超临界水中碳酸钠团簇成核与生长分子动力学模拟
张金利 何正华 韩 优,2,* 李 韡 武江洁星 甘中学 谷俊杰
(1天津大学化工学院,天津300072;2天津大学,天津市膜科学与海水淡化技术重点实验室,天津300072; 3新奥集团煤基低碳能源国家重点实验室,河北廊坊065001)
超临界水中碳酸钠团簇成核与生长分子动力学模拟
张金利1何正华1韩 优1,2,*李 韡1武江洁星1甘中学3,*谷俊杰3
(1天津大学化工学院,天津300072;2天津大学,天津市膜科学与海水淡化技术重点实验室,天津300072;3新奥集团煤基低碳能源国家重点实验室,河北廊坊065001)
应用分子动力学方法研究了碳酸钠颗粒在超临界水中的成核与生长过程.计算了温度为700-1100 K、压力在23-30 MPa下碳酸钠的团聚过程,计算时间为1 ns.对体系结合能与径向分布函数的分析表明,碳酸钠成核过程主要受静电作用的影响.在超临界态下,水分子与Na+和之间的静电作用降低,Na+与能够很容易碰撞形成Na2CO3小团簇.在Na2CO3整个成核过程中,单个离子的碰撞在前50 ps内完成,同时离子碰撞速率达到1030cm-3·s-1.另外,在成核阶段温度的影响比压力更加明显,温度越高,离子碰撞速率越快,形成的初始团簇越多.而压力对Na2CO3团簇的进一步生长影响较大.
超临界水;碳酸钠;结合能;碰撞速率;分子动力学
1 Introduction
Crystallization of salts is a phenomenon of great practical relevance.In fact,it is also one of the most important methods for industrial separation.In recent years,the nucleation of salts in supercritical water,as well as the properties of those aqueous systems under supercritical conditions,has received increasing interest because of its importance in hazardous organic waste water treatment,1natural geothermal processes,2design of the new materials,3,4and supercritical water oxidation.5-8
In the supercritical water(critical temperature:647 K and critical pressure:22.1 MPa),the salt solubility would change obviously.Therefore,supercritical water as an important solvent in salt nucleation research received extensive attention. Svishchev et al.9investigated the nucleation of strontium chloride nano-particles in supercritical water by molecular dynamic simulations.Their results showed that water molecules could reside both on the surface and in the interior of SrCl clusters, and the distribution of the clusters showed a very strong dependence on the density of the system.In their other work,10they examined the NaCl nano-particles generated by a rapid quench of supercritical aqueous solutions,and analyzed the spectroscopic signatures of NaCl clusters with different sizes and estimated their relative stabilities.Lümmen and Kvamme11,12studied the aggregation of ferrous chloride,the growth and properties of ferrous chloride with sodium chloride nano-particles in supercritical water by molecular dynamic simulations.They found that the growth rate of FeCl2particles was affected by the temperature and the density of the system.The growth rate of FeCl2particles was faster with lower temperature and higher density of the system.Finally,the disordered FeCl2crystalline was formed.
Recently,the supercritical water has been introduced in the reaction of coal catalytic gasification to enhance the efficiency.13,14The experimental results showed the Na2CO3catalyst had higher catalytic activity under supercritical water than non-supercritical water for the coal gasification reaction.But the effect mechanism of supercritical water on the catalyst as well as its contribution during the reaction process has been unclear yet.In fact,the nucleation and growth process of the Na2CO3particles in the supercritical water depend on their shape,size distribution,and loading status,which are crucial for the whole catalytic reaction process.But the nucleation process of Na2CO3particles in the supercritical water is difficult to be analyzed by using experimental method.However,with the developments of computer technology and molecular dynamics theory,theoretical studies on the micro-structure of solution system as well as the interaction mechanism of different moleculeswith moleculardynamicssimulation method have been carried out extensively.15-19Therefore,the nucleation and growth process of Na2CO3particles in supercritical water was studied in this work using molecular dynamics simulation method.The binding energy and radial distribution function (RDF)were introduced to analyze the interaction mechanism of water and Na2CO3.The effects of temperature and pressure on the distribution of Na2CO3particles as well as on ions collision rate were discussed.The important conclusions of Na2CO3nucleation in the supercritical water will shed light on the real production process.
2 Computational methods
2.1 Simulation details
The Forcite package20in the Materials Studio software21was applied here for the whole molecular dynamics simulations. The force filed of compass26,22which is suitable for the supercritical water system,23was chosen for the aqueous system.The O-H sp3hybrid orbital energy model of the o2*forcefield type,which provides a good prediction of water construction and properties,was assigned for the O atom in water molecules.The h1o forcefield type,which describes the bond between H and O,is suitable for the H atom in water molecules. The c3i forcefield type provides a good description of the C atom in the.The o2c forcefield type,which describes the O-X sp3hybrid orbital energy model in acid,is suitable for the O atom in the.The an+forcefield type was assigned for Na+.
The equations of motion were solved by Verlet leapfrog algorithm,24with the time step of 0.5 fs.Simulations have been performed in the NPT ensemble(Isobaric-Isothermal conditions). In order to determine a reasonable method to control the temperature and pressure during the dynamics simulation process, four temperature control methods(Andersen,25Berendsen,26Nose,27and Velocity28)combined with two pressure control methods(Andersen29and Berendsen25)were tested.When Andersen thermostatand Berendsen barostat were chosen,the temperature and pressure of the system can be quickly stabilized within 10 ps.Therefore,the temperature and the pressure were controlled by Andersen thermostat and Berendsen barostat methods during the whole simulation.Electrostatic interaction and van der Waals interaction were calculated using Ewald method.30The calculated density of supercritical water at 673 K and 28 MPa by the above method is 0.246 g·cm-3.It is approximate to the experimental value(0.25 g·cm-3),31indicating that the simulation method is reasonable.
A series of simulations ranging from 700 to 1100 K and from 23 to 30 MPa were explored.In each simulation,1024 water molecules,60 Na+,and 30(14.7%(w,mass fraction)Na2CO3)were randomly distributed in the simulation cell. And then,the geometry of this system was optimized with smart minimization method(which automatically combines appropriate calculation methods in a cascade.It starts with the steepest descent method,followed by the conjugate gradient method and ends with a Newton method)to prevent formation of the initial ion pairs and the unreasonably overlap with water molecules.After that,the dynamics simulations of the relaxed system at different state conditions in the supercritical region were carried out.The total simulation time was 1000 ps,and one sample could be recorded every 1000 simulation steps for follow-up analysis.
2.2 Binding energy
Based on the framework of the classical nucleation theory (CNT),32,33the whole(Gibbs)free energy of the nucleation system increased at first and then decreased as the cluster radius increased.Thus the nucleation is not a spontaneous process.It requires some impetus to overcome the energy barrier.Generally,the impetus comes from the spontaneous fluctuation of density or component in the metastable phase.34According to the CNT,a certain energy barrier is present during the nucleation process.Once the system has enough impetus,the energy barrier would be overcome and a new phase would be formed.At this moment,the thermodynamic energy is changed to the kinetic energy.As the temperature rising,the molecules move faster,which may lead single molecules to collide with each other,and then the total energy of system decreases with the new particles appearing.But the interaction of water molecules with Na2CO3is the main obstruction for the above process. Therefore,investigation of the binding energy of water and sodium carbonate is useful for better understanding of the mechanism of Na2CO3nucleation in supercritical water.
The binding energy is defined by the following equation,

where Ebindrepresents the binding energy,EH2OandENa2CO3are the energies of water and sodium carbonate in the Na2CO3solution,respectively,andEH2O+Na2CO3is the energy of the Na2CO3solution system.All the above energies are calculated in the same conditions.In the Forcite package,EH2O,ENa2CO3,Ekin,and EH2O+Na2CO3are contributed by their kinetic energy(Ekin)and potential energy(Epot),and the potential energy is defined as,

where Enon-bond,Evan,Eelect,EH-bond,Ecross,and Evalenceare the nonbond energy,van der Waals energy,electrostatic energy,hydrogen bond energy,cross term energy(which comes from the valence bond stretch,bend,and torsion),and valence interaction energy,respectively.Under the supercritical condition,the interaction of hydrogen bonds between water molecules is weak, so the hydrogen bond energy is very low,which could be ignored over the whole simulation ranges.Thus,the equation(3) was simplified as,

Therefore,
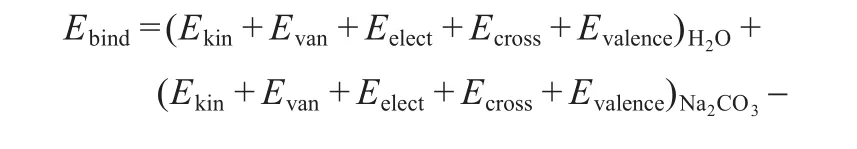

where ΔE represents the change of corresponding energies, which has been obtained by measuring the energy difference between the pure water,Na2CO3,and the Na2CO3solution at the same condition.
2.3 Cluster size
The size of Na2CO3clusters can be determined using Stillinger criteria.35That is,any two atoms belong to one cluster if their distance is less than 0.32 nm(which corresponds to the average value of the first minima peak of the Na+-Na+,Na+-,andradial distribution functions at melted state).If a continuous path which has been mentioned above is present between two atoms,they belong to the same cluster.
2.4 Collision rate
The nucleation rate is defined as the number of the clusters which is larger than the number of critical nucleus generated per unit time unit volume.The size of critical nucleus could estimate with the kinetic method reported by Yasuoka and Matsumoto.36Firstly,one threshold should be chosen.The threshold is a specific number corresponding to the number of atoms or ions in the cluster.If the atom numbers of a cluster exceed the threshold,the number of the clusters is plotted versus the simulation time,and a growth-decay evolution curve is produced.There will be a linear region at the beginning simulation time,and the slope of the linear region would reduce with the increasing threshold.When the threshold exceeds the critical value,the slope would stop decreasing.37The slope divided by the lattice volume is the nucleation rate.
3 Result and discussion
3.1 Interactions between water molecules and ions
The binding energy of water and sodium carbonate at each state point(temperature:700-1100 K,pressure:23-30 MPa) was calculated here and shown in Fig.1(a).It clearly showed that the impact of temperature on the binding energy was more obvious than that of pressure.When the pressure was constant, the binding energy decreased with temperature increasing.Especially,when the temperature was lower than 900 K,Ebindwas reduced quickly,whereas it declined slower when the temperature was higher than 900 K.The result implied that the interaction between water molecules and Na2CO3was reduced as temperature increasing.
In section 2,we introduced that the binding energy was contributed by the changes of kinetic energy,van der waals energy,electrostatic energy,the cross term energy,and the valence interaction energy(shown in formula(5)).During the simulation process,the energies of the cross terms and the valence interaction have no changes.Meanwhile,the changes of the kinetic energy and van der Waals energy are too small compared with the change of electrostatic energy.Thus,the binding energy of water and Na2CO3is mainly contributed by the change of electrostatic energy,which is shown in the Fig.1(b).That is, the electrostatic interaction between water and Na2CO3decreases at higher temperature.

Fig.1 Binding energy and the change of electrostatic energy at different temperatures and different pressures
In order to analyze the impact of interaction between water and Na2CO3on the Na2CO3nucleation process,ΔEpot,ΔEkin,and ΔEelectwere calculated during whole simulation process,their trends with simulation time at different temperatures and pressures are shown in Fig.2.Again,ΔEpotand ΔEelecthas no obvious dependence on the pressure(shown in Fig.2(a)),whereas they become lower at higher temperature(shown in Fig.2(b)) during the whole process.Besides,the change of kinetic energy almost keeps at 0 kJ·mol-1during the simulation process. The changes of potential energy and electrostatic energy decrease quickly from~800 to~150 kJ·mol-1at the initial 100 ps,indicating that the nucleation and particle initial collision of Na2CO3in supercritical water mainly occurres during this period.This period is shorter than that of FeCl2clusters,which is~200 ps.38
乔木林(纯林和混交林)碳储量3337416 t,占总碳储量的82.45%。按地类分:纯林碳储量3143808 t,混交林碳储量193608 t,纯林碳储量是混交林碳储量的16.24倍。从各龄组的碳储量分析:以中龄林、近熟林碳储量为主,其碳储量之和为2566391 t,占纯林碳储量的76.90%;中龄林的碳储量最大,其碳储量1606955 t,占纯林碳储量的48.15%。详见表2。
According to the analysis of the binding energy,the key impact of supercritical water on the Na2CO3nucleation process is the electrostatic interaction between water and Na2CO3.In the normal aqueous solution,the Na+andare surrounded by water molecules,and then hydrated Na+andform.When the system is under the supercritical condition,the electrostatic interaction between water molecules and hydrated Na+andions decreases,and the electrostatic shield interaction is weakened.Then the partial ion dehydration would result in the single ion collisions to form ion pair,which would further develop to Na2CO3clusters.During the Na2CO3nucleation process, the electrostatic interaction between the water molecules and Na2CO3decreases as the temperature increasing(shown in Fig.1(b)),which make the hydrated ions escape from the water shield easily,39-41and then collide into ion pairs.

Fig.2 Change of energies during the simulation process at 700 K(a)and 23 MPa(b)
To show the microstructure of Na2CO3aqueous solution more clearly,a series of RDF were analyzed at a wide range of temperatures and pressures.Fig.3 shows the temperature and pressure dependencies of the RDF for Na+and oxygen of the water molecules.The locations of the peaks were the same in all systems but the height of the peaks varied with the different temperatures and pressures.The first maximum peak in the curves appeared at 0.233 nm,and it was very sharp and narrow.The second peak(r=0.395 nm)and third peak(r=0.533 nm)overlapped with each other and became slightly flatter. Furthermore,their values were much smaller compared with that of the first peak.The function curves reflectes that the oxygen in the water molecules aggregates surrounding the Na+, suggesting that some water molecules are present in the initial Na2CO3particles.The first sharp peaks in these curves indicated that the Na+was besieged by a certain number of water molecules in the first coordination shell.If the value of the gNa+-O(r)peak is higher,there would be more water molecules surrounding Na+.The second and third peaks were the hint of a disorder and un-equilibrium system.When the temperature was constant,the value of firstgNa+-O(r)peak decreased gradually with the increasing pressure(shown in Fig.3(a)),indicat-ing less water molecules around Na+at higher pressure.While at a constant pressure,the values of the firstgNa+-O(r)peak increased quickly with the increasing temperature at first,and it reached the maximum at 900 K,then it only had little decrease from 900 to 1100 K.This phenomenon is partially related with the strong local density augmentation of water molecules in the supercritical conditions.42,43As temperature increasing,the degree of water agglomeration would attain maxima,while the interaction between water and Na+reduces gradually and the ion pairs are formed much easily.Because the coordination number of the Na+ions would not vary sharply,the ion pairs should be besieged by more water molecules than the single ion.Thus, initial Na2CO3particles is besieged by many water molecules when the temperature was as high as 900 K,and it would be difficult for the collision of small Na2CO3particles,which resulted in the final formation of dispersed Na2CO3clusters. The RDF curves of Na+-Na+and Na+-CO2-3(shown in Fig.4) had a maximum peak at 0.299 and 0.197 nm,respectively,and the peaks were very sharp and narrow,suggesting that the ordered and stable Na2CO3clusters are formed in the system from the first coordination shell.The second RDF peaks of Na+-Na+and Na+-CO2-3appeared at 0.405 and 0.361 nm,respectively,but the heights of the peaks were much lower than those of the first ones.The other peaks became flatter with increasing distance,indicating that there are other initial and disordered Na2CO3clusters formed from the two to three coordination shells.10The presence of these peaks reflected the heterogeneous distribution of the Na+and CO2-3in the supercritical water system,which meant that Na2CO3gathered together and the Na2CO3cluster was formed.At the constant temperature (shown in Fig.4(a,b)),the values ofgNa+-Na+(r)andgNa+-CO2-3(r) reduced slowly with the increasing pressure,while they rose quickly with the increasing temperature at the constant pressure(shown in Fig.4(c,d)).The result suggested that the Na2CO3nucleation process is more sensitive to the temperature rather than the pressure.The reason would be that the density of supercritical water system is more sensitive to the temperature rather than the pressure within our discussion range.

Fig.3 Radial ionic Na+-O distribution functions at 700 K(a)and 25 MPa(b) Insets are the enlarged ones for the main figures.(a)from top to bottom,p/MPa:23,24,25,26,27,28,29,30;(b)from top to bottom,T/K:900,800,1000,1100,700

Fig.4 Radial ionic distribution functions of Na+-Na+(a,c)and Na+-CO23-(b,d)at 700 K(a,b)and 25 MPa(c,d)Insets are the enlarged ones for the main figures.(a,b)from top to bottom,p/MPa:23,24,25,26,27,28,29,30;(c,d)from top to bottom,T/K:900,800,1000,1100,700
In order to display the point clearly,the RDFs at different system densities were analyzed and shown in Fig.5(to make the paper refining,only the RDF curves of Na+-Na+andwere shown here).Similarly,these RDF curves of Na+-Na+andhad the first maximum peaks at 0.299 and 0.197 nm, respectively,and their second RDF peaks appeared at 0.405 and 0.361 nm,respectively.The heights of the second peaks were much lower than the first one.It is noteworthy that the values ofgNa+-Na+(r)andgNa+-CO2-3(r)increased clearly with the reducing density,indicating that more stable Na2CO3clusters formed at lower system density.This conclusion was further demonstrated by the analysis of Na2CO3growth process at different system densities(Fig.6).As shown in Fig.6,when the temperature increased and the pressure decreased,the density of the supercritical water became lower,which resulted in the hydrogen bond network being broken,and the electrostatic interaction of water molecules with Na+andreduced.Therefore,the possibility and frequency of the ionsʹcollision is higher.This was also consistent with the results of the binding energy analysis.
3.2 Particle distribution
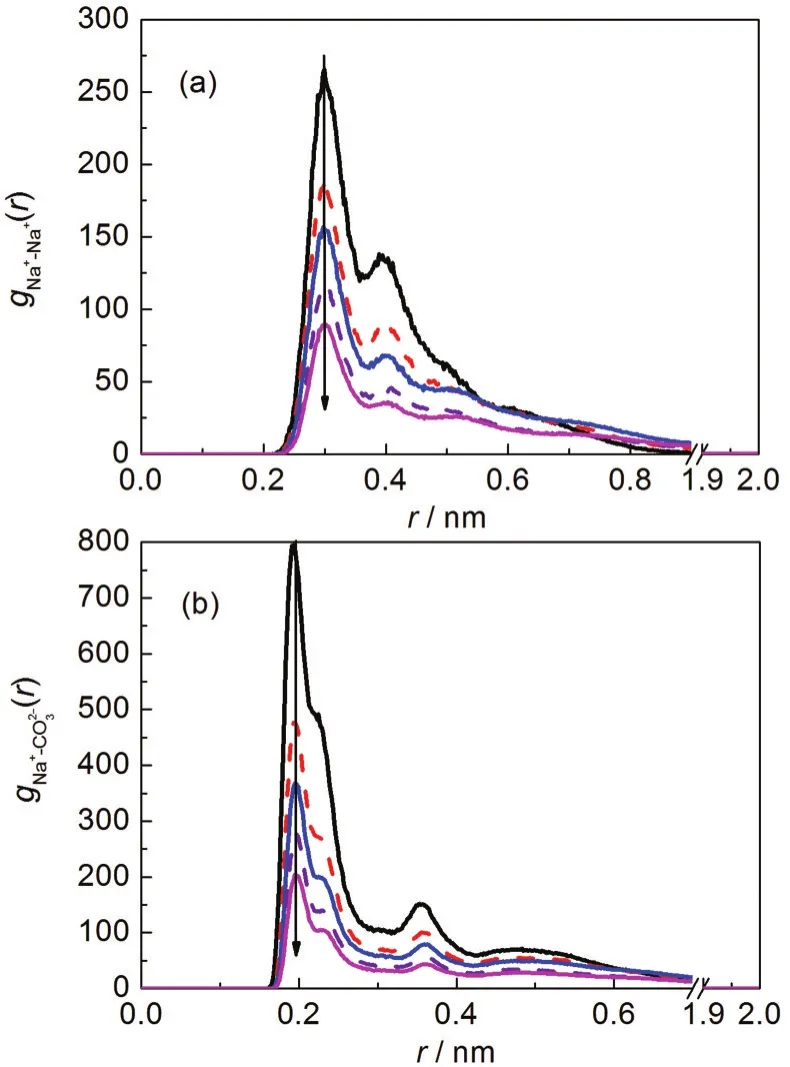
Fig.5 Radial ionic distribution functions of Na+-Na+(a)and Na+-CO2-3(b)at different system densities(ρ)from top to bottom,ρ/(g·cm-3):0.058,0.108,0.146,0.207,0.258
In this section,the initial aggregation of hydrated Na+andions,the collision and fusion of initial Na2CO3particles in the aqueous system at different temperatures and pressures were observed,and it was found that the particle distribution had a great relationship with the temperature and pressure. Herein,the change of the average size of Na2CO3cluster with the temperature at different pressures is shown in Fig.7.The average size of Na2CO3cluster decreased with temperatures increasing,this change was more rapidly at the lower temperature range.That is,the Na2CO3clusters are more dispersed with the increasing temperature in the supercritical water.But it becomes almost constant when the temperature attained 900 K,indicating that much more Na2CO3nuclei are formed when temperature is as high as 900 K.And according to the result of the Na+-O RDF at 25 MPa(shown in Fig.3(b)),the initial Na2CO3particles are surrounded by many water molecules,it is difficult for the further collision and growth.As a result,more disperse particles are formed when T≥900 K.

Fig.6 Snapshots of the last configuration at different system densitiesThe balls are denoted as Na2CO3particles,dash lines are hydrogen bonds,and the linear structures are water molecules. ρ/(g·cm-3):(a)0.058,(b)0.108,(c)0.146,(d)0.207,(e)0.258

Fig.7 Change of the average size of Na2CO3cluster with the temperature at different pressuresNaver:the average size of Na2CO3cluster
3.3 Collision rate
The simulation results showed that the nucleation rate of sodium carbonate was very fast at all the state points,and the nucleation process always completed within tens of picoseconds. In this section,the criterion of threshold method is used to investigate the collision rate.If the ion number in one Na2CO3group is higher than the threshold,this Na2CO3group is assumed to be a Na2CO3cluster.The number of Na2CO3clusters was plotted against the simulation time and shown in Fig.8.At the initial 30 ps,the number of Na2CO3clusters increased quickly and linearly,indicating that the collision and fusion of ions were very fast and it resulted in many small Na2CO3nuclei formed.When the slope of the curve in the region was divided by the volume of system,the ion collision rate could be gotten.36Here,the average volume of the system at the initial time region is used because of the system volume fluctuations.44Besides,three different thresholds are selected in Fig.8.When the threshold is 3 ions,the nucleation rate of Na2CO3cluster is 1.423×1030cm-3·s-1.When the thresholds are 6 and 9 ions,the nucleation rates are 0.733×1030and 0.690×1030cm-3·s-1,respectively.The nucleation rate decreases rapidly when the threshold changes from 3 to 6 ions,while it almost keeps constant when the threshold increases from 6 to 9 ions.Therefore,the threshold value is chosen as 6(the ions number of Na2CO3particles)here to investigate the effects of the temperature and pressure on the nucleation rate.

Fig.8 Number of Na2CO3clusters(N)versus simulation time with different thresholds
The curves of the number of Na2CO3clusters which is larger than threshold with the simulation time at different temperatures and pressures were plotted and shown in Fig.9.At the constant pressure(23 and 26 MPa),the slopes of these curves had large dependence on the temperature.Based on the linear slopes of the curves,the nucleation rates of Na2CO3were calculated and shown in the Table 1.It showed that all collision rates had a same magnitude with 1030cm-3·s-1,which is a little higher than that of sodium chloride reported by Nahtigal et al.45Besides,the nucleation rates of Na2CO3were faster at higher temperature and lower pressure,indicating the higher temperature and lower pressure could cause the ions colliding faster, which led to small primary Na2CO3particles formed46and the Na2CO3clusters more dispersed.47

Fig.9 Number of Na2CO3clusters which is larger than threshold versus simulation time

Table 1 Nucleation rate at different temperatures and pressures with threshold value of 6
3.4 Cluster growth
In order to study the growth process of Na2CO3clusters in the supercritical water,the atom number of the largest Na2CO3cluster in the system was plotted against the simulation time at different temperatures and pressures and shown in Fig.10.In these curves,there are several stages,indicating that the Na2CO3clusters need certain time to collide with each other and then congregate together to form a larger particle.The small Na2CO3clusters are kinetically favored because they are easier to nucleate at the beginning,whereas the large Na2CO3clusters are more thermodynamically stable.Within the first 50-100 ps,the curves shown in Fig.10 rose quickly,indicating that the initial nucleation is mainly the attachment of monomers or Na+-CO2-3pairs or initial Na2CO3particles.Then,the further collisions of two particles require hundreds or even thousands of picoseconds to complete(shown as the large steps in the growth curves of Fig.10).The analysis of Na2CO3growth process showed that the water molecules surrounding the particles(which could be shown in the Fig.6(f)clearly) blocked the direct connection of the initial Na2CO3particles, which induced the initial particles to take longer time to combine with each other and form larger clusters.Moreover,the size of the largest Na2CO3cluster at high temperature(lower supercritical water density)is smaller than that at low temperature(higher supercritical water density)during all the simulation time.The result further demonstrated that the high temperature or lower density was benefit for the dispersion of Na2CO3cluster.

Fig.10 Growth curves of Na2CO3particles at 23 and 26 MPa Nl:the number of atoms in the largest cluster

Fig.11 Growth process of Na2CO3particles at 700 K and 23 MPa In order to show the Na2CO3particles clearly,the water molecules were hidden in the figures.
It is noticed that the sizes of the Na2CO3clusters in the supercritical water system would never reduce once they were formed,which is different from the phenomenon reported by Römer and Kraska.44In their work,some small zinc particles which formed in supersaturated vapor would vanish when their sizes are less than the critical nucleus.In the supercritical water system,the solubility of Na2CO3sharply decreases,and the degree of super-saturation is very large,which causes a big driving force for Na2CO3nucleation.In other words,the high super-saturation of Na2CO3in supercritical water leads its fast nucleation rate and low nucleation energy barrier.
In order to display the Na2CO3growth process in supercritical water intuitively,its trajectory visualization at the state point of 700 K and 23 MPa was shown in Fig.11.The figure shows clearly that the collision of the small Na2CO3clusters needs a long simulation time for further growth.Furthermore, the Na2CO3particles formed in the supercritical water are amorphous.In fact,no Na2CO3crystalline structure is observed in present work.

Fig.12 Scheme of Na2CO3nucleation and growth mechanism in the supercritical water system
From the analysis above,we summarized the nucleation and growth processes of Na2CO3catalyst in supercritical water shown in Fig.12.The nucleation and growth processes of Na2CO3particles in the supercritical water system could divide into two stages.(1)Initial collision process of the single-ions. In the supercritical condition,the electrostatic interaction of water molecules with Na+andions decreases swiftly,then Na+andcould collide with each other easily to form small Na2CO3clusters.At this stage,the effect of temperature is more important than the pressure.Higher temperature would cause faster collision rate,and more initial particles are formed.(2)The further collision and fusion processes of small Na2CO3clusters.The collision of the small Na2CO3particles leads to larger clusters formed,and high pressure would be slightly benefit for this process.
4 Conclusions
In this work,the sodium carbonate nucleation and growth processes in supercritical water were investigated using molecules dynamics simulation method.The interaction of water
molecules with hydrated Na+and,the binding energy, RDF of the ions in the system,Na2CO3particles distribution, the initial collision rate of ions as well as particles growth under different temperatures and pressures were explored and discussed.As the temperature increasing,the electrostatic interac-
tion of water molecules and hydrated Na+anddecreases, causing a lower binding energy between water and Na2CO3. Thus,the initial collision rate of ions is very fast at higher temperature,and the fast nucleation would result in forming many small Na2CO3particles,which is crucial for the whole nucleation and growth processes.At lower pressure,the initial Na2CO3particles are besieged by more water molecules,which would partially prevent the further growth of Na2CO3particles. Therefore,the dispersed and small Na2CO3clusters would be formed in the supercritical water system with high temperature (~900 K)and low pressure,resulting in a low-density system, which will be very helpful for the experimenters to optimize the operating conditions to enhance the catalytic activity of Na2CO3catalyst and improve the efficiency of the supercritical coal catalytic gasification reaction.
(1) Shaw,R.W.;Brill,T.B.;Clifford,A.A.;Eckert,C.A.;Franck, E.U.Chem.Eng.News 1991,69,26.
(2) Valeriani,C.;Sanz,E.;Frenkel,D.J.Chem.Phys.2005,122,
194501.doi:10.1063/1.1896348
(3) Reverchon,E.;Adami,R.J.Supercrit.Fluids 2006,37,1.doi: 10.1016/j.supflu.2005.08.003
(4) Cansell,F.;Aymonier,C.J.Supercrit.Fluids 2009,47,508.doi: 10.1016/j.supflu.2008.10.002
(5) Hodes,M.;Marrone,P.A.;Hong,G.T.;Smith,K.A.;Tester,J. W.J.Supercrit.Fluids 2004,29,265.doi:10.1016/S0896-8446 (03)00093-7
(6) Kritzer,P.;Dinjus,E.Chem.Eng.J.2001,83,207.doi:10.1016/ S1385-8947(00)00255-2
(7) Bermejo,M.D.;Martín,A.;Queiroz,J.P.S.;Bielsa,I.;Ríos, V.;Cocero,M.J.Chem.Eng.J.2010,158,431.doi:10.1016/ j.cej.2010.01.013
(8)Kim,K.;Son,S.H.;Kim,K.S.;Kim,K.;Kim,Y.C.Chem. Eng.J.2010,165,170.doi:10.1016/j.cej.2010.09.012
(9) Svishchev,I.M.;Zasetsky,A.Y.;Nahtigal,I.G.J.Phys.Chem. C 2008,112,20181.doi:10.1021/jp803705z
(10) Nahtigal,I.G.;Svishchev,I.M.J.Supercrit.Fluids 2009,50, 169.doi:10.1016/j.supflu.2009.05.006
(11)Lümmen,N.;Kvamme,B.Phys.Chem.Chem.Phys.2009,11, 9504.
(12)Lümmen,N.;Kvamme,B.J.Phys.Chem.B 2008,112,12374. doi:10.1021/jp710156b
(13) Li,Y.L.;Guo,L.J.;Zhang,X.M.;Jin,H.;Lu,Y.J.Int.J. Hydrog.Energy 2010,35,3036.doi:10.1016/j.ijhydene. 2009.07.023
(14) Jin,H.;Lu,Y.J.;Liao,B.;Guo,L.J.;Zhang,X.M.Int.J. Hydrog.Energy 2010,35,7151.doi:10.1016/j.ijhydene. 2010.01.099
(15) Xiao,H.Y.;Zhen,Z.;Sun,H.Q.;Cao,X.L.;Li,Z.Q.;Song, X.W.;Cuo,X.H.;Liu,X.H.Acta Phys.-Chim.Sin.2010,26, 422.[肖红艳,甄 珍,孙焕泉,曹绪龙,李振泉,宋新旺,崔晓红,刘新厚.物理化学学报,2010,26,422.]doi:10.3866/ PKU.WHXB20100216
(16) Zhou,J.;Lu,X.H.;Wang,Y.R.;Shi,J.Acta Phys.-Chim.Sin. 1999,15,1017.[周 健,陆小华,王延儒,时 钧.物理化学学报,1999,15,1017.]doi:10.3866/PKU.WHXB19991112
(17) Liao,R.J.;Zhu,M.Z.;Zhou,X.;Yang,L.J.;Yan,J.M.;Sun, C.X.Acta Phys.-Chim.Sin.2011,27,815.[廖瑞金,朱孟兆,周 欣,杨丽君,严家明,孙才新.物理化学学报,2011,27, 815.]doi:10.3866/PKU.WHXB20110341
(18) Chen,C.;Li,W.Z.Acta Phys.-Chim.Sin.2009,25,507. [陈 聪,李维仲.物理化学学报,2009,25,507.]doi:10.3866/ PKU.WHXB20090318
(19) Shen,Q.C.;Liang,W.C.;Hu,X.B.;Li,H.R.Acta Phys.-Chim.Sin.2008,24,1169.[沈秋婵,梁婉春,胡兴邦,李浩然.物理化学学报,2008,24,1169.]doi:10.3866/PKU. WHXB20080709
(20) Allen,M.P.;Tildesley,D.J.Computer Simulation of Liquids; Clarendon:Oxford,1987.
(21) MaterialsStudioOverview.http://accelrys.com/products/ materials-studio/(accessed May 02,2012)
(22) Sun,H.J.Phys.Chem.B 1998,102,7338.doi:10.1021/ jp980939v
(23) Sun,H.Macromolecules 1995,28,701.doi:10.1021/ ma00107a006
(24) Allen,M.P.;Tildesley,D.J.Computer Simulation of Liquids; Oxford University Press:Oxford,1989.
(25) Frenkel,D.;Smit,B.Understanding Molecular Simulations: from Algorithms to Applications;Academic Press:San Diego, 1996.
(26) Berendsen,H.J.;Postma,J.P.J.Chem.Phys.1984,18,3684.
(27) Hoover,W.G.Phys.Rev.A 1985,31,1695.doi:10.1103/ PhysRevA.31.1695
(28) Hoffmann,K.H.;Schreiber,M.Computational Physics 1996, 268.
(29)Anderson,H.C.J.Chem.Phys.1980,72,2384.
(30) Ewald,P.P.Annales de Physique.1921,64,253.
(31)Sun,W.;Huang,S.Y.;Wang,C.W.;Chi,R.A.J.Huazhong Univ.of Sci.&Tech.2008,36,103.[孙 炜,黄素逸,王存文,池汝安.华中科技大学学报,2008,36,103.]
(32) Becker,R.;Döring,W.Annales de Physique.1935,24,719.
(33)Volmer,M.;Weber,A.Z.Z.Phys.Chem.1926,119,277.
(34) Debenedetti,P.G.Metastable Liquids:Concept and Principles; Princeton Press:NJ,1996.
(35) Stillinger,F.H.J.Chem.Phys.1963,38,1486.doi:10.1063/ 1.1776907
(36)Yasuoka,K.;Matsumoto,M.J.Chem.Phys.1998,109,8451. doi:10.1063/1.477509
(37) Rozas,R.;Kraska,T.J.Phys.Chem.C 2007,111,15784.doi: 10.1021/jp073713d
(38)Lümmen,N.;Kvamme,B.J.Chem.Phys.2010,132,014702. doi:10.1063/1.3270158
(39) Guo,G.J.;Zhang,Y.G.;Li,M.J.Chem.Phys.2008,128, 194504.doi:10.1063/1.2919558
(40)Wernet,P.;Testemale,D.;Hazemann,J.L.;Argoud,R. J.Chem.Phys.2005,123,154503.doi:10.1063/1.2064867
(41) Skarmoutsos,I.;Guardia,E.J.Chem.Phys.2010,132,074502. doi:10.1063/1.3305326
(42) Kalinichev,A.G.;Bass,J.D.J.Phys.Chem.A 1997,101,9720. doi:10.1021/jp971218j
(43) Skarmoutsos,I.;Samios,J.J.Phys.Chem.B 2006,110,21931. doi:10.1021/jp060955p
(44) Römer,F.;Kraska,T.J.Supercrit.Fluids 2010,55,769.doi: 10.1016/j.supflu.2010.08.010
(45) Nahtigal,I.G.;Zasetsky,A.Y.;Svishchev,I.M.J.Phys.Chem. B 2008,112,7537.doi:10.1021/jp709688g
(46) Römer,F.;Kraska,T.J.Chem.Phys.2007,127,234509.doi: 10.1063/1.2805063
(47) Sue,K.;Kawasaki,S.;Suzuki,M.;Hakuta,Y.;Hayashi,H.; Arai,K.;Takebayashi,Y.;Yoda,S.;Furuya,T.Chem.Eng.J. 2011,166,947.doi:10.1016/j.cej.2010.11.080
March 28,2012;Revised:May 2,2012;Published on Web:May 3,2012.
Nucleation and Growth of Na2CO3Clusters in Supercritical Water Using Molecular Dynamics Simulation
ZHANG Jin-Li1HE Zheng-Hua1HAN You1,2,*LI Wei1WU Jiang-Jie-Xing1GAN Zhong-Xue3,*GU Jun-Jie3
(1School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,P.R.China;2Tianjin Key Laboratory of Membrane Science and Desalination Technology,Tianjin University,Tianjin 300072,P.R.China;3State Key Laboratory of Coal-Based Low Carbon Energy,ENN Group,Langfang 065001,Hebei Province,P.R.China)
The nucleation and growth of Na2CO3particles in supercritical water were investigated using molecular dynamics simulation.The clustering process of Na2CO3was studied for 1 ns at a series of state points,across temperature and pressure ranges of 700 to 1100 K and 23 to 30 MPa,respectively.The binding energy and radial distribution function analysis showed that the electrostatic interaction was the main factor affecting the whole Na2CO3nucleation process.Under supercritical conditions,the electrostatic interaction of water molecules with Na+andions rapidly decreased,allowing Na+andions to readily collide with each other to form small Na2CO3clusters.During the initial Na2CO3nucleation process, all the single-ion collisions were complete within 50 ps and the ionic collision rates appeared to be of the order of 1030cm-3·s-1.Furthermore,the effect of temperature was found to be more important than that of the pressure at the nucleation stage and a higher temperature led to an enhanced collision rate and the formation of more initial Na2CO3particles.The further growth of the Na2CO3particles was more dependent on the pressure.
Supercritical water;Sodium carbonate;Binding energy;Collision rate; Molecular dynamics
10.3866/PKU.WHXB201205032
∗Corresponding authors.HAN You,Email:yhan@tju.edu.cn;Tel:+86-22-27401476.GAN Zhong-Xue,Email:ganzhongxue@enn.cn;
Tel:+86-316-2596900.
The project was supported by the National High-Tech Research and Development Program of China(863)(2011AA05A201),National Natural Science Foundation of China(21106094,20836005),and National Key Basic Research Program of China(973)(2010CB736202).
国家高技术研究发展计划项目(863)(2011AA05A201),国家自然科学基金(21106094,20836005)和国家重点基础研究发展规划项目(973) (2010CB736202)资助
O641;O645

