet al.,2011).Recently,several anticoagulant polysaccharides with novel structures have been isolated from marine green algae (Rodrigueset al.,2011).Maedaet al.(1991) reported that the anticoagulant activity of sulfated polysaccharide fromMonostroma nitidumis 6-fold higher than that of heparin,whereas Liet al.(2011) isolated an anticoagulantactive sulfated polysaccharide fromM.latissimum.In addition,Matsubaraet al.(2001) reported that the anticoagulant-active polysaccharide fromCodium cylindricumis a sulfated galactan.OtherCodiumspecies such asC.fragileandC.vermilarahave also been found to yield sulfated polysaccharides with anticoagulant (Cianciaet al.,2007).
Enteromorpha linzais one of the most common green seaweeds in China.It has long been used for drugs in traditional Chinese medicine.E.linzacommonly occurs during algal proliferation in eutrophicated coastal and lagoon waters.Several studies have focused on the cellwall polysaccharides fromEnteromorpha(Athukoralaet al.,2007; Chattopadhyayet al.,2007; Jiaoet al.,2009; Kimet al.,2011; Ray,2006).However,the chemical characteristics and biological activities of sulfated polysaccharides fromE.linzahave not been well documented.In addition,several studies have found that the sampling time influences chemical characteristics of algal polysaccharides(Ma- rinho-Soriano and Bourret,2003).Thus,the sulfated polysaccharides fromE.linzacollected at different periods of the year are worth being investigated.The present study focused on the chemical characteristics of two anticoagulant-active sulfated polysaccharides isolated fromE.linzacollected at different periods in 2008.
2 Material and Methods
2.1 Materials
Enteromorpha linzawas collected in May and September,2008 on the coast of Qingdao,Shandong,China(approximate seawater temperature 20℃).The raw material was thoroughly washed with tap water; air-dried and milled,then kept in plastic bags at room temperature.Dialysis membranes (flat width 44 mm,molecular weight cut off 3500) were purchased from Lvniao (Yantai,China),Q Sepharose Fast Flow and Sephacryl S-400/HR purchased from Pharmacia Bioscience (Uppsala,Sweden),standard dextrans (Mw: 5.9,9.6,21.1,47.1,107,200,and 708 kDa) purchased from Showa Denko K.K.(Tokyo,Japan),standard heparin,D-glucose,L-rhamnose,D-xylose,L-arabinose,D-mannose,L-fucose,D-galactose,D-glucuronic acid,D-galacturonic acid andN-acetyl-β-D-glucosamine purchased from Sigma (St.Louis,Missouri,USA),reagents for activated partial thromboplastin time(APTT) and prothrombin time (PT) assays purchased from Shanghai Sun (Shanghai,China),and reagents for thrombin time (TT) assay purchased from Dade Behring(Deerfield,Illinois,USA).
2.2 Isolation and Purification of Sulfated Polysaccharides from E.linza
The air-dried algal samples were milled and suspended in 30 volumes of H2O at room temperature for 1 h,and then homogenized and refluxed at 100℃ for 4 h.After cooling to the room temperature,the supernatant was recovered by centrifugation at 3600 g for 10 min,concentrated under reduced pressure,dialyzed in a cellulose membrane against flowing distilled water for 72 h.The recovered retention was concentrated under reduced pressure,precipitated by 4 volumes of 95% (v : v) ethanol,and then dried.The protein in the fraction was removed using the method of Sevag (Matthaeiet al.,1962).The crude polysaccharide was dissolved in distilled water and fractionated by anion exchange chromatography on a Q Sepharose Fast Flow column (30 cm × 3 cm),which was coupled to an AKTA FPLC system and eluted with a step-wise gradient of 0 – 4.0 mol L−1NaCl.The fractions were assayed for carbohydrate content using the phenol-sulfuric acid method (Duboiset al.,1956).The fractions containing abundant polysaccharides were pooled,dialyzed and further purified by gel filtration chromatography on a Sephacryl S-400/HR (100 cm × 3 cm) eluted with 0.2 mol L−1NH4HCO3.The major polysaccharide fractions were pooled,concentrated,desalted and freezedried.In the following text,MP refers to the sulfated polysaccharide fromE.linzacollected in May,and SP refers to that fromE.linzacollected in September.
2.3 Analytical Techniques
Total sugar content was determined using the phenolsulfuric acid method with rhamnose as the standard (Duboiset al.,1956),protein content determined following the method of Bradford (1976),sulfate ester content estimated according to Therho and Hartiala (1971),and uronic acid content determined by the carbazole-sulfuric acid method (Bitter and Muir,1962).Desulfation of the sulfated polysaccharide was achieved according to Liet al.(2011).The desulfated products were designated as dsMP and dsSP,respectively.
2.4 Analysis of Monosaccharide Composition
Monosaccharide composition was determined by reversed-phase HPLC after pre-column derivatization with a XDB-C18column (4.6 mm × 250 mm) and UV detector(Agilent 1200 Series) (Sunet al.,2009).Sugar was identified by comparison with reference sugars (D-glucose,L-rhamnose,D-xylose,L-arabinose,D-mannose,L-fucose,D-galactose,D-glucuronic acid,D-galacturonic acid andN-acetyl-β-D-glucosamine).The molar percent of monosaccharide was calculated based on the peak area of monosaccharide.
2.5 Determination of Purity and Molecular Weight
Purity and molecular weight were determined by high performance gel permeation chromatography (HPGPC)using a Shodex OHpak SB-804 HQ column and a refractive index detector (Agilent 1100 Series).The column was eluted with 0.2 mol L−1Na2SO4at a flow rate of 0.5 mL min−1,and 20 μL of 1% sample solutions in 0.2 molL−1Na2SO4was injected.The molecular weight was estimated according to a calibration curve constructed by a set of standard dextrans (Mw: 5.9,9.6,21.1,47.1,107,200,and 708 kDa) (Chenet al.,2011).
2.6 Methylation Analysis
Methylation analysis was performed according to Hakomori (1964).Two micrograms of polysaccharide was dissolved in 2 mL of DMSO,to which was added 100 –200 mg of anhydrous NaH.The mixture was stirred at room temperature for 1.5 h.Then,CH3I was added to the mixture,which was stirred for another 1.5 h.The reaction was terminated with water,and the residue was extracted with CHCl3,washed with distilled water and then driedviaevaporation.The completeness of methylation was confirmed by IR spectroscopy as the disappearance of OH bands.Methylated samples were hydrolyzed with 2 molL−1TFA at 105℃ for 6 h.The methylated products were converted to the corresponding alditols by NaBH4reduction and subsequent acetylation.The products were analyzed by gas chromatography-mass spectrometric (GCMS) on a HP6890II / 5973 instrument using a DB 225 fused silica capillary column (0.25 mm × 30 m) (Agilent Technologies Co.Ltd.,USA).The temperature gradient was:100 – 240℃ at 5℃ min−1followed by 240℃ for 15 min.Identification of partially methylated alditol acetates was carried out based on the retention time and mass fragmentation patterns.
2.7 Spectroscopy Analysis
The Fourier-transform infrared (FTIR) spectrum of polysaccharide was measured on a Nicolet Nexus 470 spectrometer.The polysaccharide was mixed with KBr powder,ground and pressed into a 1-mm pellet for FTIR measurement in the frequency range of 4000 – 5000 cm–1.1H nuclear magnetic resonance (NMR) and13C NMR spectral analyses were performed at 23℃ on a JEOL ECP 600 MHz spectrometer.Forty milligrams of polysaccharide was deuterium-exchanged by 3 successive freezedrying steps in 99% D2O and then dissolved in 0.5 mL of 99.98% D2O.Chemical shifts are expressed in × 10−6using acetone as the internal standard at 2.225 × 10−6for1H and 31.45 × 10−6for13C.
2.8 Anticoagulant Activity Assay
The APTT assay was carried out according to Mourânoet al.(1996).Briefly,90 µL of citrated normal human plasma and 10 µL of sample solution (0,10,20,50,100,and 200 µg mL–1) were incubated at 37℃ for 60 s.Then,100 µL of prewarmed APTT assay reagent was added to allow the reaction at 37℃ for 2 min.Thereafter,100 µL of 0.25 molL–1pre-warmed calcium chloride was added and APTT was recorded as the time of clot formation.The TT assay was performed as follows: 90 µL of citrated normal human plasma was mixed with 10 µL of polysaccharide solution (0,10,20,50,100,and 200 µg mL–1) and incubated at 37℃ for 60 s.Then,200 µL of pre-warmed TT assay reagent (37℃) was added to the mixture and the clotting time was recorded.For PT clotting assay,90 µL of citrated normal human plasma was mixed with 10 µL of polysaccharide solution (0,10,20,50,100,and 200 µg mL–1) and incubated at 37℃ for 1 min.Then,200 µL of pre-incubated PT assay reagent (37℃,10 min) was added to the mixture and the clotting time was recorded.The clotting assays were performed in triplicate and results are expressed as mean values ± standard deviations (SD).
3 Results and Discussion
3.1 Isolation and Chemical Compositions of Sulfated Polysaccharides from E.linza
Two polysaccharides,designated MP and SP,were obtained fromE.linzahot water extraction and chromatographic purification (Fig.1).The yields of MP and SP from dry alga were 15.1% and 12.3%,respectively.Both MP and SP gave a single peak in the HPGPC chromatogram,and their molecular weights were estimated to be 535 and 502 kDa,respectively (Fig.2).The sulfate ester content of MP was higher than that of SP,consistent with result obtained from uronic acid assay.MP and SP contained minor amounts of protein,possibly contaminated in polysaccharides.Reversed-phase HPLC analysis indicated that both MP and SP were composed of abundant rhamnose with small amounts of xylose and glucuronic acid,but SP also contained a small amount of galactose(Fig.3).As shown in Table 1,the polysaccharides MP and SP fromE.linzawere high rhamnose-containing sulfated polysaccharides,and their chemical compositions were different from those of the sulfated polysaccharides isolated fromE.compressa(Chattopadhyayet al.,2007).The variation in chemical compositions for MP and SP was likely related to different sampling time,as suggested by Marinho-Soriano and Bourret (2003).

Fig.1 Isolation and purification of sulfated polysaccharides from E.linza. A: Crude polysaccharides applied to a Q Sepharose Fast Flow column.The fraction eluted with 0.9 molL−1 NaCl was designated MP,and that eluted with 0.7 molL−1 NaCl designated SP;and B: MP and SP applied to a Sephacryl S-400/HR column and eluted with 0.2 molL−1 NH4 HCO3.

Fig.2 HPGPC chromatograms and the molecular weight standard curve of two sulfated polysaccharides(MP and SP) from E.linza.

Fig.3 Chromatograms of the monosaccharides of two sulfated polysaccharides (MP and SP) from E.linza determined by HPLC.

Table 1 Chemical compositions of two sulfated polysaccharides (MP and SP) isolated from E.linza
3.2 Methylation Analysis
In order to determine the linkage pattern of sugar residues,MP and SP were subjected to methylation analysis.Generally,a comparative analysis between sulfated polysaccharides and their desulfated products provided important information on linkages and sulfate substitution position of sugar residues.The identification and the proportions of methylated alditol acetates of the two polysaccharides are listed in Table 2.A large amount of 1,3,4,5-tetra-O-acetyl-2-O-methyl-rhamnitol and small amounts of 1,4,5-tri-O-acetyl-2,3-di-O-methyl-rhamnitol,1,2,4,5-tetra-O-acetyl-3-O-methyl-rhamnitol and 1,4,5-tri-O- acetyl-2,3-di-O-methyl-xylitol were detected in MP,suggesting that MP mainly consisted of (1→3,4)-linked rhamnose with small amounts of (1→4)-linked rhamnose and (1→2,4)-linked rhamnose and (1→4)-linked xylose residues.By comparison,large amounts of(1→4)-linked rhamnose residues and small amounts of(1→3,4)-linked rhamnose residues were detected in dsMP,suggesting that the sulfate substitution was at the C-3 of (1→4)-linked rhamnose residues.
Compared with SP,dsSP had substantially larger amounts of 1,4,5-tri-O-acetyl-2,3-di-O-methyl-rhamnitol and smaller amounts of 1,3,4,5-tetra-O-acetyl-2-O-methyl-rhamnitol.Results showed that the sulfate substitution was at the C-3 of (1→4)-linked rhamnose residues.In addition,1,3,5-tri-O-acetyl-2,
4-di-O-methyl-rhamnitol,indicated by the (1→3)-linked rhamnose residue,was found in SP.1,4,5-tri-O-acetyl-2,3-di-O-methyl-xylitol,1,3,5-tri-O-acetyl-2,4,6-tri-O-methyl-galactitol and 1,2,4,5-tetra-O-acetyl-3-O-methyl-rhamnitol were also detected,indicating the presence of (1→4)-linked xylose,(1→3)-linked galactose and (1→2,4)-linked rhamnose residues.The linkage patterns of SP were different from those of MP.It was likely that collection time influenced the structural characteristics of relevant polysaccharides(Marin ho-Soriano and Bourret,2003).In addition,the linkage patterns of MP and SP were found different from those of sulfated polysaccharides isolated fromE.compressa(Ray,2006).

Table 2 Results of methylation analyses of the two sulfated polysaccharides (MP and SP)isolated from E.linza and their desulfated polysaccharides
3.3 Spectroscopy Analysis
The IR spectrum of MP was similar to that of SP(Fig.4).The signal at 3445 cm–1was due to the stretch vibration of O-H in the hydrogen bond of the polymer.The signal at 2938 cm–1was assigned to the stretch vibration of C-H,and the signal at 1059 cm–1was due to the vibration of C-O.The peaks at 851 cm−1and 1256 cm−1were derived from the bending vibration of sulfate C-O-S in the axial position and stretching vibration of sulfate SO,respectively (Maoet al.,2009).The peak at 1650 cm−1was attributed to the asymmetric stretch vibration of COO−.The peak at 1450 cm−1was due to the symmetric stretch vibration of COO−and the stretch vibration of C-O within COOH.
1H NMR spectrum of MP showed five signals for anomeric protons corresponding to five different residues(Fig.5A) The proton signals at 5.12 × 10−6and 5.01 × 10−6were due to H-1 of (1→2,4)-linked a-rhamnopyranose and (1→4)-linked a-rhamnopyranose residues,respec-tively (Lahaye and Ray,1996; Liet al.,2011).The anomeric proton signals at 4.93 × 10−6,4.68 × 10−6,and 4.63 ×10−6were assigned to (1→4)-linked 3-sulfated-a- rhamnopyranose,(1→4)-linked b-glucuronic acid and (1→4)-linked b-xylopyranose residues,respectively (La- hayeet al.,1997; Lahaye,1998).The signal at 1.33 × 10−6was attributed to the proton of CH3group of the rham- nose residues.In the13C NMR spectrum of MP (Fig.5B),the signals at 98.6 × 10−6and 101.4 × 10−6were assigned to(1→4)-linked 3-sulfate-a-rhamnopyranose residues,which linked with O-4 of (1→4)-linked b-xylopyranose and(1→4)-linked b-glucuronic acid residues,respectively.The signals at 103.6 × 10−6and 104.3 × 10−6were ascribed to (1→4)-linked b-glucuronic acid residues.The signal at 104.7 × 10−6was due to (1→4)-linked b-xylopyranose residues.The signals at 18.0 × 10−6and 175.2 × 10−6were attributed to the C-6 of rhamnose and glucuronic acid,respectively (Lahaye and Ray,1996; Lahayeet al.,1997;Lahaye,1998; Cassolatoet al.,2008; Liet al.,2011).
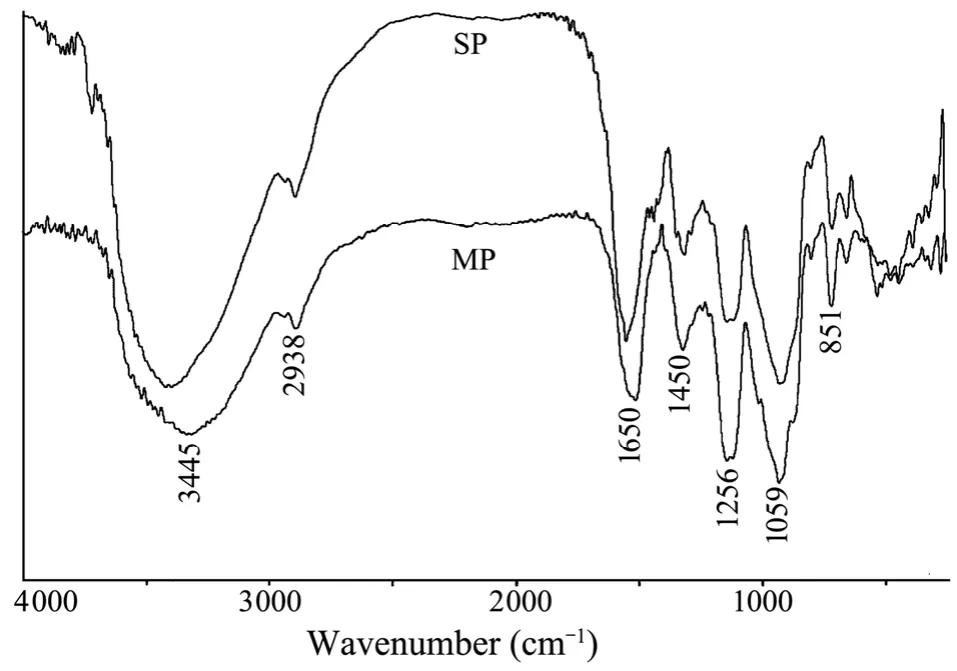
Fig.4 IR spectra of two sulfated polysaccharides (MP and SP) isolated from E.linza.
In the1H NMR spectrum of SP (Fig.5C),the anomeric proton signals at 5.15 × 10−6,5.06 × 10−6,4.94 × 10−6,4.69 ×10−6,and 4.64 × 10−6were assigned to (1→2,4)-linked a-rhamnopyranose,(1→3)-linked a-rhamnopyranose,(1→4)-linked 3-sulfated-a-rhamnopyranose,(1→4)-linked b-glucuronic acid,and (1→4)-linked b-xylopyranose residues,respectively (Cassolatoet al.,2008).The signal at 1.33 × 10−6was attributed to the proton of CH3group of the rhamnose residues (Liet al.,2011).In the13C NMR spectrum of SP (Fig.5D),the signals at 98.6 × 10−6and 101.4 × 10−6were due to (1→4)-linked 3-sulfate-a-rhamnopyranose residues which linked with O-4 of (1→4)-linked b-xylopyranose and (1→4)-linked b-glucuronic acid residues,respectively (Lahayeet al.,1997; Lahaye,1998).The signal at 104.3 × 10−6was ascribed to (1→4)-linked b-glucuronic acid residues.The signal at 104.7 ×10−6was due to (1→4)-linked b-xylopyranose residues(Cassolatoet al.,2008).The signals at 18.0 and 175.2 ×10−6were attributed to the C-6 of rhamnose and glucuronic acid,respectively (Liet al.,2011).
Presently,the complete assignments of NMR spectra of sulfated polysaccharides remain difficult,and further elucidation of the precise structures of the two sulfated polysaccharides (MP and SP) is mainly limited by their structural heterogeneity regarding linkage pattern and sulfate position.

Fig.5 NMR spectra of two sulfated polysaccharides (MP and SP) isolated from E.linza.Spectral analyses were performed at 23℃ on a JEOL ECP 600 MHz spectrometer using acetone as internal standard.Chemical shifts are referred to internal acetone at 2.225 × 10−6 for 1H and 31.45 ×10−6 for 13C.A: 1H NMR spectrum of MP; B: 13C NMR spectrum of MP; C: 1H NMR spectrum of SP; and D: 13C NMR spectrum of SP.
The structural characteristics of MP and SP were different from those of sulfated polysaccharides previouly isolated fromE.compress(Ray,2006).The latter were composed of (1→4)- and (1→2,4)-linked rhamnopyranosyl,(1→4)-linked xylopyranosyl and (1→4)- and terminally-linked glucuronosyl residues,with sulfate groups mainly located at C-3 of (1→4)-linked rhamnose units and C-2 of (1→4)-linked xylose units.In addition,the structural characteristics of MP and SP were different from sulfated polysaccharides previously isolated fromMonostromaceae(Liet al.,2011; Harada and Maeda,1998).For example,the sulfated polysaccharide fromM.latissimumconsisted of (1→3)-linked α-rhamnopyranose,(1→2)-linked α-rhamnopyranose and (1→2,3)-linked α-rhamnopyranose residues in a molar ratio of 4 : 1 : 1,and the sulfate groups were substituted at C-2 of the (1→3)-linked α-rhamnopyranose and C-3 of the (1→2)-linked α-rhamnopyranose residues (Liet al.,2011).Ha- rada and Maeda (1998) reported that a water-soluble sulfated polysaccharide fromM.nitidumwas composed of (1→3)-linked rhamnose and the sulfate group was located at C-2.The present study suggested that marine green algae are a potential source of sulfated polysaccharides with novel structures.
3.4 Anticoagulant Activity of Sulfated Polysaccharides
Anticoagulant activities of the two sulfated polysaccharides (MP and SP) isolated fromE.linzawere evaluated by APTT,TT and PT assays,then compared with that of heparin,a classical anticoagulant.As shown in Fig.6,APTT and TT were effectively prolonged by MP.The signals for clotting time of MP were more than 200 s for APTT and 120 s for TT,at 200 µg mL−1.The effects of SP on APTT and TT were weaker than those of MP,and the signals for clotting time of SP were 126.8 s for APTT and 77.4 s for TT,at 200 µg mL−1.Different from heparin,MP and SP had no prolongation effects on PT.The coagulation process includes the intrinsic and/or common pathway and extrinsic pathway.Prolongation of APTT indicates inhibition of the intrinsic and/or common pathway,prolongation of TT suggests inhibition of thrombin activity or fibrin polymerization,and the lack of PT activity demonstrates no inhibition of the extrinsic pathway of coagulation.Thus,we considered that the anticoagulant effects of MP and SP affected the intrinsic and/or common pathways of coagulation and thrombin activity or conversion of fibrinogen to fibrin,and did not inhibit the extrinsic pathway of coagulation.The extrinsic pathway activation led to fibrin clot formation in response to tissue injury,while the red thrombus or clot in the absence of tissue injury was the result of the intrinsic pathway.
MP showed higher anticoagulant activity than SP,suggesting that the biological activity was increased by the high sulfate ester.In addition,uronic acid content,monosaccharide composition and linkage patterns of sugar residues could influence the interaction of polysaccharide with coagulation inhibitors and their target proteases (Meloet al.,2004; Pereiraet al.,1999; Pominet al.,2005).Athukoralaet al.(2007) also reported that anticoagulant activity largely depends on the molecular size of polysaccharides.Together these results indicate that complex relationships exist between the structure and anticoagulant properties of sulfated polysaccharides.Further investigation on the relationship between the fine structure and anticoagulant activity is needed for the two sulfated polysaccharides isolated fromE.linza.In addition,the effects of MP and SP on APTT and TT were found substantially different from that of heparin.The APTT and TT activities were slowly increased by MP and SP,while APTT and TT activities were rapidly increased by heparin,with the clotting time of > 200 s at 20 µg mL−1for APTT and 120 s at 50 µg mL−1for TT.These suggest that MP and SP had lower bleeding risks than heparin at the same therapeutic doses,thus is a promising antithrombotic agent (Rodrigueset al.,2011).

Fig.6 Anticoagulant activities of two sulfated polysaccharides (MP and SP) from E.linza analyzed by APTT,TT and PT.Results are expressed as means values ± standard deviation (SD).All assays were performed in triplicate.
4 Conclusions
Two sulfated polysaccharides,designated MP and SP,were successfully isolated from the marine green algaE.linza.Both MP and SP are high rhamnose-containing sulfated polysaccharides but associated chemical characteristics are substantially different from each other.Compared with SP,MP has higher anticoagulant activity and specifically interferes with the coagulation system at several stages,thus is a potential source of anticoagulant.Further investigation is needed to demonstrate whether MP is a candidate for food supplements and/or ingredi-ents in pharmaceutical industry.In addition,complex relationships exist between the structure and anticoagulant property MP and SP.An in-depth anticoagulant activity study on these two sulfated polysaccharides with different structural characteristic will contribute to understanding of their anticoagulant activity mechanisms.The two sulfated polysaccharides fromE.linzacollected at different periods of the year are worth investigating further.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.41076086),the National Oceanographic Center of Qingdao,China and the Science and Technology Development Program of Shandong Province,China (No.2010GHY10509).
Athukorala,Y.,Lee,K.W.,Kim,S.K.,and Jeon,Y.J.,2007.Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea.Bioresource Technology,98(9): 1711-1716.
Bitter,T.and Muir,H.M.,1962.A modified uronic acid carbazole reaction.Analytical Biochemistry,4 (4): 330-334.
Bradford,M.M.,1976.A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Analytical Biochemistry,72(1-2): 248-254.
Cassolato,J.E.F.,Noseda,M.D.,Pujol,C.A.,Pellizzari,F.M.,Damonte,E.B.,and Duarte,M.E.R.,2008.Chemical structure and antiviral activity of the sulfated heterorhamnan isolated from the green seaweedGayralia oxysperma.Carbohydrate Research,343 (18): 3085-3095.
Chattopadhyay,K.,Mandal,P.,Lerouge,P.,Driouich,A.,Ghosal,P.,and Ray,B.,2007.Sulphated polysaccharides from Indian samples ofEnteromorpha compressa(Ulvales,Chlorophyta): Isolation and structural features.Food Chemistry,104 (3): 928-935.
Chen,Y.,Mao,W.J.,Tao,H.W.,Zhu,W.M.,Qi,X.H.,Chen,Y.L.,Li,H.Y.,Zhao,C.Q.,Yang,Y.P.,Hou,Y.J.,Wang,C.Y.,and Li.N.,2011.Structural characterization and antioxidant properties of an exopolysaccharide produced by the mangrove endophytic fungusAspergillussp.Y16.Bioresource Technology,102 (17): 8179-8184.
Ciancia,M.,Quintana,I.,Vizcargüénaga,M.I.,Kasulin,L.,de Dios,A.,Estevez,J.M.,and Cerezo,A.S.,2007.Polysaccharides from the green seaweedsCodium fragileandC.vermilarawith controversial effects on hemostasis.International Journal of Biological Macromolecules,41 (5): 641-649.
Dubois,M.,Gilles,K.A.,Hamilton,J.K.,Rebers,P.A.,and Smith,F.,1956.Colorimetric method for determination of sugars and related substances.Analytical Chemistry,28 (3):350-356.
Fonseca,R.J.C.,Oliveira,S.N.M.C.G.,Melo,F.R.,Pereira,M.G.,Benevides,N.M.B.,and Mourão,P.A.S.,2008.Slight differences in sulfation of algal galactans account for differences in their anticoagulant and venous antithrombotic activities.Thrombosis and Haemostasis,99 (3): 539-545.
Hakomori,S.,1964.A rapid permethylation of glycolipid,and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide.Journal of Biochemistry,55 (2): 205-208.
Harada,N.and Maeda,M.,1998.Chemical structure of antithrombin-active rhamnan sulfate fromMonostrom nitidum.Bioscience,Biotechnology,and Biochemistry,62 (9): 1647-1652.
Jiao,L.L.,Li,X.,Li,T.B.,Jiang,P.,Zhang,L.X.,Wu,M.J.,and Zhang,L.P.,2009.Characterization and antitumor activity of alkali-extracted polysaccharide fromEnteromorpha intestinalis.International Immunopharmacology,9 (3): 324-329.
Kim,J.K.,Cho,M.L.,Karnjanapratum,S.,Shin,I.S.,and You,S.G.,2011.In vitro and in vivoimmunomodulatory activity of sulfated polysaccharides fromEnteromorpha prolifera.International Journal of Biological Macromolecules,49 (5):1051-1058.
Lahaye,M.,1998.NMR spectroscopic characterisation of oligosaccharides from twoUlva rigidaulvan samples (Ulvales,Chlorophyta) degraded by a lyase.Carbohydrate Research,314 (1-2): 1-12.
Lahaye,M.,Brunel,M.,and Bonnin,E.,1997.Fine chemical structure analysis of oligosaccharides produced by an ulvanlyase degradation of the water-soluble cell-wall polysaccharides from Ulva sp.(Ulvales,Chlorophyta).Carbohydrate Research,304 (3-4): 325-333.
Lahaye,M.and Ray,B.,1996.Cell-wall polysaccharides from the marine green algaUlva "rigida"(Ulvales,Chlorophyta) –NMR analysis of ulvan oligosaccharides.Carbohydrate Research,283 (1): 161-173.
Li,H.Y.,Mao,W.J.,Zhang,X.L.,Qi,X.H.,Chen,Y.,Chen,Y.L.,Xu,J.,Zhao,C.Q.,Hou,Y.J.,Yang,Y.P.,Li,N.,and Wang,C.Y.,2011.Structural characterization of an anticoagulant – active sulfated polysaccharide isolated from green algaMonostroma latissimum.Carbohydrate Polymers,85 (2):394-400.
Li,H.Y.,Mao,W.J.,Hou,Y.J.,Gao,Y.,Qi,X.H.,Zhao,C.Q.,Chen,Y.,Chen,Y.L.,Li,N.,and Wang,C.Y.,2012.Preparation,structure and anticoagulant activity of a low molecular weight fraction produced by mild acid hydrolysis of sulfated rhamnan fromMonostroma latissimum.Bioresource Technology,114: 414-418.
Mao,W.J.,Li,H.Y.,Li,Y.,Zhang,H.J.,Qi,X.H.,Sun,H.H.,Chen,Y.,and Guo,S.D.,2009.Chemical characteristic and anticoagulant activity of the sulfated polysaccharide isolated fromMonostroma latissimum(Chlorophyta).International Journal of Biological Macromolecules,44 (1): 70-74.
Maeda,M.,Uehara,T.,Harada,N.,Sekiguchi,M.,and Hiraoka,A.,1991.Heparinoid-active sulfated polysaccharides fromMonostroma nitidumand their distribution in the Chlorophyta.Phytochemistry, 30 (11): 3611-3614.
Marinho-Soriano,E.and Bourret,E.,2003.Effects of season on the yield and quality of agar fromGracilariaspecies (Gracilariaceae,Rhodophyta).Bioresource Technology,90 (3): 329-333.
Matthaei,J.H.,Jone,O.W.,Martin,R.G.,and Nirenberg,M.W.,1962.Characteristics and composition of RNA coding units.Proceedings of the National Academy of Sciences, 48(4): 666-667.
Matsubara,K.,Matsuura,Y.,Bacic,A.,Liao,M.L.,Hori,K.,and Miyazawa,K.,2001.Anticoagulant properties of a sulfated galactan preparation from a marine green alga,Codium cylindricum.International Journal of Biological Macromolecules,28 (5): 395-399.
Melo,F.R.,Pereira,M.S.,Foguel,D.,and Mourão,P.A.S.,2004.Antithrombin-mediated anticoagulant activity of sulfated polysaccharides.The Journal of Biological Chemistry,279 (20): 20824-20835.
Mourâno,P.A.S.,Pereira,M.S.,Pavão,M.S.G.,Mulloy,B.,Tollefsen,D.M.,Mowinckel,M.C.,and Abildgaard,U.,1996.Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm.The Journal of Biological Chemistry,271 (39): 23973-23984.
Pereira,M.G.,Benevides,N.M.B.,Melo,M.R.S.,Valente,A.P.,Melo,F.R.,and Mourão,P.A.S.,2005.Structure and anticoagulant activity of a sulfated galactan from the red alga,Gelidum crinale.Is there a specific structural requirement for the anticoagulant action?Carbohydrate Research,340 (12):2015-2023.
Pereira,M.S.,Mulloy,B.,and Mourão,P.A.S.,1999.Structure and anticoagulant activity of sulfated fucans.Comparison between the regular,repetitive,and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae.The Journal of Biological Chemistry,274(12): 7656-7667.
Pomin,V.H.,Pereira,M.S.,Valente,A.,Tollefsen,D.M.,Pavão,M.S.G.,and Mourão,P.A.S.,2005.Selective cleavage and anticoagulant activity of a sulfated fucan: stereospecific removal of a 2-sulfate ester from the polysaccharide by mild acid hydrolysis,preparation of oligosaccharides,and heparin cofactor Ⅱ-dependent anticoagulant activity.Glyco biology,15 (4): 369-381.
Ray,B.,2006.Polysaccharides fromEnteromorpha compressa:Isolation,purification and structural features.Carbohydrate Polymers, 66 (3): 408-416.
Rodrigues,J.A.G.,Queiroz,I.N.L.,Quinderé,A.L.G.,Vairo,B.C.,Mourão,P.A.S.,and Benevides,N.M.B.,2011.An antithrombin-dependent sulfated polysaccharide isolated from the green algaCaulerpa cupressoideshasin vivoanti- and prothrombotic effects.Ciencia Rural, 41 (4): 634-639.
Sun,H.H.,Mao,W.J.,Chen,Y.,Guo,S.D.,Li,H.Y.,Qi,X.H.,Chen,Y.L.,and Xu,J.,2009.Isolation,chemical characteristics and antioxidant properties of the polysaccharides from marine fungusPenicilliumsp.F23-2.Carbohydrate Polymers,78 (1): 117-124.
Therho,T.T.,and Hartiala,K.,1971.Method for determination of the sulfate content of glycosamino glycans.Analytical Biochemistry,41 (2): 471-476.
Xie,L.,Chen,M.H.,Li,J.,Yang,X.M.,and Huang,Q.J.,2011.Antithrombotic effect of a polysaccharide fraction fromLaminaria japonicafrom the South China Sea.Phytotherapy Research,25 (9): 1362-1366.
 Journal of Ocean University of China2013年1期
Journal of Ocean University of China2013年1期
- Journal of Ocean University of China的其它文章
- Isolation and Expression Analysis of FTZ-F1 Encoding Gene of Black Rock Fish (Sebastes schlegelii)
- Optimization of the Purification Methods for Recovery of Recombinant Growth Hormone from Paralichthys olivaceus
- Seasonal Changes in Food Uptake by the Sea Cucumber Apostichopus japonicus in a Farm Pond: Evidence from C and N Stable Isotopes
- Purification and Characterization of a New Thermostable κ-Carrageenase from the Marine Bacterium Pseudoalteromonas sp. QY203
- Growth,Metabolism and Physiological Response of the Sea Cucumber,Apostichopus japonicus Selenka During Periods of Inactivity
- What Depth Should Deep-Sea Water be Pumped up from in the South China Sea for Medicinal Research?
