哮喘患者血清高敏C-反应蛋白与哮喘控制测试评分相关性研究
余雷,资文杰
(1.重庆市九龙坡区第一人民医院内科,重庆 400000;2.第三军医大学新桥医院,重庆 404100)
高敏C-反应蛋白(hs-CRP)可用于评估慢性轻度炎症。研究表明,hs-CRP是健康人群中独立预测心肌梗塞、卒中、外周动脉病变和心源性猝死的指标,也是已知冠心病患者未来心血管病发病和死亡的预测指标[1,6]。哮喘是涉及多种炎症细胞相互作用、许多介质和细胞因子参与的一种慢性气道炎症疾病,其中参与炎症的细胞因子如白细胞介素1(IL-1)、白细胞介素6(IL-6)等和核因子能调节hs-CRP的水平[6,7]。研究表明,哮喘患者的hs-CRP水平显著高于健康对照组的水平,非控制哮喘患儿血清中hs-CRP水平显著高于哮喘控制的和健康的儿童[9]。成年人hs-CRP水平与哮喘的存在和严重程度呈正相关[10-14],但 hs-CRP水平在非控制和受控的哮喘患者之间的比较研究尚不多。本研究的目的旨在评估hs-CRP水平与哮喘控制测试(ACT)评分之间的关系。
1 资料与方法
1.1 一般资料
45例哮喘患者为2007年1月至2010年9月在本院住院的患者。其中,男性36例,女性9例;年龄52.2岁(19~72岁);轻度哮喘19例,中度哮喘26例。45例对照者为同期健康体检者,其中,男性29例,女性16例;年龄49.5岁(22~71岁)。两组间性别构成及年龄差异均无统计学意义(P=0.15,0.69)。哮喘的诊断和严重程度的评估根据GINA指南[14]。所有对象都进行了病史采集、体检、哮喘评估、呼吸功能测试(RFT)、ACT评分和血清hs-CRP水平测量。
1.2 方法
1.2.1 血清hs-CRP的测定
采清晨空腹静脉血3ml,室温静置30min后分离血清。采用日立7600全自动生化分析仪,免疫比浊法测定hs-CRP。hs-CRP试剂为英国朗道公司产品。
1.2.2 RFT的检测
采用ZJ15AS-7肺功能检查仪,记录用力肺活量(FVC)、第1秒用力呼气容积(FEV1)及呼气峰流速(PEF)。
1.2.3 ACT评分
[15,16],回忆过去4周内与哮喘病症相关情况,回答5个问题,每个问题有5个选项,分别对应1、2、3、4、5分。各得分相加即为 ACT评分,可用于准确评估哮喘控制情况。
1.3 统计学方法
用SPSS 16软件进行统计,数据以中位数(最小值-最大值)表示。两个独立群体之间的差异分析用Mann-Whitney U检验,相关性分析采用Spearman秩相关检验。P<0.05为差异有统计学意义。
2 结果
2.1 哮喘患者和对照者的hs-CRP及RFT结果比较
哮喘患者的 hs-CRP、FEV1%、PEF、PEF%显著高于对照者(表1)。
2.2 血清hs-CRP水平与哮喘严重程度之间的关系
轻度和中等哮喘患者的血清hs-CRP水平分别为0.70(0.15~5.6)和2.19(0.35~10.1),中度哮喘组的血清hs-CRP水平显著高于轻度哮喘的hs-CRP水平(P=0.03)。hs-CRP水平与哮喘的严重程度之间呈显著正相关(R=0.32,P=0.03)。然而,hs-CRP 与 FEV1(R =-0.13,P =0.56)、FEV1%(R=0.03,P=0.94)、PEF(R=0.16,P=0.45)、PEF%(R=-0.12,P=0.92)无相关性。
2.3 Hs-CRP和ACT评分之间的关系
哮喘患者的ACT评分为17.5(7~25)。依据ACT评分将哮喘患者分为两组,其中,ACT评分≥20者15例,ACT评分<20者30例。ACT评分<20组的 HS-CRP水平为2.19(0.31~10.4),显著高于 ACT≥20组的0.5(0.15~3.28)(P=0.01)。Hs-CRP水平与 ACT 评分(R=-0.81,P<0.001)呈现显著负相关。
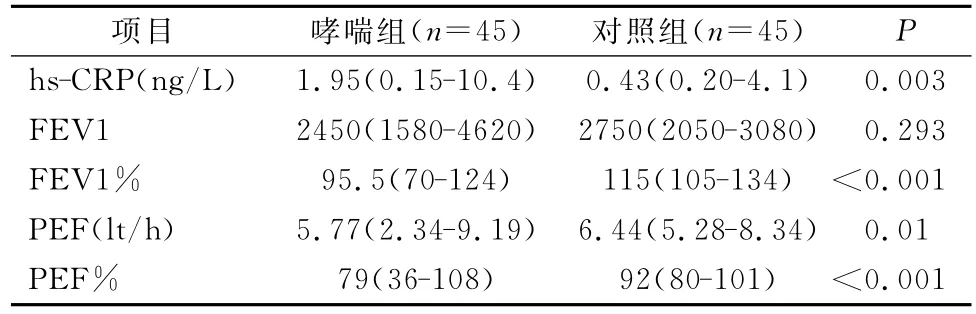
表1 哮喘患者和对照者的hs-CRP及RFT结果比较研究
3 讨论
Hs-CRP水平与哮喘的严重程度的关系已被众多研究证明[21-22]。我们的研究显示hs-CRP和哮喘的严重程度呈正相关,hs-CRP水平和ACT评分之间呈负相关,但在hs-CRP水平与RFT的参数之间没有相关性。hs-CRP水平与哮喘的严重程度之间的关系表明hs-CRP是一个促炎症因子。不同的炎性细胞因子如IL-1、IL-6、白细胞介素8(IL-8)和白细胞介素8(IL-18)可被hs-CRP激活,从而进一步加重哮喘炎症。最近的一项研究证明在严重的哮喘组和无任何呼吸道症状的对照组之间hs-CRP水平差异有统计学意义[26]。我们的研究表明中度哮喘患者的hs-CRP水平明显高于轻度哮喘患者的hs-CRP水平。但我们没能发现哮喘患者的hs-CRP水平与RFT参数之间有显著关系,这与以往的报道一致[21,27,28],提示hs-CRP不宜作为评估和预测 RFT的相关指标。
哮喘症状是哮喘控制的必需因素,因此评估炎症和哮喘症状之间的关系就显得很重要。研究发现,CRP水平增加与呼吸系统症状如喘息、气急和夜间咳嗽之间有显著关系[31,32]。哮喘患者和非哮喘患者的系统炎症标志物水平均较健康者的系统炎症标志物水平高,且在哮喘患者中显著增高[34]。这些研究表明,哮喘症状和炎症之间有明确的相关关系,大部分的研究表明未控制哮喘的炎症较控制哮喘的炎症重[25]。治疗哮喘的主要目标是有效控制急性发作症状并维持最轻的症状,甚至无任何症状。评价哮喘的严重程度和控制有许多种方法,如哮喘控制测试 (ACT)[16]和哮喘控制问卷的形式(ACQF)[35]。哮喘控制确定后,可以进行治疗。我们的研究采用ACT评分,发现哮喘患者血清hs-CRP水平与ACT评分之间呈负相关。hs-CRP水平在未控制哮喘与控制哮喘者间差异有统计学意义。
综上,本研究表明,hs-CRP水平与哮喘的严重程度和ACT评分相关,hs-CRP可以作为一个反映哮喘的严重程度和哮喘控制的敏感指标。
参考文献
[1]Sahoo RC,Acharya PR,Noushad TH,Anand R,et al.A study of high-sensitivity C-reactive protein in bronchial asthma[J].Indian J Chest Dis Allied Sci,2009,51(4):213-216.
[2]Pepys MB,Baltz ML.Acute phase protein with special reference to C-reactive protein and related proteins(pentaxins)and serum amyloid a protein[J].Adv Immunol,1983,34:141-212.
[3]Anderson GP.COPD,asthma and C-reactive protein[J].Eur Respir J,2006,27(5):874-876.
[4]Pepys MB,Hirshfield GM.C-reactive protein:a critical update[J].J Clin Invest,2003,111(12):1805-1812.
[5]Aziz N,Fahey JL,Detels R,et al.Analytical performance of a highly sensitive C-reactive protein-based immunoassay and the effects of laboratory variables on levels of protein in blood[J].Clin Diagn Lab Immunol,2003,10(4):652-657.
[6]Bassuk SS,Rifai N,Ridker PM.High-sensitivity C-reactive protein:clinical importance[J].Curr Probl Cardiol,2004,29(8):439-493.
[7]Elbehidy RM,Amr GE,Radwan HM.High sensitivity-C reactive protein as a novel marker for airway inflammation and steroid responsiveness in asthmatic children[J].Egypt J Bronchol,2010,4:79-87.
[8]Kony S,Zureik M,Driss F,et al.Association of bronchial hyperresponsiveness and lung function with C-reactive protein(crp):apopulation based study[J].Thorax,2004,59(10):892-896.
[9]Navratil M,Plavec D,Dodig S,et al.Markers of systemic and lung inflammation in childhood asthma[J].J Asthma,2009,46(8):822-828.
[10]Tracy RP.Inflammation in cardiovascular disease:cat,horse,or both?[J].Circulation,1998,97(20):2000-2002.
[11]Rifai N,Tracy RP,Ridker PM.Clinical efficacy of an automated high-sensitivity C-reactive protein assay [J].Clin Chem,1999,45(12):2136-2141.
[12]Roberts WL,Sedrick R,Moulton L,et al.Evaluation of four automated high-sensitivity C-reactive protein methods:implications for clinical and epidemiological applications[J].Clin Chem,2000,46(4):461-468.
[13]Roberts Wl,Moulton L,Law TC.Evaluation of nine automated high sensitivity C-reactive protein methods:implications for clinical and epidemiological applications[J].Clin Chem,2001,47(3):418-425.
[14]Ridker PM,Hennekens CH,Buring JE,Rifai N.C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women[J].N Engl J Med,2000,342(12):836-843.
[15]Global Initiative For Asthma(GINA).Global Strategy For Asthma Management And Prevention.Nhlbi/Who Workshop Report.National Institute Of Health.National Heart,Lung And Blood Institute.Revised 2007.
[16]Nathan RA,Sorkness CA,Kosinski M,et al.Development of the asthma control test:a survey for assessing asthma control[J].J Allergy Clin Immunol,2004,113(1):59-65.
[17]Takemura M,Matsumoto H,Niimi A,Ueda T,et al.High sensitivity C-reactive protein in asthma[J].Eur Respir J,2006,27(5):908-912.
[18]Bochner BS,Busse WW.Allergy and asthma[J].J Allergy Clin Immunol,2005,115(5):953-959.
[19]Joppa P,Petrasova D,Stancak B,et al.Systemic inflammation in patients with COPD and pulmonary hypertension[J].Chest,2006,130(2):326-333.
[20]Malo O,Sauleda J,Busquets X,et al.Systemic inflammation during exacerbations of chronic obstructive pulmonary disease[J].Arch Bronconeumol,2002,38(4):172-176.
[21]Qian FH,Zhang Q,Zhou LF,eta l.High-sensitivity C-reactive protein:apredictive marker in severe asthma [J].Respirology,2008,13(5):664-669.
[22]Sävykoski T,Harju T,Paldanius M,et al.Chlamydia pneumoniae infection and inflammation in adults with asthma[J].Respiration,2004,71(2):120-125.
[23]Pepys MB,Hirschfield GM,Tennent GA,et al.Targeting C-reactive protein for the treatment of cardiovascular disease.Nature,2006,440(7088):1217-1221.
[24]Ballou SP,Lozanski G.Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein[J].Cytokine,1992,4(5):361-368.
[25]Tillie-Leblond I,Montani D,Crestani B,et al.Relation between inflammation and symptoms in asthma[J].Allergy,2009,64(3):354-367.
[26]Giannini D,Di Franco A,Cianchetti S,et al.Analysis of induced sputum before and after withdrawal of treatment with inhaled corticosteroids in asthmatic patients[J].Clin Exp Allergy,2000,30(12):1777-1784.
[27]Büyüköztürk S,Gelincik AA,GençS,et al.Acute phase reactants in allergic airway disease[J].Tohoku J Exp Med,2004,204(3):209-213.
[28]Ramirez D,Patel P,Casillas A,et al.Assessment of highsensitivity C-reactive protein as a marker of airway inflammation in asthma[J].Ann Allergy Asthma Immunol,2010,104(6):485-489.
[29]Fujita M,Ueki S,Ito W,et al.C-reactive protein levels in the serum of asthmatic patients[J].Ann Allergy Asthma Immunol,2007,99(1):48-53.
[30]Kim MH,Kim SH,Kang HR,Park HW,Chang YS,Kim DH,et al.High-sensitivity C-reactive protein as a marker of bronchial hyperresponsiveness and lung function [J].J Asthma Allergy Clin Immunol,2009,29:112-116.
[31]Kony S,Zureik M,Driss F,et al.Association of bronchial hyperresponsiveness and lung function with C-reactive protein(Crp):apopulation based study[J].Thorax,2004,59(10):892-896.
[32]Qlafsdottir IS,Gislason T,Thjodleifsson B,et al.C-reactive protein levels are increased in non-allergic but not allergic asthma:a multicentre epidemiological study[J].Thorax,2005,60(6):451-454.
[33]Lönnkvist K,Hellman C,Lundahl J,et al.Eosinophil markers in blood,serum,and urine for monitoring the clinical course in childhood asthma:impact of Budesonide treatment and withdrawal[J].J Allergy Clin Immunol,2001,107(5):812-817.
[34]RytiläP,Metso T,Heikkinen K,et al.Airway inflammation in patients with symptoms suggesting asthma but with normal lung function[J].Eur Respir J,2000,16(5):824-830.
[35]Juniper EF,Buist AS,Cox FM,et al.Validation of a standardized version of Quality Of Life Questionnaire[J].Chest,1999,115(5):1265-1270.

