Cloning and Expression Analysis of vasa During the Reproductive Cycle of Korean Rockfish,Sebastes schlegeli
MU Weijie,WEN Haishen,,HE Feng,LI Jifang,LIU Miao,MA Ruiqin,ZHANG Yuanqing,HU Jian,and QI Baoxia
1) Fisheries College,Ocean University of China,Qingdao 266003, P.R.China
2) College of Animal Science and Technology,Inner Mongolia Nationality University,Tongliao 028000,P.R.China
1 Introduction
Germ cells are highly specialized to undergo meiosis and play an important role in maintaining species-specific genomic information through generations (Liet al.,2010).They are derived from primordial germ cells (PGCs),which are segregated from the somatic cell lineage during early embryonic stages (Saffman and Lasko,1999) and migrate to the developing gonad to form germline stem cells (Lin,1997).The molecular characteristics of germ cell determinants have been studied inDrosophila(Rongoet al.,1995),and several molecular components were identified,e.g.,oskar,vasa,nanosandtudor.Of these,the best-characterizedvasagene first identified inDrosophilais responsible for PGCs formation and oocyte differentiation (Schüpbach and Wieschaus,1986; Hayet al.,1988).Thevasagene encodes a putative ATP-dependent RNA helicase of the DEAD-box (asparagines (D),glutamine (E),alamine (A),and asparagines (D)) family(Hayet al.,1988; Lasko and Ashburner,1988; Lianget al.,1994).The DEAD-box proteins,presented in a wide range of organisms and shared in 8 conserved amino acid motifs,are commonly involved in RNA metabolism such as RNA splicing,editing,rRNA processing,translation initiation,nuclear mRNA export,and RNA degradation (Lükinget al.,1998; Rocak and Linder,2004).
Sequence analysis ofvasain diverse animal species indicates that thevasagene sequence is highly conserved across animal phyla,including zebrafish (Olsenet al.,1997),planarian (Shibataet al.,1999),trout (Yoshizakiet al.,2000),tilapia (Kobayashiet al.,2000),medaka(Shinomiyaet al.,2000),gibel carp (Xuet al.,2005),hydra (Mochizukiet al.,2000) and mouse (Fujiwaraet al.,1994).As its mRNA is only expressed in the PGCs or germ cells in fish and several other species such as honeybee and sea urchin (Dearden,2006; Voroninaet al.,2008),thevasagene has been used as a molecular marker of PGCs (Braatet al,1999).
Research ofvasamRNA expression was first reported in zebrafish,Danio rerio(Yoonet al,1997; Olsenet al.,1997),and then in tilapia,Oreochromis niloticus(Kobayashiet al.,2000),and gilthead bream,Sparus aurata(Cardinaliet al.,2004).It has been shown that the expression ofvasaoccurs at each stage of oogenesis in fe-male tilapia,and at early stages rather than late stages of testis development in male tilapia (Kobayashiet al.,2000).Thevasais only expressed at the primary spermatocytes stage in Pacific bluefin tuna (Nagasawaet al.,2009),and its transcript levels decline from spermatogenesis to spermiation stages in catfish (Raghuveer and Senthilkumaran,2010).However,Blázquezet al.(2010)have recently found that thevasaexpression level increases during germ cell proliferation in European sea bream.In addition,Raghuveer and Senthilkumaran (2010)have indicated thatvasamRNA can be up-regulated by human chorionic gonadotropin in the recrudescing ovary(in vivo) and testicular (in vitro).
The Korean rockfish,Sebastes schlegeli,is a viviparous teleost mainly inhabiting in coastwaters of Korea,Japan,and China.It is a commercially important marine fish species with great aquacultural potential,as well as a typical ovoviviparous species for studying the evolutionary processes of reproductive control mechanism from oviparity to viviparity.In this study,thevasacDNA was cloned from Korean rockfish (Sebastes schlegeli) to examine its distribution in tissues and temporal expression patterns during the gonadal reproductive cycle in male and female fish.Results will provide information on the reproductive physiology and endocrinology of viviparous fish and other marine fish,thus are valuable for optimization of the artificial propagation of ovoviviparous fish.In addition,the study of seasonal changes invasaexpression levels during the reproductive cycle will contribute to understanding of the role of vasa in spermatogenesis and oogenesis.
2 Materials and Methods
2.1 Experimental Fish
During November 2008 – September 2009,20 mature male and female Korean rockfish were obtained every two months from the Penglai coastal area of Shandong Province,China.The gonads were excised and the sexual maturity was determined based on the presence of mature ova and sperm.Thereafter,all fish were anesthetized in tricaine methane sulfonate (MS-222,Sigma,St.Louis,MO) (Liuet al.,2011).Tissue samples of the brain,heart,caeca,liver,gill,head kidney,bowel,stomach,spleen,gonad and pituitary samples were collected at various growth stages,immediately frozen in liquid nitrogen and stored at −80℃ prior to total RNA extraction.Parts of the gonads were stored in Bouin’s solution for hematoxylin and eosin (HE) staining so as to identify the developmental stages of gonad.
2.2 Histological Analysis
Fixed gonad segments were dehydrated in a 30% – 100%ethanol series,cut into 5 – 8 μm-thick paraffin sections by microtome (LEICA-RM2016),stained with HE,and photographed by light microscopy (Nikon-E200,Japan).
2.3 Total RNA Extraction and Reverse Transcription-PCR (RT-PCR)
Total RNA was extracted from Korean rockfish tissues using RNAiso reagent (Takara,Japan) following the manufacturers’ instructions.The concentration and purity of extracted RNA were determined by UV spectroscopy at 260 and 280 nm.Reverse transcription was carried out with RNA extracts (1 μg) and oligo d(T)18primers in 10 μL reactions at 70℃ for 5 min using MMLV reverse transcriptase following the manufacturer’s protocol (Promega,USA).
2.4 Isolation and PCR Amplification of Vasa cDNA Fragments
For amplification of thevasacDNA fragments,two pairs of degenerate primers (VasaF1/VasaR1,Table 1)were designed from highly conserved amino acid sequences ofvasain fish species using a web-based primer design program,Code Hop online (Roseet al.,1998; Chenet al.,2009).The PCR reaction (50 μL) contained 2 μL of cDNA from ovarian and testis tissues following the manufactures’ instructions (Takara,Japan).The touchdown PCR cycling conditions were as follows: pre-denaturation at 94℃ 5 min; 10 cycles of denaturation at 94℃ for 30 s,annealing at a decreasing temperature from 70℃ to 60℃for 30 s (decreasing 1℃ each cycle),and extension at 72℃ for 35 s,followed by additional 30 cycles of 94℃for 30 s,62℃ for 30 s and 72℃ for 35 s; and a final extension at 72℃ for 10 min.The PCR products were separated by a 1.5% agarose gel,purified using a TIAN gel midi Purification Kit (QIAGEN,China),cloned into pGEM-T vector (QIAGEN,China),and propagated inE.coliDH5α (QIAGEN,China).The clones were sequenced using an ABI3730XL sequencer (ABI,USA).

Table1 Primers and probes used for cloning,RT-PCR,and qPCR of vasa gene fragments
2.5 5′and 3′ RACE-PCR
The 5′ and 3′ RACE reactions were performed using a SMARTTMRACE cDNA Amplification Kit (Clontech,USA) with primers listed in Table 1.The PCR reactions(10 µL) for first-strand cDNA synthesis and reverse transcription required 1 μg of total RNA and 1 μmol L−1each primer.Subsequently,the full-length cDNA was clonedviaa nested PCR using the following thermal cycling conditions: pre-denaturation at 94℃ for 5 min; 40 cycles of denaturation at 94℃ for 30 s,annealing at 69℃ for 30 s(Vasa-5-1,Vasa-3-1,Vasa-3-2) or 68℃ for 30 s (Vasa-5-2),and extension at 72℃ for 1 min; and a final extension at 72℃ for 10 min.The PCR products were run on a 1.5% agarose gel.DNA bands of the target length were excised,purified and cloned into vectors.Selected clones were sequenced on an ABI3730XL sequencer (ABI,USA).
2.6 Phylogenetic Analysis
Multiple protein sequences ofvasacDNA were obtained from Genbank (Altschulet al.,1990) and aligned with ClustalX 1.81 (Thompsonet al.,1997).Phylogenetic analyses of full-length amino acid sequences were conducted using MEGA 4.0 (Tamuraet al.,2007).Amino acid regions that remained unalignable were deleted using default parameters on the Gblocks serve (http://molevol.-ibmb.csic.es/Gblocks.html).Phylogenetic trees were constructed using the maximum likelihood method (1000 bootstrapping replicates).The trees were rooted with amino acid sequences of mammals as outgroup.
2.7 Tissue Distribution Pattern of Vasa Transcripts
The expression level ofvasatranscripts was examined in various tissuesviaRT-PCR assays.Total RNA was extracted from ovary,liver,kidney,head kidney,brain,heart,spleen,caeca,stomach,fat,gills,intestinal,pituitary of a female fish at late-vitellogenic stage and testis at spermiated stage using Trizol reagent.To avoid genomic contamination,extracted RNA was treated with RNasefree DNase I before reverse transcription.Total RNA was reversely transcribed using M-MLV RT (Promega,USA)following the manufactures’ instructions,and the primers used for examination of tissue expression pattern are listed in Table 1.The tissue expression was normalized using 18S rRNA as a reference gene with the sense primer 5′-CCTGAGAAACGGCTACCATC-3′ and the antisense primer 5′-CCAATTACAGGGCCTCGAAAG-3′.PCR cycling conditions were as follows: 95℃ for 5 min; 40 cycles of 95℃ for 5 s,58℃ for 30 s,and 72℃ for 30 s; and 72℃ for 10 min.The PCR products were checked by 1.5% agarose gel electrophoresis.The gel was pre-stained with ethidium bromide and visualized on a Gel system(Tanon,China).
2.8 Quantitative Real-Time PCR (qPCR)
The relative expression ofvasamRNA was determinedviaqPCR using total RNA extracted from gonads of Korean rockfish.The PCR assays were performed using Multicolor Real-Time PCR Detection System (Roche Lightcycler480,German) and iQ™ SYBR Green Supermix (Takara,Japan) according to the manufacturers' protocol.The sequences of primers targetingvasa(V-e-F and V-e-R) are listed in Table 1.The mRNA was treated with DNase I (Takara,Japan) and Ribonuclease Inhibitor (Takara,Japan) to remove trace genomic DNA and prevent potential genomic DNA amplification.ThevasaqPCR conditions were as follows: 1 cycle of denaturation at 95℃ for 5 min,40 cycles of denaturation at 95℃ for 30 s,annealing at 58℃ for 30 s,and extension at 72℃ for 30 s.As an internal control,18S rRNA was amplified under the same conditions using Korean rockfish-specific primers (Table 1,see 2.7),and no significant changes were observed in the 18S rRNA expression level during gonadal development.The cycle threshold (Ct) values were obtained from the exponential phase of qPCR amplification,and results were analyzed using the comparative Ct method.Thevasaexpression level was normalized against 18S rRNA expression level to generate a ΔCt value (ΔCt = target gene Ct − reference gene Ct),and the relative expression ofvasa/18Swas analyzed according to the expression 2−ΔΔCt(Livak and Schmittgen,2001).The initial stage of samples was used as calibrator for comparative relative qPCR.
3 Results
3.1 Histological Characteristics of Gonads
According to previous research regarding gonadal histology ofSebastiscus marmoratusandSebastes schlegeli(Lin and You,2000; Linet al.,2000; Yanget al.,2010;Shiet al.,2011),the testes in male Korean rockfish were classified into four categories (Fig.1).In the primary spermatogonia stage,the testes mainly had primary spermatocyte (Fig.1A); in the immature sperm stage,the primary spermatogonia divided and developed into secondary spermatogonia (Fig.1B); in the mature testes stage,germ cells in the spermatogenic cysts developed into sperms (Fig.1C); and in the post-spermiation stage,the sperms degenerated and died (Fig.1D).As for the ovary development,five stages were identified in female Korean rockfish,including the immature stage (perinucleolus stage,oocyte in the 2ndphase with clear nucleus)(Fig.1E); vitellogenic stage (primary yolk stage,oocyte in the 3rdphase with an increasing oocyte volume and decreasing nucleus volume) (Fig.1F); ovulation stage (secondary yolk stage,oocyte in the 4thphase with visible,pink- stained yolk granules) (Fig.1G) and zona radiate)(Fig.1H); and ovulation stage (tertiary yolk stage,oocyte in the 5thphase full of mature yolk granules) (Fig.1I); and gestational ovary stage (embryo formed) (Fig.1J).
3.2 Isolation and Characterization of Korean Rockfish Vasa cDNAs

Fig.1 The histology and morphology of gonad in Sebastes schlegeli.A: Primary spermatogonia testis,bar = 24 µm; B: Testis filled with immature sperm,bar = 24 µm; C: Mature testis,bar = 24 µm; D: Post-spermiation testis,bar = 24 μm; E: Immature stage,perinucleolus stage oocyte at the 2nd phase,bar = 100 μm; F: Vitellogenic stage,early-oocyte at the 3rd phase,bar = 70 μm; G: Ovulation stage,post-oocyte at the 4th phase,bar = 100 μm; H: The zona radiate,bar = 100 μm; I:Ovulation stage,post-oocyte at the 5th phase,bar = 100 μm; J: Gestational ovary stage,oocyte at the 6th phase,showing the embryo,bar = 100 μm; Sg I: primary spermatogonium; ScI: primary spermatocytes; ScII: second spermatocytes; St:spermatid; Sz: spermatozoa; MC: Mesangial cell; SC: Sertoli cell; and Bs: brown substance.
The full-lengthvasacDNA is 2443 bp.It consists a 1950 bp open reading frame (ORF) that encodes a 650-amino-acid protein.The predicted protein contains 8 conserved signature domains of the DEAD-box protein family,including the ATP-binding motifs (Figs.2 and 3)and the N-terminal regions with arginine-glycine (RG)and arginine-glycine-glycine (RGG) repeats.The nucleotide sequence has been deposited in Genbank (JN634874).A consensus tree was constructed with Mega 4 using the maximum-likelihood method (Hasegawa and Kishino,1994).Phylogenetic analysis of the Vasa proteins from different fish species (Fig.4) showed the existence of 7 main clades,including mammals,birds,amphibians,teleosts,insects,mollusks and ascidians.Sequence comparison showed that the amino acid sequence of Korean rockfish Vasa had the highest homology with that ofOsphronemus goramy(84%).

Fig.2 Nucleotide sequences and deduced amino acid sequences of Korean rockfish (Sebastes schlegeli) vasa cDNA.DEAD box shown in the gray open box; 8 conserved regions of the DEAD-box protein family shown in closed boxes; arginine-glycine (RG) repeats and arginine-glycine-glycine (RGG) repeats in the N-terminal region underlined and double underlined,respectively; acidic amino acid residues (aspartic acid,D; and glutamic acid,E) and tryptophan (W) in the N-terminal and C-terminal regions shown by triangle; asterisk (*) indicates the stop codon.
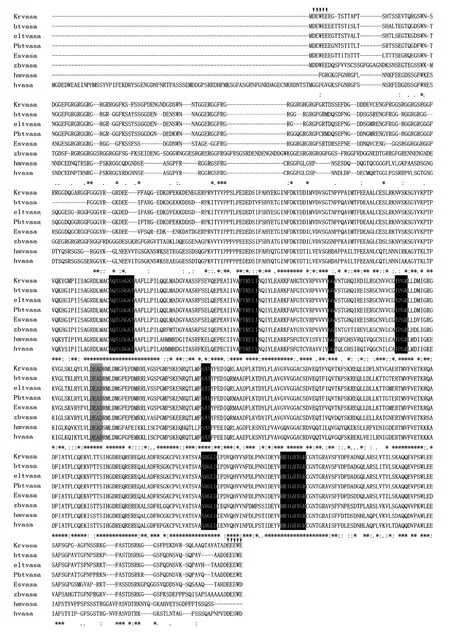
Fig.3 Amino acid sequence alignment of Vasa in Korean rockfish and other teleosts.Asterisks (*) and dots (:) marked for completely conserved and highly conserved amino acids,respectively; GenBank protein ID numbers for the sequences alignment are: Sebastes schlegeli vasa (Krvasa),JN634874; Auxis rochei vasa (btvasa),ADD81193; Euthynnus affinis vasa (eltvasa),ADD81191; Thunnus orientalis vasa (Pbtvasa),ABY77970; Dicentrarchus labrax vasa (Esvasa),ADK79106;Danio rerio vasa (zbvasa),AAL89410; Mus musculus vasa (hmvasa),BAA03584; Homo sapiens vasa (hvasa),AAF72705;acidic amino acid residues (mentioned above) in the N-terminal and C-terminal regions shown by triangle; functional(conserved) DEAD region in gray boxes; 8 conserved boxes of Vasa in black boxes.
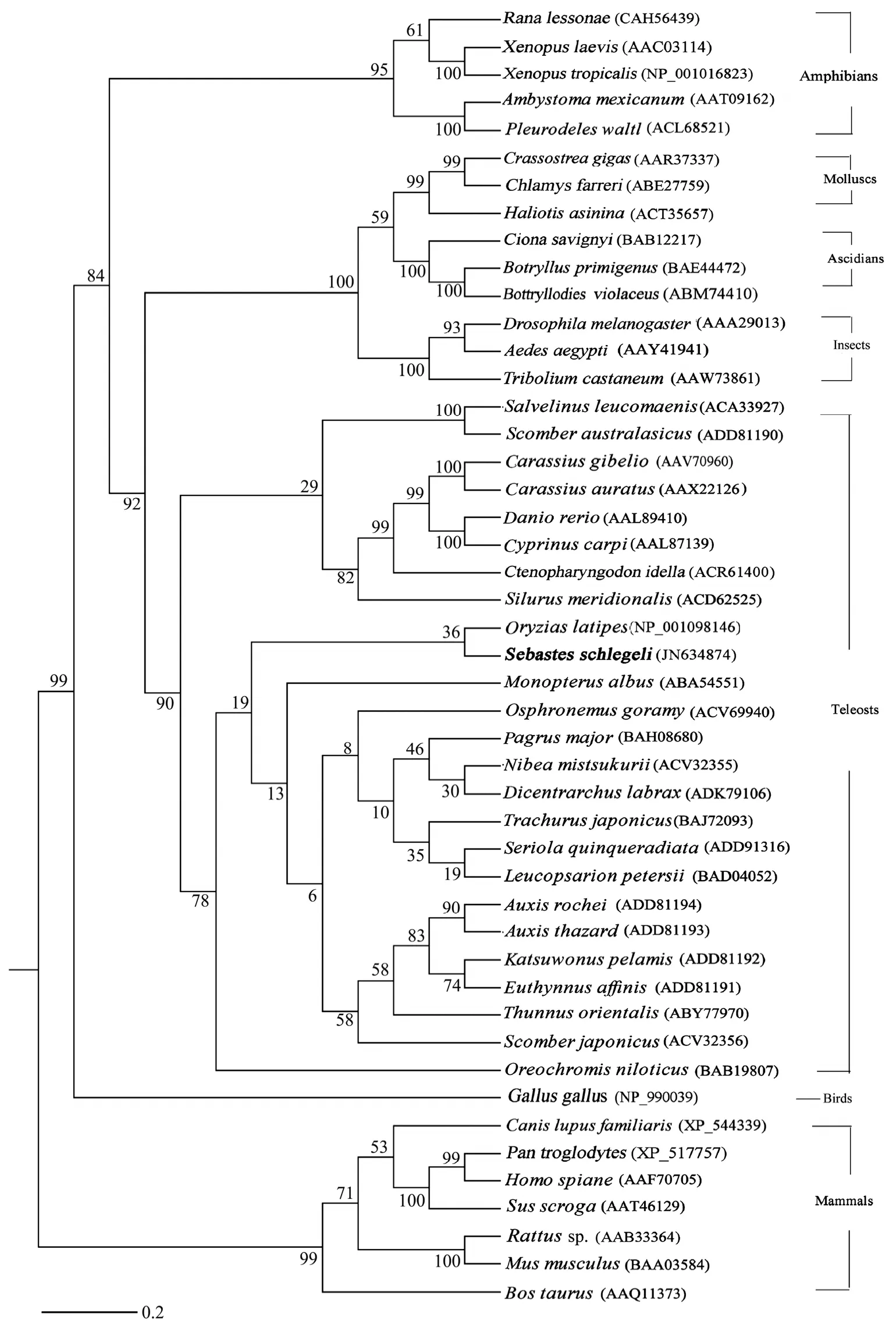
Fig.4 Phylogenetic tree based on Vasa amino acid sequences of teleost and other animal species.Accession numbers of amino acid sequences from GenBank databases shown in brackets; tree constructed using the maximum-likelihood method;bootstrap values (1000 replicates) shown on the branches; sequences containing gaps and dubious alignments are eliminated; mammal amino acid sequences used as the out-group; branch lengths recalculated for the resulting consensus tree.
3.3 Tissue Distribution of Vasa Gene
Primers of Vasa-e-F and Vasa-e-R were used to determine specific tissue expression ofvasa,and primers 18SF and 18SR were applied to normalize the PCR products ofvasamRNA and produce semi-quantitative results.RTPCR assays revealed that thevasagene was only expressed in the testis and ovary,but undetectable in other tissues (Fig.5).
3.4 Expression Pattern of vasa at Gonad Developmental Stages during the Reproductive Cycle
Expression ofvasamRNA was observed in gonads of both male and female Korean rockfish throughout the reproductive cycle (Fig.6).In male fish,thevasaexpression level increased from 0.73 ± 0.14 in the primary spermatogonia stage to 1.2 ± 0.38 in the immature sperm stage,and then substantially decreased to 0.11 ± 0.1 during the mature testis stage,with the lowest value (0.02 ± 0.01)observed in the post-spermiation stage (P< 0.05).Differentvasaexpression levels were observed in gonads of female fish,that is,thevasaexpression level continuously decreased from the immature stage to vitellegenic stage,and then stabilized at a low level during the ovulation stage,with the lowest level observed in the gestational stage (P< 0.05).

Fig.5 Expression patterns of vasa in different tissues of adult Korean rockfish detected by RT-PCR assay.18S rRNA expression as internal control; A,marker; B,heart; C,liver; D,spleen; E,head kidney; F,ceaca; G,testis; H,ovary; I,stomach; J,fat; K,kidney; L,gill; M,brain; N,pituitary; and O,intestine.

Fig.6 Relative vasa mRNA expression levels in gonads of male (A) and female (B) Korean rockfish during the annual reproduction cycle.Samples analyzed by qPCR; data presented as the mean normalized gene expression (MNE)levels ± standard error of the mean of triplicate samples;values normalized against the 18S rRNA expression level;different letters above the error bar showing statistical differences (P < 0.05) among samples collected in different periods.
4 Discussion
In the present study,the full-lengthvasacDNA was isolated from ovarian tissue of Korean rockfish using RT-PCR and RACE amplification strategies.The Vasa protein is known to be a member of the DEAD protein family,which possesses ATP-dependent RNA helicase activity (Hayet al.,1988; Lianget al.,1994).The deducedvasaamino acid sequence contained 8 consensus sequences including the ATP-A (AXXXXGKT) and ATP-B motifs (DEAD) of the DEAD protein family(Linderet al.,1989; Pause and Sonenberg,1992),whereas a glycine-rich region in the N-terminal region of Korean rockfish Vasa was shown to contain 7 arginine-glycine repeats and 9 arginine-glycineglycine repeats.The glycine-rich region is considered to be a characteristic of single-stranded nucleic acid-binding proteins such as RNA helicase (Hayet al.,1988; Kiledjian and Dreyfuss,1992; Lianget al.,1994).In addition,a variety of amino acids such as glutamate (E) and aspartate (D) are acidic residues in the C-terminal region,as indicated by Fujiwaraet al.(1994) and Castrillonet al.(2000).Together these demonstrate that: (1) Thevasagene encodes a DEAD protein with ATP-dependent RNA helicase activity; (2) The Vasa protein is highly conserved during evolution; and (3) Conserved motifs potentially play an important role in sustaining the structure and function of the Vasa protein.
In this study,we found that the Vasa protein of Korean rockfish shares high homology with those of other fish species (76% – 84%),as well as the Vasa homologs ofOsphronemus goramy(84%).These indicate that the cDNA clone ofvasain Korean rockfish is a member of the Vasa family.The phylogenetic tree based on protein distances contains 7 main branches,including mammals,birds,amphibians,teleosts,insects,mollusks and ascidians (Fig.4).This suggests that the teleost Vasa protein is a single group from all other species.In addition,RT-PCR assays demonstrated thatvasawas specifically expressed in the testis and ovary of Korean rockfish(Fig.5).Similarly,a number of reports have shown thatvasa-related genes are specifically expressed in germline cells in different animal species,such as Drosophila (Hayet al.,1988; Lasko and Ashburner,1988),Kuruma shrimp(Sellarset al.,2007),zebrafish (Olsenet al.,1997; Yoonet al.,1997; Krøvel and Olsen,2004) and mouse (Fujiwaraet al.,1994).These findings illustrate the prominent role ofvasain the germline development (Braatet al.,1999).However,it has been shown thatvasamRNA is weakly expressed in extra-gonadal tissues such as heart and brain in rainbow trout (Yoshizakiet al.,2000).Ikenishi and Tanaka (2000) suggested thatvasamight be implicated in the translational regulation of mRNA,which is important for specification and differentiation of specific cell types in non-gonadal tissues.
In this study,qPCR assays demonstrated different expression patterns ofvasamRNA in gonads of adult male and female Korean rockfish during the reproductive cycle.ThevasamRNA levels were found higher during the spermatogonia and immature sperm stages compared with the mature testes and post-spermiation stages in male fish.A similar decreasing expression pattern has been reported in tilapia (Kobayashiet al.,2000) and rainbow trout (Yoshizakiet al.,2000).A study of tilapia byin situhybridization showed that thevasasignal was strong during spermatogonia and weakened with testis development(Kobayashiet al.,2000).In medaka,the hybridization signals have been found stronger in spermatogonia and spermatocytes at early stages,but substantially weaker in spermatocytes at later stages with no signals in spermatids (Shinomiyaet al.,2000).These demonstrate that thevasagene potentially plays an important role in the division and development of early germ cells,with a relatively minor role played in the development and maturation of late germ cells.
In female catfish,thevasamRNA level has been found highest in the early ovary stage,and drastically declined with gonadal development (Raghuveer and Senthilkumaran,2010).Similarly,our study showed that the level in female Korean rockfish decreased from the perinucleolus oocyte stage.A similar phenomenon has been observed in tilapia and gibel carp byin situhybridization,and one explanation is that the amount of maternally inheritedvasatranscript is diluted during the individual growth(Kobayashiet al.,2000; Xuet al.,2005).These findings indicate thatvasaplays a key role in gametogensis.
In summary,the present study obtained the full-length sequence ofvasagene in Korean rockfish (Sebastes schlegeli).Spatial expression analysis substantiates thatvasais only expressed in gonads,with transcripts in nongonadal tissues under the minimum detection level,including stomach,intestine,gill,and spleen.In addition,we compared thevasamRNA expression at each stage of gonadal development,and thevasatranscript was detected at high levels at the spermatogonia and immature sperm stages in male fish and the perinucleolus stage in female fish.These suggest thatvasasynthesis is predominant during the early reproductive stages in Korean rockfish.Future study regarding examination of Vasa in gonads at the protein level by immunohistochemistry will explore the function of Vasa in the whole reproductive cycle of gonadal development.
Acknowledgements
This research was supported by The National Natural Science Foundation of China (No.41176122) and the Key Program of Natural Science Foundation of Shandong,China (No.Z2008D03).
Altschul,S.F.,Gish,W.,Miller,W.,Myers,E.W.,and Lipman,D.,1990.Basic local alignment search tool.Journal of Molecular Biology,215: 403-410.
Blázquez,M.,González,A.,Mylonas,C.C.,and Piferrer,F.,2010.Cloning and sequence analysis of avasahomolog in the European sea bass (Dicentrarchus labrax): Tissue distribution and mRNA expression levels during early development and sex differentiation.General and Comparative Endocrinology,170 (2): 322-333.
Braat,A.K.,Speksnijder,J.E.,and Zivkovic,D.,1999.Germ line development in fishes.The International Journal of Developmental Biology,43 (7): 745-760.
Cardinali,M.,Gioacchini,G.,Candiani,S.,Pestarino,M.,Yoshizaki,G.,and Carnevali,O.,2004.Hormonal regulation ofvasa-like messenger RNA expression in the ovary of the marine teleostSparus aurata.Biologyof Reproduction,70 (3):737-743.
Castrillon,D.H.,Quade,B.J,Wang,T.Y.,Quigley,C.,and Crum,C.P.,2000.The humanVASAgene is specifically expressed in the germ cell lineage.Proceedings of the National Academy of Sciences of the United States of America,97 (17):9585-9590.
Chen,C.F.,Wen,H.S.,He,F.,and Dong,S.L.,2009.Programming design of degenerate primers and cloning halfsmooth tongue soleCynoglossus semilaevisCYP17gene.Periodical of Ocean University of China,39 (6): 1213-1218 (in Chinese with English abstract).
Dearden,P.K.,2006.Germ cell development in the honeybee(Apis mellifera):VasaandNanosexpression.BMC Developmental Biology,6 (6): 1-14.
Fujiwara,Y.,Komiya,T.,Kawabata,H.,Sato,M.,Fujimoto,H.,Furusawa,M.,and Noce,T.,1994.Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophilavasaand its specific expression in germ cell lineage.Proceedings of the National Academy of Sciences of the United States of America,91 (25): 12258-12262.
Hasegawa,M.,and Kishino,H.,1994.Accuracies of the simple methods for estimating the bootstrap probability of a maximum-likelihood tree.Molecular Biology and Evolution,11(1): 142-145.
Hay,B.,Jan,L.Y.,and Jan,Y.N.,1988.A protein component of Drosophila polar granules is encoded byvasaand has extensive sequence similarity to ATP-dependent helicases.Cell,55 (4): 577-587.
Ikenishi,K.,and Tanaka,T.S.,2000.Spatio-temporal expression ofXenopus vasahomolog,XVLG1,in oocytes and embryos: the presence ofXVLG1RNA in somatic cells as well as germline cells.DevelopmentGrowth and Differentiation,42 (2): 95-103.
Krøvel,A.V.,and Olsen,L.C.,2004.Sexual dimorphic expression pattern of a splice variant of zebrafishvasaduring gonadal development.Development Biology,271 (1): 190-197.
Kiledjian,M.,and Dreyfuss,G.,1992.Primary structure and binding activity of the hnRNP U protein: binding RNA through RGG box.The EMBO Journal,11 (7): 2655-2664.
Kobayashi,T.,Kajiura-Kobayashi,H.,and Nagahama,Y.,2000.Differential expression ofvasahomologue gene in the germ cells during oogenesis and spermatogenesis in a teleost fish,tilapia,Oreochromis niloticus.Mechanismsof Development,99 (1-2): 139-142.
Lasko,P.F.,and Ashburner,M.,1988.The product of theDrosophilagenevasais very similar to eukaryotic initiation factor-4A.Nature,335: 611-617.
Li,C.J.,Liu,L.,Chen,X.H.,Zhang,T.,Gan,F.,and Cheng,B.L.,2010.Identification of avasahomologue gene in grass carp and its expression pattern in tissues and during embryogenesis.Comparative BiochemistryandPhysiology B,157 (2):159-166.
Liang,L.,Diehl-Jones,W.,and Lasko,P.,1994.Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities.Development,120:1201-1211.
Lin,D.J.,and You,Y.L.,2000.The ovarian cyclical development of ovoviviparous teleost,Sebastiscus marmoratus.Zoological Research,21 (4): 269-74 (in Chinese with English abstract).
Lin,D.J.,You,Y.L.,and Chen,L.Y.,2000.The testicular cycle development of ovoviviparous of teleost,Sebastiscus marmoratus.Zoological Research,21 (5): 337-342 (in Chinese with English abstract).
Linder P,Lasko P.F.,Ashburner,M.,Leroy,P.,Nielsen,P.J.,Nishi,K.,Schnier,J.,Slonimski,P.P.,1989,Birth of the D-EA-D box.Nature,337 (6203): 121-122.
Lin,H.,1997.The tao of stem cells in the germline.Annual Review of Genetics,31 (1): 455-491.
Liu,M.,Wen,H.S.,He,F.,Li,J.F.,Shi,D.,Hu,J.,Zhang,Y.Q.,Ma,R.Q.,Mu,W.J.,and Qi,B.X.,2011.CompExpression patterns of cytochrome P450 aromatase genes during ovary development and their responses to temperature stress in female yellow catfish (Pelteobagrus fulvidraco).Journalof Ocean University of China, 10 (4): 409-416.
Livak,K.J.and Schmittgen,T.D.,2001.Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CTmethod.Methods,25 (4): 402-408.
Lüking,A.,Stahl,U.,and Schmidt,U.,1998.The protein family of RNA helicases.Critical Reviewsin BiochemistryandMolecular Biology,33 (4): 259-296.
Mochizuki,K.,Nishimiya-Fujisawa,C.,and Fujisawa,T.,2000.Universal occurrence of thevasa-related genes among metazoans and their germline expression inHydra.Development Genes and Evolution,211 (6): 299-308.
Nagasawa,K.,Takeuchi,Y.,Miwa,M.,Higuchi,K.,Morita,T.,Mitsuboshi,T.,Miyaki,K.,Kadomura,K.,and Yoshizaki,G.,2009.cDNA cloning and expression analysis of avasa-like gene in Pacific bluefin tunaThunnus orientalis.Fisheries Science,75 (1): 71-79.
Olsen,L.C.,Aasland,R.,and Fjose,A.,1997.Avasa-like gene in zebrafish identifies putative primordial germ cells.Mechanisms of Devevopment,66 (1-2): 95-105.
Pause,A.and Sonenberg,N.,1992.Mutational analysis of a DEAD box RNA helicase: the mammalian translation initiation factor eIF-4A.The EMBO Journal,11 (7): 2643-2654.
Raghuveer,K.,and Senthilkumaran,B.,2010.Cloning and differential expression pattern of vasa in the developing and recrudescing gonads of catfish,Clarias gariepinus.Comparative Biochemistry and Physiology A,157 (1): 79-85.
Rongo,C.,Gavis,E.R.,and Lehmann,R.,1995.Localization of oskar RNA regulates oskar translation and requires Oskar protein.Development,121: 2737-2746.
Rocak,S.,and Linder,P.,2004.DEAD-box proteins: the driving forces behind RNA metabolism.NatureReviewsMolecularCell Biology,5: 232-241.
Rose,T.M.,Schultz,E.R.,Henikoff,J.G.,Pietrokovski,S.,McCallum,C.M,and Henikoff,S.,1998.Consensus-degenerate hybrid oligonucleotide primers for amplification of distantly related sequences.Nucleic Acids Research,26 (7): 1628-1635.
Saffman,E.E.,and Lasko,P.,1999.Germline development in vertebrates and invertebrates.CellularandMolecularLife Sciences,55: 1141-1163.
Schüpbach,T.,and Wieschaus,E.,1986.Maternal-effect mutations altering the anterior-posterior pattern of theDrosophilaembryo.Development Genes and Evolution,195: 302-317.
Sellars,M.J.,Lyons,R.E.,Grewe,P.M.,Degnan,B.M.,and Preston,N.P.,2007.Isolation of avasa-like gene from the Kuruma shrimpMarsupenaeus japonicusand its differential expression pattern during embryonic,larval and post larval development.Aquaculture,272: 309-310.
Shi,D.,Wen,H.S.,and Yang,Y.P.,2011.The Annual Change of Ovarian Development in FemaleSebastes schlegel.Periodical of Ocean University of China,41 (9): 25-30 (in Chinese with English abstract).
Shibata,N.,Umesono,Y.,Orii,H.,Sakurai,T.,Watanabe,K.,and Agata,K.,1999.Expression ofvasa(vas)-related genes in germline cells and totipotent somatic stem cells of planarians.DevelopmentalBiology,206 (1): 73-87.
Shinomiya,A.,Tanaka,M.,Kobayashi,T.,Nagahama,Y.,and Hamaguchi,S.,2000.Thevasa-like gene,olvas,identifies the migration path of primordial germ cells during embryonic body formation stage in the medaka,Oryzias latipes.DevelopmentGrowth and Differentiation,42 (4): 317-326.
Tamura,K.,Dudley,J.,Nei,M.,and Kumar,S.,2007.MEGA4:Molecular evolutionary genetics analysis (MEGA) software version 4.0.MolecularBiologyandEvolution,24 (8): 1596-1599.
Thompson,J.D.,Gibson,T.J.,Plewniak,F.,Jeanmougin,F.,and Higgins,D.G.,1997.The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools.NucleicAcidsResearch,25 (24):4876-4882.
Voronina,E.,Lopez,M.,Juliano,C.E.,Gustafson,E.,Songa,J.L.,Extavour,C.,George,S.,Oliveri,P.,McClay,D.,and Wessel,G.,2008.Vasa protein expression is restricted to the small micromeres of the sea urchin,but is inducible in other lineages early in development.DevelopmentalBiology,314(2): 276-286.
Xu,H.Y.,Gui,J.F.,and Hong,Y.H.,2005.Differential expression ofvasaRNA and protern during spermatogenesis and oogenesis in the gibel carp (Carassius auratus gibelio),a bisexually and gynogenetically reproducing vertebrate.Development Dynamics,233 (3): 872-882.
Yang,Y.P.,Wen,H.S.,He,F.,Li,J.F.,Shi,D.,Chen,C.F.,Zhang,J.R.,Jin,G.X.,Chen,X.Y.,and Shi,B.,2010.The morphology and developmental histology of testis in rockfishSebastes schlegeli.Journal of Dalian Oceanuniversity,25 (5):391-396 (in Chinese with English abstract).
Yoon,C.,Kawakami,K.,and Hopkins,N.,1997.Zebrafishvasahomologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells.Development,124: 3157-3165.
Yoshizaki,G.,Sakatani,S.,Tominaga,H.,and Takeuchi,T.,2000.Cloning and characterization of avasa-like gene in rainbow trout and its expression in the germ cell lineage.Molecular Reproduction and Development,55 (4): 364-371.
 Journal of Ocean University of China2013年1期
Journal of Ocean University of China2013年1期
- Journal of Ocean University of China的其它文章
- Isolation and Expression Analysis of FTZ-F1 Encoding Gene of Black Rock Fish (Sebastes schlegelii)
- Chemical Characteristics and Anticoagulant Activities of Two Sulfated Polysaccharides from Enteromorpha linza(Chlorophyta)
- Optimization of the Purification Methods for Recovery of Recombinant Growth Hormone from Paralichthys olivaceus
- Seasonal Changes in Food Uptake by the Sea Cucumber Apostichopus japonicus in a Farm Pond: Evidence from C and N Stable Isotopes
- Purification and Characterization of a New Thermostable κ-Carrageenase from the Marine Bacterium Pseudoalteromonas sp. QY203
- Growth,Metabolism and Physiological Response of the Sea Cucumber,Apostichopus japonicus Selenka During Periods of Inactivity
