制备方法对锰Mn掺杂钾六铝酸盐催化剂催化甲烷燃烧性能的影响
郑建东任晓光葛秀涛
(1滁州学院材料与化工学院,滁州 239000)
(2北京石油化工学院,北京 102617)
制备方法对锰Mn掺杂钾六铝酸盐催化剂催化甲烷燃烧性能的影响
郑建东*,1任晓光2葛秀涛1
(1滁州学院材料与化工学院,滁州 239000)
(2北京石油化工学院,北京 102617)
以甲烷催化燃烧为目标反应,通过共沉淀法、溶胶凝胶法和反相微乳液法制备了Mn掺杂六铝酸盐催化剂,用XRD和TG-DTA技术对催化剂进行了物理性能表征,通过BET模型计算了其比表面积。结果说明3种方法所制备催化剂经1 200℃焙烧4h后均可以形成完整的六铝酸盐晶型,同时都具有高的催化性能和高温稳定性,其中反相微乳液法制备的K2MnAl11O19催化剂具有较高的比表面积和甲烷催化燃烧活性,起燃温度T10%为458℃,至676℃甲烷完全转化。
材料科学;六铝酸盐;催化活性
0 Introduction
The world is facing the ever-increasing challenges of energy shortage and environmental deterioration,mainly resulting from an over-dependence of our society on fossil energy[1].As the temperature of flame combustion raises,nitrogen oxide emission is increased.NOxcan cause atmospheric pollution,especially acid rain.The catalytic combustion is an effective method to suppress the nitrogen oxide emission from combustors.This potential application has attracted many researchers in recent decades.Catalytic combustion of hydrocarbons is an important technology both for energy production and for environmental pollution abatement.For heat generation process using natural gas as fuel,catalytic combustion instead ofthe conventional combustion has several advantages such as having higher efficiency and demanding lower temperature which effectively suppresses thermal NOx formation[2-4].
In order to obtain a high energy transforming efficiency and low emission of air pollutants,the catalyst with excellent ignition activity and high heatresistant is still urgently needed[5-10].Noble metal catalysts have high catalytic activity.However,their high cost and low resistance are obvious disadvantages.Metal oxide catalysts are also not ideal in high temperature stability.Hexaaluminate is now considered as one of the most suitable materials for hightemperature catalytic combustion of methane due to its excellent thermal stability and high activity.
Hexaaluminate compounds containing alkali,alkaline earth,or rare earth metals have β-A12O3or magnetoplumbite-type crystal structure.This structure consists of alternated stacking of a spinel block with close packed oxide ions and a mirror plane with the large cation along the c axis[11-13].This structure results in high resistance to high temperature sintering,because the large cation in mirror plane can suppress the crystal growth along the c axis[14-16].Many Mnsubstituted hexaaluminate catalysts were investigated and experimental results indicated that they were the most efficient catalysts for combustion of methane[17-20].At the same time their catalytic activity could be further improved by mirror plane cation substitution with ions of approximate radius[21].
We report here a new hexaaluminate K2MnAl11O19prepared by co-precipitation method,sol-gel method or reverse microemulsion-mediated method to catalyze combustion ofmethane.The propertiesofthese catalysts were characterized by XRD,low temperature nitrogen adsorption-desorption and TG-DTA techniques.Their catalytic activities were evaluated for methane combustion in a fix bed micro-reactor.
1 Experimental
1.1 Preparation of materials
1.1.1 Co-precipitation method
K2MnAl11O19sample was prepared by coprecipitation method (carbonates route)[22].The appropriate amounts of potassium nitrate (0.516 g,0.005 1 mol,Sinopharm Chemical Reagent Co.,Ltd),manganese nitrate (0.457 g,0.915 mL)and aluminum nitrate(10.541 g,0.028 mol)solution were mixed(0.25 mol·L-1)and added into a well-stirred container by addition of(NH4)2CO3(6.805 g,0.028 mol)at constant temperature (90 ℃)and pH value of 7~8.After filtering and washing with water several times,the solid product was dried at 120℃for 3 h and then calcined at 1 200 ℃ in Muffle furnace for 4 h under air.The sample thus prepared is referred to as 1﹟hereafter.
1.1.2 Sol-gel method
The same K2MnAl11O19sample was also prepared by sol-gel method[23].Al(OC3H7)(99.5%,5.739g,0.028 mol,Beijing Reagents Chemicals)was dissolved in isopropanol (99.5%,100 mL,Beijing Reagents Chemicals).The solution was heated to 80 ℃ and kept for 3 h in dry N2.The solution was then cooled to room temperature.An aqueous solution of manganese nitrate and potassium was dropped to the isopropanol solution and a gel was formed rapidly.After kept 12 h at room temperature,the solvent was removed by evaporation under reduced pressure in a rotary evaporator.The obtained powder was dried for 3 h at 120℃in oven,and then calcined at 1 200 ℃ for 4 h in air.The sample thus prepared is referred to as 2﹟ hereafter.
1.1.3 Reverse microemulsion-mediated method
For comparison,the K2MnAl11O19sample was prepared by the microemulsion technique.Ionic surfactants may contaminate the system,therefore we use nonionic surfactant Triton X-100 as the surfactant,n-hexanol as the co-surfactant and cyclohexane as the oil phase.The microemulsion was composed of oil phase (34wt%),surfactant (23wt%),co-surfactant(21wt%)and water phase(22wt%)[5].Al(OC3H7)3(99.5%,5.739 g,0.028 mol)was dissolved in cyclohexane.An aqueous solution of manganese nitrate and potassium was added into the reverse microemulsion.After aging the precipitated particles for 24 h at room temperature,the suspended particles were recovered by means of centrifugation and washed with methanol in order toremove most of the surfactant.The obtained powder was dried for 24 h at 100℃in oven,and then calcined at 500℃for 3 h under oxygen flow to remove the surfactant.At last the powder was calcined at 1 200 ℃for 4 h under oxygen flow.The sample thus prepared is referred to as 3#hereafter.
1.2 Characterization
The phase composition of the calcined samples was determined by X-ray powder diffraction(XRD)(7000X diffractometer,Japan)using a Ni filter andCu Kα radiation(λ=0.154 18 nm),at 40 kV and 30 mA.The data were collected between 15°and 75°(the 2θ value range).The specific surface area and the pore volume of the samples were measured on an AUTOSORB-I-MP Series Instrument using N2adsorption at liquid N2temperature.The specific surface area was determined according to the Brunauer-Emmett-Teller theory and the analysis of the average pore diameter and pore volume was carried out according to BJH equation.
TG and DTA were carried out on a BÄHRSTA503 thermal analyzer at a constant heating rate of 10 ℃·min-1.
The reaction of methane combustion was carried out in a conventional microreactor under atmospheric pressure.Catalyst(300 mg,420~841 μm (20~40 mesh))was loaded in a quartz reactor (i.d.8 mm),with quartz wool sealed at both ends of the catalyst bed.A mixture of 1vol%methane and 99vol%air was fed into the catalyst bed at GHSV=50,000 h-1.The output gas compositions were analyzed by an on-line gas chromatography(GC9890,Instrument of shanghai Linghua Co.Ltd.)with a capillary column(30 m×0.32 mm×0.5 μm,LanZhou Institute of Chemical Physics,Chinese Academy of Sciences)and a flame ionization detector(Temperature of column:150℃,Temperature of injector:200℃,Temperature of detector:230℃).
2 Results and discussion
2.1 Crystalline phases
The XRD patterns of the samples are shown in Fig.1.One single hexaaluminate phase is formed after the catalysts are calcined at 1 200℃.We can see that three samples behave similarly.The microcrystalline Potassium hexaaluminate phase (PDF 84-0819)is the main component in three samples.The characteristic diffraction peaks of Potassium hexaaluminate are at 25.45°,5.73°,37.92°,43.22°,52.42°,57.72°and 66.79°.

Fig.1 XRD patterns for K2Mn Al11O19prepared by different methods
The diffraction intensity of K2MnAl11O19is weakened for the sample from carbonate precipitation method.The catalyst 3#has higher intensity than those of the others.The diffraction peaks of Mn oxides could not be observed.It reveals that the Mn ion could easily enter the hexaaluminate and occupy the proper lattice position to promote crystal shaping.
The diffraction peaks of K3AlO3is observed in 1#.It is worthy of noting that those spinel can form hexaaluminate via the solid state reaction with γ-Al2O3.At the same time there are no disturbing peaks in the X-ray diffractions of 2#and 3#.This implies that the preparation method is important in the formation of hexaaluminate crystal.Reverse microemulsionmediated method renders the precursormixture homogenous and enables facile mass transfer,resulting in a formation of pure hexaaluminate during subsequent calcination at 1 200℃.
The a and c unit cell parameters have been calculated(Table 1)for K2MnAl11O19catalysts prepared by different methods.Unit cell parameters value of a and c seems to be rather independent of the preparation methods.

Table 1 Properties of the catalysts obtained from different methods
2.2 Adsorption/desorption isotherm,pore size and specific surface area
The adsorption/desorption isotherms of samples are shown in Fig.2.They all show the type-Ⅳisotherm with a type-H3 desorption hysteresis loop according to IUPAC classification[24].The shapes of the hysteresis loops have often been identified with specific pore structures.The type-Ⅳisotherm is associated with particles giving rise to slit-shaped pores.
The specific surface area and pore volume as well asaverage pore diameterofthree samples are summarized in Table 1.We can see that 3#possesses much larger pore than those of 1#and 2#,which is in line with the characterization of the specific surface area.As seen from Table 1,the specific surface area of 3#is larger than those of 1#and 2#.The trend for the variation of pore volume is identical to that of the specific surface area.So we believe that preparation method plays an important role in the structure of the pores and specific surface area.
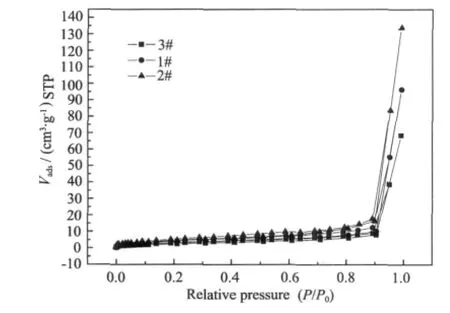
Fig.2 Effect of the preparation method on the isotherms of K2MnAl11O19catalyst
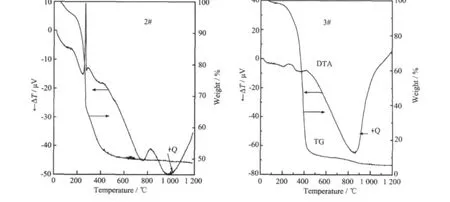
Fig.3 TG-DTA curves of 2#and 3#after 100℃ageing
2.3 TG-DTA analysis
TG-DTA results of 2#and 3#after 100℃ageing are shown in Fig.3.When the samples are heated from room temperature to 1 200℃at a constant heating rate of 10℃·min-1,2#and 3#loose about 50%and 95%of weight,respectively.Itshowsthatmore organic compounds such as surfactants and co-surfactants are removed during the reverse microemulsion-Mediated process.The samples of 2#and 3#behave differently when the temperature is under 700℃.For 2#sample,DTA curve presents two lower endothermic peaks and a stronger exothermic peak before 700℃.The first endothermic peak below 200℃is due to the removal of the adsorbed water.The second peak between 350℃and 450℃corresponds to the decomposition of MnCO3and K2CO3.At the same time the water is removed from AlOH3.The stronger exothermic peak between 220 and 320℃is caused by the burning of some oxides.For 3#sample,the peak is very weak.
When the temperature is above 700℃,no weight loss is observed.The stronger endothermic peak(+Q)from 800 to 1 200℃for 2#and 3#may be related to the transformation of metal oxides into hexaaluminate.So we think,the formation of the hexaaluminate phase starts at 800℃and is completed at 1 200℃.
2.4 Catalytic activity
The catalytic activity for methane combustion over different catalysts is shown in Fig.4.The results indicate that the catalysts behave similarly.The T10%and T90%(T10%and T90%corresponding to 10%and 90%CH4conversion)of 3#catalyst are 458℃and 670℃,respectively.Compared with the 3#catalyst,the 1#catalyst shows lower activity,its T10%and T90%are 502℃and 700℃,respectively.2#catalyst has the activity with T10%at 490℃and T90%at 670℃.It is obvious that the difference in catalytic activity is attributed to the difference in the preparation.3#and 2#have the same complete conversion temperature.3#shows the best ignition activity.The samples prepared by reverse microemulsion-mediated method or sol-gel method have higher activity than that of the sample prepared by carbonate precipitation method.Thus we believe that K3AlO3is existed in 1#,when the temperature is higher,it will be sintered.Reverse microemulsion-mediated method renders the precursor mixture homogenous.

Fig.4 Catalytic activities ofcatalysts in the combustion of methane
3 Conclusions
Hexaaluminate K2MnAl11O19catalyst was prepared by co-precipitation method,sol-gel method and reverse microemulsion-mediated method.The catalytic property ofthecatalystswasstrongly dependenton the preparation method.The formation of the hexaaluminate phase starts at 800℃and completes at 1 200℃.The catalyst of K2MnAl11O19prepared by reverse microemulsion-mediated method possesses the highest specific surface area (34 m2·g-1)and has the lowest temperature for initial and complete conversion of methane than those prepared by co-precipitation method or sol-gel method.
Acknowledgements:We are very grateful to the Natural Science Foundation of China (No.21076025)and the Applied Chemistry Key Project of Anhui Province(No.200802187C)for the financial support.
[1]Yan X Y,Crookes R J.Prog.Energ.Combust.,2010,36:651-676
[2]Gao Z M,Wang R Y.Appl.Catal.B:Environ.,2010,98:147-153
[3]Tian C X,Ahmad H,Andrew P.E.Catal.Today,2009,147:196-202
[4]Yin F X,Ji S F,Wu P Y,et al.J.Mol.Catal.A,2008,294:27-36
[5]Andrey J Z,Yin G Y.Langmuir,2000,16:3042-3049
[6]Erik E S,Magali B.Appl.Catal.B,2008,84:241-250
[7]Li S Q,Wang X I.J.Alloys Compd.,2006,432:333-337
[8]Todd H,Gardner J J,Spivey A C.Catal.Today,2010,157:166-169
[9]XU Jin-Guang(徐金光),TIAN Zhi-Jian(田志坚),ZHANG Pei-Qing(张培青),et al.Chem.J.Chinese Universities(Gaodeng Xuexiao Huaxue Xuebao),2005,11:2103-2107
[10]Ren X G,Zheng J D,Song Y J.Catal.Commun.,2008,9:807-810
[11]Ersson A,Persson K,Adu I K.Catal.Today,2006,112:157-163
[12]Wang J W,Tian Z J,Xu J G.Catal.Today,2003,83:213-222
[13]Baylet A,Royer S,Mare P,et al.Appl.Catal.B:Environ.,2008,81:88-96
[14]Teng F,Yi M,Liang S H.J.Non-cryst Solids,2007,56:4806-4812
[15]Zhang K,Zhou G D,Li J.Catal.Lett.,2009,130:246-253
[16]Yeh T F,Lee H G.Mater.Sci.Eng.A,2004,384:324-330
[17]Tian M,Wang A,Wang X D.Appl.Catal.B:Environ.,2009,92:437-444
[18]Woo S,Min S.J.Appl.Catal.B:Environ.,1998,18:317-324
[19]Wang Y H,Ouyang J H,Liu Z G.Mater.Des.,2010,31:3353-3357
[20]Dupeyrat C B,Martinez F O.Appl.Catal.A:General,2001,206:205-215
[21]Zheng J D,Ren,X G.React.Kinet.Catal.Lett.,2008,93:3-9
[22]Jang B W,Nelson R M,Spivey J J.Catal.Today,1999,47:103-113
[23]Groppi G,Cristiani C.Appl.Catal.B:Environ.,2001,35:137-141
[24]Parfitt G D,Sing K S.J.Colloid Interf.Sci.,1975,53:187-193
Effect of Preparation Method on Catalytic Property of Mn-substituted Potassium Hexaaluminate for Methane Combustion
ZHENGJian-Dong*,1REN Xiao-Guang2GE Xiu-Tao1
(1College of Material and Chemical Engineering,Chuzhou University,Chuzhou,Anhui 239012,China)
(2Beijing Institute of Petrochemical Technology,Beijing 102617,China)
Manganese substituted potassium hexaaluminate catalyst was prepared by co-precipitation method,solgel method and reverse microemulsion-mediated method.The title catalyst was calcined at 1 200℃and characterized by XRD and TG-DTA techniques.The catalytic activity was evaluated for methane combustion.The specific surface area of them was calculated using the BET model.The samples exhibit significant catalytic activity for methane combustion at 800℃.Upon calcination at 1 200℃ the K2MnAl11O19prepared by reverse microemulsion-mediated method retains a specific surface area of 34 m2·g-1and shows an excellent activity for methane combustion(the conversions of 10%and 90%are obtained at 458 and 670℃,respectively).
materials science;hexaaluminate;catalytic activity
O643
A
1001-4861(2012)04-0823-06
2011-10-17。收修改稿日期:2011-12-25。
国家自然科学基金(No.21076025),安徽省应用化学重点学科(No.200802187C),安徽省教育厅自然科学研究重点项目(No.KJ2012A213)资助项目。
*通讯联系人。E-mail:zjd071@126.com

