不对称大环双核Ni(Ⅱ)配合物的DNA切割性能研究
丁 超 陈云峰 刘 明 宋会婷 周 红 潘志权
(武汉工程大学绿色化工过程教育部重点实验室,武汉 430073)
不对称大环双核Ni(Ⅱ)配合物的DNA切割性能研究
丁 超 陈云峰 刘 明 宋会婷 周 红 潘志权*
(武汉工程大学绿色化工过程教育部重点实验室,武汉 430073)
本文利用模板导向的方式合成了一种含有不对称侧链的大环双核金属Ni(Ⅱ)配合物(大环配体由3-溴甲基-5-甲基水杨醛,乙二胺,N,N-二(氨丙基)-2呋喃甲胺分步合成得到),其结构通过红外光谱、元素分析、X-射线单晶衍射进行了表征。利用紫外光谱、分子荧光和粘度试验对配合物与DNA的相互作用进行了研究,结果表明配合物与CT-DNA的结合常数K=4.8×104mol-1·L荧光淬灭常数Ksv=7.12×103mol-1·L;同时机理研究表明配合物与CT-DNA结合方式为插入模式。
同双核配合物;大环配合物;结合常数;粘度;DNA切割
Thechemistryofmulti-metalcomplexesasartificial nucleases has received much.attention because of their generally higher DNA cleavage activity provided that the ligand hold the metal centers in an appropriate geometry[1]and their importance in cancer therapy[2]as well as in molecular biology[3].In general,the design of the new ligand is the key to find high biological activity metal complexes. The macrocyclic polyamine compounds have been demonstrated to be popular ligand for researches on biological inorganic chemistry because of their multifarious geometry and the strong ability to stabilize of the central metal ions[4].It was demonstrated that the macrocyclic polyamine Zn(Ⅱ)and Cu(Ⅱ)homodinuclear or heterodinulcear complexesshow good activities in the binding and the cleavage DNA[5].In general,the method for synthesis of macrocyclic polyamine metal complexes is one-pot condensation of diamine with dialdehyde,which gives the symmetric macrocyclic polyamine frameworks[6].By contrast,the researches about the synthesis and biological activities of the unsymmetrical macrocyclic polyamine complexes were rarely reported because of the complexity and difficulty oftheirsynthesis.However,it was found that the substitutents in amines play an important role on the biological activity for their metal complexes.Previously,our group reported the synthesis of a new nickel(Ⅱ)complex of a symmetrical side chains macrocyclic polyamine ligand by one-pot template synthesis,in which the complex was shown to have an efficient DNA binding activity[7].Within our ongoingstudieson therelationship between the structure and the biological activities of macrocyclic polyamine complexes,a nickel(Ⅱ)complex of an unsymmetricalsidechainsmacrocyclicpolyamine ligand is synthesized by a stepwise macrocyclization,and the DNA binding activities are also studied in this paper.The synthesis of the unsymmetrical macrocyclic polyamine Ni(Ⅱ)complex is shown in Scheme 1.

Acetate ligand is omitted for clarityScheme 1 Synthesis of the title complex
1 Experimental
1.1 Materials and preparation
All chemicals were purchased from commercial sources and used as received.Solvents were dried according to standard procedures and distilled prior to use.3-(bromomethyl)-2-hydroxy-5-methylbenzaldehyde,N1,N2-dibenzylethane-1,2-diamine and N1-(3-aminopropyl)-N1-(2-(furan-2-yl)ethyl)propane-1,3-diaminewere synthesized according to a procedure reported previously[8].
1.2 Physical measurement
IR spectra were recorded on a vector 22 FIIR spectrophotometer using KBr disk.1H NMR spectra were recorded on a Varian Mercury VX-400 spectrometer with TMS as the internal reference.Elemental analyses were performed on a Perkin-Elmer 240 analyzer.Electrospray mass spectra(ES-MS)were determined on a Finnigan LCQ ES-MS mass spectrograph using methanol as the mobile phase with a sample concentration of about 1.0 mmol·dm-3.UV-Vis spectra were recorded on an UV-2450 spectrophotometer.Circular dichroic spectra of DNA were obtained using a Jasco J-810 spectropolarimeter.Fluorescence spectra were recorded on a Jasco FP-6500 spectrophotometer.
1.3 Preparation of the complexes
1.3.1 Synthesis of 3,3′-(ethane-1,2-diylbis(benzylazanediyl))bis(methylene)bis(2-hydroxy-5-methyl-benzaldehyde)
To a solution of 3-(bromomethyl)-2-hydroxy-5-methylbenzaldehyde (13.0 g,0.055 mol)in THF(150 mL)was added K2CO3(20.7 g,0.15 mol),then a solution of N1,N2-dibenzylethane-1,2-diamine(0.025 mol)in THF (200 mL)was added by dropwise.The mixture was stirred overnight at room temperature and then the precipitate was filtered.After removal of the solvent,the residue was added an aqueous solution of 32%HCl(15 mL).The mixture was then extracted with methylene chloride(3×150 mL).The obtained aqueous phase was made alkaline by addition of a saturated solution of NaHCO3and the resultant mixture wasextracted with methylene chloride (3 ×150 mL).The combined CH2Cl2fractions were dried overwith anhydrous Na2SO4.After filtration and evaporation,the residue was purified by recrystallization from acetone gave the product as white solid(4.85 g,yield:36.2%).1H NMR (400 Hz,CDCl3),10.16(s,2H),7.17~7.35(m,14H),3.59(s,4H),3.51(s,4H),2.65(s,4H).13C NMR(100 Hz,CDCl3),193.1,158.6,137.2,129.3,129.2,128.3,127.4,124.9,121.6,58.5,54.9,53.9,50.1,20.2.
1.3.2 Preparation of the complexes[Ni2L(μ-OAc)]
ClO4·CH3OH
To a solution of 3,3′-(ethane-1,2-diylbis(benzylazanediyl))bis(methylene)bis-(2-hydroxy-5-methylbenzaldehyde)(0.2 mmol,0.107 g)in absolute methanol(10 mL)was added a solution of hydrated nickel acetate(0.2 mmol,0.05 g).The solution was stirred vigorously while a methanol solution (10 mL)containing N1-(3-aminopropyl)-N1-(furan-2-ylmethyl) propane-1,3-diamine (0.2 mmol,0.0422 g)was added by dropwise over a period of 2 h,the resulting solution was stirred for another 3 hat ambient temperature following by the addition of 1 mL triethylamine.Then the nickel(Ⅱ)perchlorate hexahydrate (0.2 mmol,0.067 7 g)was added to above solution,the resulting mixture was stirred for 4 hours.The resulting solution was allowed to evaporate slowly,the tea green crystals was collected,washed with ether,dried in air(yield:0.152 g,57%),Anal.found(%):C,57.6;H,5.3;N,6.2.calcd.(%):C 56.7;H 5.7;N 6.9.IR(KBr,cm-1):2 924,1 621,1 568,1 462,1 310,1 088,742.The green block crystals of[Ni2L(μ-OAc)]ClO4·CH3OHsuitableforX-raydiffraction were obtained by slow evaporation of the clarified solution at ambient temperature for one week.
1.4 Determination of the crystal structure
Diffraction intensity data were collected on a SMART CCD area-detector diffractometer at 298 K using graphite monochromatic Mo Kα radiation(λ=0.071073 nm).Date reduction and cell refinement were performed by SMART and SAINT programs[9].The structure was solved by direct methods(Bruker SHELXTL)and refined on F2by full-matrix least squares(Bruker SHELXTL)using all unique data[10].The non-H atoms in the structure were treated as anisotropic.Hydrogen atoms were located geometrically and refined in a riding mode.
1.5 DNA binding experiments
CT-DNA(20 mg)was dissolved in 100 mL of Tris-HCl buffer(50 mmol·L-1Tris-HCl,50 mmol·L-1NaCl,pH=7.4)and keep at 4 ℃ for less than 4 d.A260,A280of the above solution were determined on UV-Vis spectrophotometer and A260/A280should be between the ranges of 1.8~2.0.The DNA concentration was determined via absorption spectroscopy using the molar absorption coefficient of 6600 L·mol-1·cm-1(260 nm)for CT-DNA[11].
The nickel complex was dissolved in DMF at a concentration of 5.0×10-5mol·L-1.The UV absorption titrations were performed by keeping a concentration of the complex while varying the DNA concentration.Complex-DNA solutions allowed to incubate for 30 min before measurements were made.The intrinsic binding constant Kbwas calculated according to the literature[12].Fluorescence quenching experiments were performed by adding the solution of complex (1.5 μL)into EB-bound CT-DNA solution (1.5 μL)atdifferent concentrations(from 0 to 200 μmol·L-1).All samples were excited at 520 nm,and emission was recorded at 450~750 nm.
Circular dichroism (CD)spectra of CT-DNA were recorded in the absence and presence of the complexes.The CD spectra were run from 320 to 220 nm at a speed of 300 nm·min-1.Data were recorded at an interval of 0.1 nm.The CD spectrum of CT-DNA alone was recorded as the control experiment.
Viscosity measurements of CT-DNA were carried outusing a capillary viscometerata constant temperature(23℃±0.1℃).Flow times were measured with a digital stopwatch and each sample was measured three times and an average flow time was calculated.Data are presented as(F/F0)1/3vs molar ratio of complex to DNA[13],where F is the viscosity of DNA in the presence of complex and F0is the viscosity of DNA in the absence of complex.
1.6 DNA photocleavage experiments
The cleavage of pBR322 DNA by the complexes was examined by gel electrophoresis experiments.Negative supercoiled pBR322 DNA (0.5 μL,0.5 μg·μL-1)was treated with different concentration of complexes(1 μL)in Tris-HCl buffer(1 μL,50 mmol·L-1Tris-HCl,50 mmol·L-1NaCl,pH=7.2).After mixing,the DNA solutions were incubated at 37℃ for 3 h.The reactions were quenched by the addition of sterile solution(1 μL,0.25%bromophenol blue and 40%w/v sucrose).The samples were then analyzed by electrophoresis for 1 h at 80 V on agarose gel in TAE buffer(40 mmol·L-1Tris-base,40 mmol·L-1acetic acid and 1 mmol·L-1EDTA,pH=7.4).The gel was stained with EB(1 μg·μL-1)for 0.5 h after electrophoresis and then photographed.
2 Results and discussion
An unsymmetrical macrocyclic dinuclear Ni(Ⅱ)complex was synthesized by Schiff base condensation of 3,3′-(ethane-1,2-diylbis(benzylazanediyl))bis(methylene)bis(2-hydroxy-5-methyl-benzaldehyde)with N1-(3-aminopropyl)-N1-(2-(furan-2-yl)ethyl) propane-1,3-diamine in the presence of Ni(OAc)2·4H2O and Ni(ClO4)2·6H2O.The synthetic pathway to the complex is shown in Scheme 1.In the IR spectrum of the complex,the sharp C=N stretching vibration corresponding to the imine groups of the macrocyclic framework is observed at 1621 cm-1,indicating that amacrocyclic complex has been synthesized.The ES-MS spectrum of the nickel(Ⅱ)complex in methanol solution is dominated by a peak at m/z 886.63,corresponding to[Ni2(OAc)L]+(C49H57O7N5Ni2,calcd.886.39).
2.1 Crystal structure of[Ni2L(μ-OAc)]ClO4·CH3OH
A perspective view of[Ni2L(μ-OAc)]ClO4·CH3OH is given in Fig.1,together with the atom labeling scheme.Crystallographic data and details about the data collection are presented in Table 1.This metal macrocycle exhibits a twisted molecular clip conformation,where two phenol motifs serve as two sidewalls and two metal ions serve as the linkers.Two benzyl groups and 2-furanylmethyl group are located in the convex of the clip.
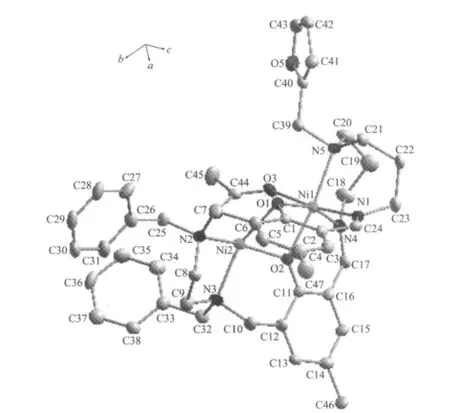
Fig.1 Molecular structure of the title complexe with ellipsoid drawn at 30%probability
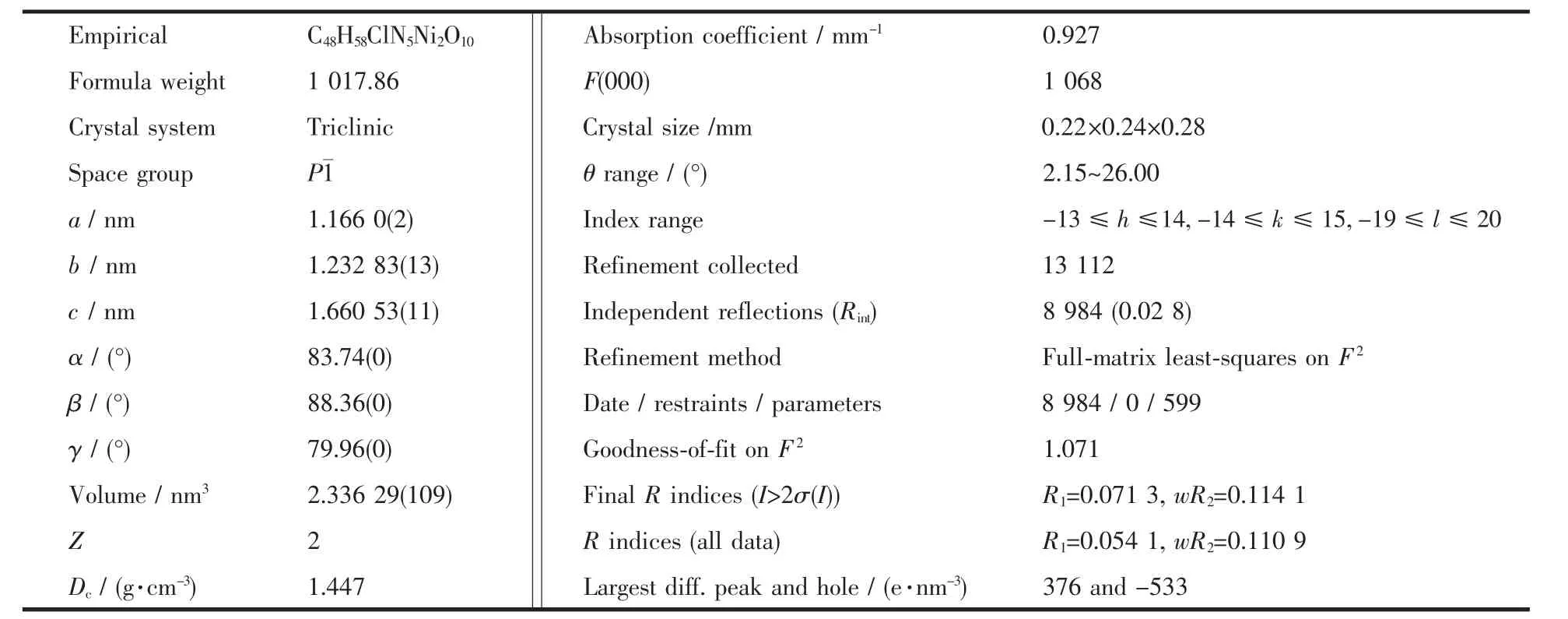
Table 1 Crystal data and details of the structure determination for the title complexe
CCDC:835716.where two phenolic oxygen and one μ2-OAc linked two nickel(Ⅱ) atoms,the distance of two nickel(Ⅱ) atoms is about 0.3038 nm.However,the two Ni2+were located in different coordination environments,one Ni2+coordinated with another two nitrogen atoms to form a fivecoordinate square pyramid configuration,whereas the other one coordinated with another three nitrogen atoms to give a six-coordinate distorted octahedral configuration.The selected bond lengths and angles relevant to the nickel(Ⅱ)coordination spheres of the complex are listed in Table 2.

Table 2 Selected bond distances(nm)and angles(°)for nickel complex
2.2 DNA-binding activity
The absorption spectra of the complex in the absence and presence ofCT-DNA atdifferent concentrations (0~150 μmol·L-1)are given in Fig.2.The spectrum of the complex shows a very strong absorption at 371 nm,which is attributed to a metal-toligand charge transfer[14].The band just shows a litter bathochromic shift of about 1 nm together with a hypochromism of 32.6%after adding DNA.The value of Kbwas obtained from the ration of slope to the intercept from the plot of cDNA/(εa-εf)versus cDNA(Fig.3).The Kbvalue is 4.8×104mol-1·L,is larger than previousreported symmetric dinuclearnickel(Ⅱ)complex[7].These findings support the complex binds to DNA by a moderate intercalation.
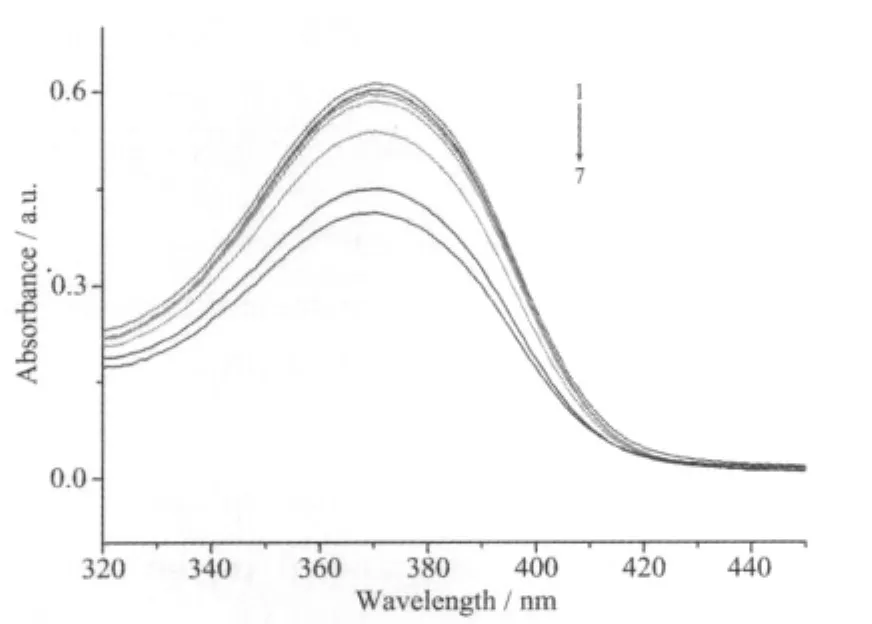
Fig.2 UV-visible spectra of the Ni(Ⅱ) complex in thepresence of increasing amounts of CT-DNA

Fig.3 Plot of cDNA/(εa-εf)versus cDNAfor absorption titration of CT-DNA with the complex
The mixed solutions of DNA and EB show very strongly enhanced fluorescence emission because of the intercalation of EB to DNA,so if the additive can displace the EB to bind to DNA,the emission will be reduced[15].When the Ni(Ⅱ) complex was added to the EB-DNA system,a significant reduction in fluorescenceintensity of the EB-DNA solution was observed,the emission spectra of EB bound to DNA in the absence and the presence of the complex are given in Fig.4,and Stern-Volmer quenching plot show the corresponding quenching constant K is 7.12 ×103mol-1·L(Fig.5),which is very close to the previous results[7],suggesting the complex have a moderate ability to intercalate DNA.
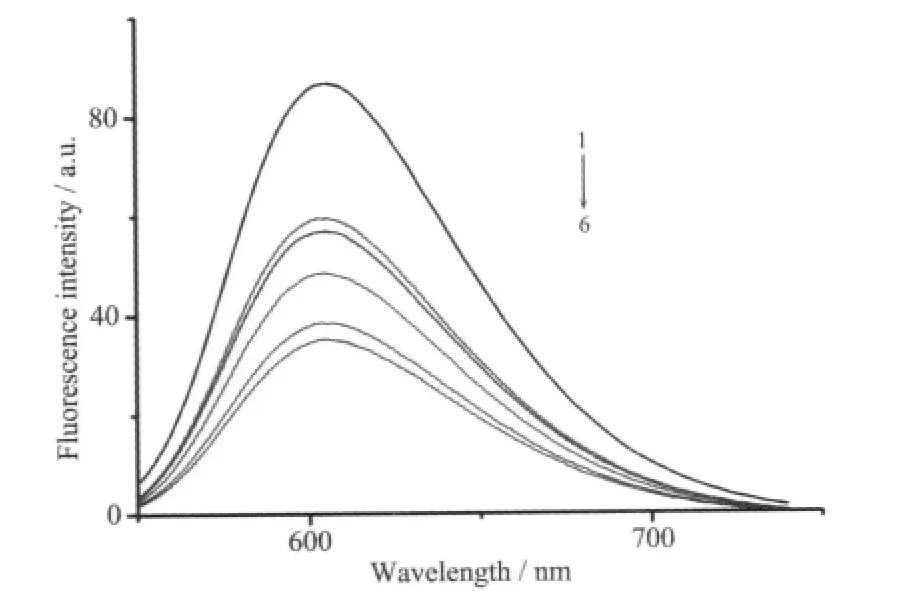
Fig.4 Emission spectra of EB bound to DNA in the absence(1)and presence(2~6)of the complex

Fig.5 Stern-Volmer quenching plot of EB bound to DNA by Ni(Ⅱ)complex
The effect of the complex by circular dichrosim spectroscopy was also investigated.In general,the CTDNA is normally present in the B-DNA form and shows a negative CD band at 245 nm caused by the helical conformation and a positive CD band at 275 nm due to base stacking[16].In this case,we found a positive bands increase in intensity (Fig.6)when the complex was added to the CT-DNA solution,which suggested that the complex can unwind the DNA helix.

Fig.6 Circular dichromism spectra of CT-DNA in the absence(a)and presence of the complex(b)
A viscosity study was also carried out to further confirm the interaction mode of the complex with DNA(Fig.7).An obvious increase of viscosity of the DNA with increasing concentration of the complex was found,this again suggested that the binding mode between the complex and DNA may be intercalation[17].
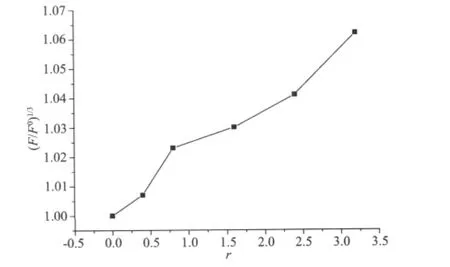
Fig.7 Effects of increasing amounts of the complex on the relative viscosities of CT-DNA
2.3 DNA cleavage activity
The cleavage of supercoiled plasmid pBR322 DNA by the complex was studied in the absence of H2O2or other reducing agents.As shown in Fig.8,with the increasing concentrations of the complex,the amount of Form Ⅰ (supercoiled form)of DNA diminished obviously,whereas FormⅡ (nicked form)increased.In contrast with previous results,a new form(FormⅢ)is also appeared in this case especially with the increasing concentrations of the complex.We propose this form is the smaller nicked DNA fragment.These results suggest that this complex have a larger DNA cleavage efficiency than the symmetric dinuclear Ni(Ⅱ) macrocyclic complex[7].At the same time,time dependence of the cleavage ability of DNA with the complex reveal that the cleavage of DNA increased with the increasing incubation time(Fig.9).Furthermore,the comparison experiments with typical scavengers also identify that this complex cleave the DNA by a hydrolytic pathway not by an oxidative pathway(Fig.10).

Fig.8 Agarose gel electrophoresis of pBR322 plasmid DNA in the presence of different concentrations of the complex

Fig.9 Time dependence of the cleavage of DNA with the complex

Fig.10 Agarose gel showing cleavage of pBR322 DNA incubated with the complex
3 Conclusions
In conclusion,an unsym metricalside chain macrocyclic dinuclear nickel(Ⅱ)complex has been synthesized and structurally characterized.The biological activities of this complex towards CT-DNA were studied by UV absorption, fluorescent spectroscopy,CD spectra and viscosity measurements.This complex binds to DNA by a moderate intercalation similar to the previous reported the symmetrical side chains macrocyclic polyamine dinuclear nickel(Ⅱ)complex,and this complex shows a litter better efficient cleavage activity towards supercoiled DNA(pBR322 DNA)in the absence of any external agents.These slight differences may be ascribed to their similar coordination environments of the metal ions.The detailed relationship between structure and biological activates is underway in our lab.
[1](a)Jiang Q,Xiao N,Shi P F,et al.Coord.Chem.Rev.,2007,251(16):1951-1972(b)Mancin F,Scrimin P,Tecilla P,et al.Chem.Comm.,2005,20:2540-2548(c)Prusis P,Dambrova M,Andrianov V,et al.J.Med.Chem.,2004,47:3105-3110(d)Ren S,Wang R,Komatsu K,et al.J.Med.Chem.,2002,45:410-419(e)Liu Q,Zhang J,Wang M Q,et al.J.Eur.Med.Chem.,2010,45:5302-5308(f)Zhao Y M,Zhu J H,Guo Z J,et al.Chem.Eur.J.,2006,12:6621-6629(g)Li J H,Wang J T,Zhang L Y,et al.Inorg.Chem.Acta,2009,362:1918-1924(h)Rao T,Zhou H,Pan Z Q,et al.Transition.Met.Chem.,2010,35:985-989
[2](a)Marzano C,Pellei M,Tisato F,et al.Anti-cancer Agent Me.,2009,9:185-211(b)Teicher B A.Biochem.Pharmacol.,2008,75:1262-1271(b)Shao R G,Zhen Y S.Anti-cancer Agent Me.,2008,8:123-131(c)Kiehlbauch J A,Hannett G E,Salfinger M,et al.J.Clin.Microbiol.,2000,38:3341-3348
[3](a)Chmielewski P J,Latos-Grazynski L.Coord.Chem.Rev.,2005,249:2510-2533(b)Schroder F C,Farmer J J,Attygalle A B,et al.Science.,1998,281:428-431(c)Malina J,Hannon M J,Brabec V.Chem.Eur.J.,2008,14:10408-10414(d)Otero L,Smircich P,Vieites M,et al.J.Inorg.Biochem.,2007,101:74-79
[4](a)Vigato P A,Tamburini S,Beltolo L.Coord.Chem.Rev.,2007,251:1311-1492(b)Mewis R E,Archibald S J.Coord.Chem.Rev.,2010,54:1686-1712(c)Yu G F,Zhang J,Yu X Q,et al.Bioorg.Med.Chem.,2007,15(2):696-701(d)Huang Q D,Chen H,Zhou L H,et al.Chem.Biol.Drug.Des.,2008,71:224-229
[5](a)Chen T T,Wang X Y,Wang J J,et al.Inorg.Chem.,2009,48:5801-5809(b)Bazzicalupi C,Bencini A,Biagini S,et al.Chem.Eur.J.,2009,15:8049-8063(c)Benetollo F,Di Bernardo P,Tamburini S,et al.Inorg.Chem.Comm.,2008,11:246-251(d)Li K,Zhou L H,Yu X Q,et al.J.Eur.Med.Chem.,2009,44:1768-1772(e)Anbu S,Kandaswamy M,Varghese B,et al.J.Inorg.Biochem.,2009,103:401-410
[6](a)BAI-Jun Lin(白君林),ZHOU-Hong(周红),PAN Zhi-Quan(潘 志 权),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24:1994-2001(b)Luo Y X,Lu L D,Qian M,et al.Transition.Met.Chem.,2002,27(5):469-472(c)Zeng Q D,Qian M,Gou S H,et al.Inorg.Chim.Acta,1999,294:1-7[7]Hu H,Chen Y F,Pan Z Q,et al.Transition.Met.Chem.,2011,36:395-402
[8](a)Hamid G,Hamid R M.Bull.Chem.Soc.Ethiop.,2010,24:151-155(b)Azim G,Hamid G,Rahman H.J.Chin.Chem.Soc.,2005,52:531-534
[9]Smart and Saint Area Detector Control and Integration Software,Siemens Analytical X-Ray Systems Inc,Madison,1996.
[10]Sheldrick G M.SHELXTL V5.1 Software Reference Manual,Bruker AXS Inc.,Madison,1997.
[11]Reichmann M E,Rice S A,Doty P,et al.J.Am.Chem.Soc.,1954,76:3047-3053
[12]Pyle A M,Qian M,Gou S H,et al.Inorg.Chim.Acta,1999,294:1-7
[13]Cohen G,Eisenberg H.Biopolymers,1969,8:45-49
[14](a)Neves A,de Brito M A,Vencato I,et al.Inorg Chem.,1996,35:2360-2368(b)Gaber B P,Miskowski V,Spiro T G.J.Am.Chem.Soc.,1974,96:6868-6873
[15]Meyer-Almes F J,Porschke D.Biochem.,1993,32:4246-4253
[16]Maheswari P U,Palaniandavar M.J.Inorg.Biochem.,2004,98:219-230
[17](a)Sun J,Yan A,Liu J,et al.Polyhedron.,2008,27:2845-2850(b)Liu J G,Ye B H,Li J N,et al.J.Inorg.Biochem.,1999,76:266-271
Studies on an Unsymmetrical Macrocyclic Dinuclear Ni(Ⅱ)Complex Interaction with DNA
DING Chao CHEN Yun-Feng LIU Ming SONG Hui-Ting ZHOU Hong PAN Zhi-Quan*
(Key Laboratory for Green Chemical Process of Ministry of Education,Wuhan Institute of Technology,Wuhan 430073,China)
An unsymmetrical side chains macrocyclic dinuclear Ni(Ⅱ)complex has been obtained by templatedirected synthesis (the macrocyclic ligand was synthesized by 3-bromomethyl-2-hydroxy-5-methylbenzaldehyde,ethane-1,2-diamine,and N1-(3-aminopropyl)-N1-(furan-2-ylmethyl)propane-1,3-diamine),the structure has been characterized by IR spectrum,elemental analysis and X-ray crystallography determination.The interactions of the complex with DNA have been measured by UV spectroscopy,fluorescence spectroscopy and viscosity experiments.Absorption spectroscopic investigation reveals of intercalative binding of the complex with DNA with a binding constant of 4.8×104mol-1·L.Fluorescence spectroscopy shows that the complex displaces EB and bind to DNA with a quenching constant of 7.12×103mol-1·L.The appearance of both increased CD bands nearing 371 nm gives evidence for effective complex-DNA binding.The agarose gel electrophoresis studies show that the complex displays effective DNA cleavage activity in the absence of any external agents.CCDC:835716.
homodinuclear complex;macrocyclic complex;binding constant;viscosity;DNA cleavage
O614.81+3
A
1001-4861(2012)04-0801-08
2011-09-22。收修改稿日期:2011-10-28。
国家科学自然基金(No.20971102)和湖北省教育厅中青年人才项目(No.Q20111507)资助。
*通讯联系人。E-mail:zhiqpan@163.com

