Menstrual Factors, Reproductive Factors and Lung Cancer Risk: A Meta-analysis
Yue ZHANG, Zhihua YIN, Li SHEN, Yan WAN, Baosen ZHOU
1Department of Epidemiology, School of Public Health, China Medical University, Shenyang 110001, China; 2Key Laboratory of Cancer Etiology and Intervention, Liaoning University, Shenyang 110036, China; 3China Medical University Center For Evidencebased Medicine, Shenyang 110001, China
Abstract
Key words Lung neoplasms; Age at menarche; Length of menstrual cycle; Female sex hormones
Introduction
Lung cancer remains the leading cause of death from cancer among women in the United States[1,2]. The incidence of lung cancer is increasing in women, in contrast to that seen in men. Interestingly, the proportion of lung cancer cases in females attributable to smoking is approximately half of that seen in males[3]. In addition, there is new evidence suggesting that estrogen receptors (ERs) ERα and ERβ have been detected on lung cancer cells in females[4,5].Laboratory evidence proves that estrogen acts as a promoter for lung adenocarcinoma in a mouse model based on genetic alterations that are relevant to the human condition[6].These findings have suggested a hypothesis that female sex hormones, and consequently menstrual and reproductive factors, may play an important role in lung carcinogenesis.
Resultsfrom epidemiological studies, however, have been controversial. To evaluate the relationship between risk of lung cancer and menstrual and reproductive factors, we conducted a meta-analysis of these studies.
Materials and methods
Literature search
We attempted to report this meta-analysis in accordance with the meta-analysis of Observational Studies in Epidemiology guidelines[7]. We conducted a systematic literature search of the PubMed database, CNKI,WANFANG DATA and VIP INFORMATION through January 2012 by using the following search strategy:(menstrual factors OR reproductive factors) AND (lung cancer OR lung neoplasms OR pulmonary cancer OR pulmonary neoplasms), with no restrictions. Reference lists of the chosen papers were also reviewed for other potential articles that may have been missed in the database search.We did not contact the authors of the primary studies to request addition information.
Study selection
Studies were included in our analysis if they met the following criteria: 1) the study design was a case-control study or a cohort study; 2) the endpoint of cohort study was incidence of lung cancer; 3) the risk estimate of lung cancer related to menstrual or reproductive factors and the corresponding 95% confidence intervals (CIs) were reported;studies without existing RRs were also eligible if the relevant data presented in the study we can use to calculate crude RRs instead; 4) of the studies with the same or overlapping population published in more than one study, we included the most recent ones.
Data extraction and quality assessment
Two authors independently reviewed the articles and extracted the data, any disagreement was resolved by discussion. Information was recorded for each study as follows: last name of the first author, year of publication,study location, type of study design, length of follow-up (if applicable), total person-years of observation (if applicable),studied outcome, exposure variables and categories, number of lung cancer cases, number of controls, cohort size, age range (if applicable), menopausal status of the participants(if applicable), smoking status of the participants (if applicable), adjusted RR estimates from multivariable model with corresponding 95%CIs and statistical adjustment for potential confounders of interest.
Instead of providing aggregate scores, we assessed the quality of individual studies by recording the key components of study designs[7], including characteristics of study populations, assessments of exposure and outcome and confounding variables controlled.
Statistical analysis
In this reanalysis, the odds ratio (OR) and the hazard ratio (HR) were directly considered as RR. The categories of menstrual and reproductive exposure measures varied across studies, so we performed this meta-analysis of the comparison of the highest versus the lowest category in each study. In addition, exposures to OC use and HRT use were analyzed as dichotomous variables. Because some studies reported associations for different durations of OC use and HRT use in comparison with never use, we pooled those RRs to evaluate the total effect for ever versus never use.
Pooled risk estimates were calculated for exposure variables that were reported in at least five studies, which included age at menarche, length of menstrual cycle, number of pregnancies, parity (number of live births), age at first live birth, age at menopause, oral contraceptive (OC) use and hormone replacement therapy (HRT) use.
Other menstrual and reproductive variables reported in less than five studies included menopause status, other hormones, type of surgery, number of difficult labour,positive history of hysterectomy; miscarriage; spont;abortion, breast feeding, period length, menstrual regularity,quantity of menstrual flow, mental tension or pains related to menses, age at first use OC, age at first use HRT, years of menstruation, time since first use OC, time since last use OC,type of HRT, lifetime estrogen dose from HRT, intrauterine device use, age at last birth, estrogen plus progestin pills,calendar year of first use OC, HRT and number of menstrual cycles up to the reference date.
Heterogeneity test was performed with the use of Q statistic at the P<0.05 level of significance[8]. We also calculated the I2statistic, a quantitative measure of inconsistency across studies, which was classified as low(25%), moderate (50%) and high (50%)[9]. RRs from different studies were pooled using the fixed effect model and the random effect model based on the Mantel-Haenszel method and the Dersimonian and Laird method respectively. If there is no heterogeneity between studies, we used a fixed effect model; otherwise, we adopt the random effect model.
Subgroup analyses according to geographic region and study design were carried out to assess the potential impacts on the association. In addition, we conducted a sensitivity analysis to examine the influence of individual studies on the overall meta-analysis RR by sequentially omitting each one before pooling study-specific RRs.
Potential publication bias was assessed by visual inspection of Begg's funnel plots and formally by Egger's linear regression test[10,11]. All statistical analyses were done using STATA version 11.0 software. A P value<0.05 was considered statistically significant and all statistical tests were two-sided.
Results
Literature search
The literature search initially identified 926 publications from PubMed, CNKI, WANFANG and VIP INFORMATION; most were excluded because they were reviews or because the exposure or endpoint was not relevant to our analysis. After scanning, 33 were retrieved for further evaluation that also let to identification of 5 more articles from their collective references. Thus, 38 articles reported associations of at least one menstrual or reproductive variable with lung cancer risk. However, we excluded the articles by Fan et al[12], Zheng et al[13], Dorjgochoo et al[14]and Liu et al[15]because they provided insufficient data. Articles by Zhong et al[16], Qin et al[17], Yin et al[18], and Fang et al[19]were excluded because only point estimates and 95%CI were reported without categorical variables. We further excluded one study[20]which studied on the survival of lung cancer.Two articles reported risk estimates based on the partially overlapping Chinese population[22,47], so we extracted data from Zheng et al[22], the more recent reference, for all menstrual and reproductive variables. Two articles reported partially overlapping data from the Czech Women's Lung Cancer Study[27,48], however, Zatloukal et al[48]reported the results separately by cell types of lung cancer, so we extracted all menstrual and reproductive data from Kubik et al[27]. Two articles based on the China Gansu Province population database overlapped[31,49], so data from the more recent reference were used[31]. One report analyzed unpublished data collected in a long-standing hospital-based case-control study[24], which materials and methods were described in detail by Wynder et al[25], so the information of study was extracted from Wynder et al[25]. Seow et al[28]reported the results separately by smoking status, so we extracted two data sets from the study. Two articles reported partially overlapping data from the prospective Shanghai Women's Health Study[38,39], we extracted data from Chen et al[39], the more recent reference, for all menstrual and reproductive variables except number of children and age at first live birth,which were only available from Weiss et al[38]. Two articles based on the population from Fujian reported overlapping results on age at menarche[37,43], so those results were extracted from the more recent reference[43], other related variables were extracted from Chen et al[37]. Therefore, a total of 25 articles (18 written in English and 7 in Chinese),representing 24 independent studies, were included in this meta-analysis. A flow chart showing the study selection process is presented in Fig 1.
The characteristics of the included studies are presented in Tab 1. The 25 studies were published between 1988 and 2012. Of them, 13 studies had been carried out in Asia, 9 in North America and 3 in Europe. Eighteen studies reported case-control comparisons and 7 were analyses of cohorts.
Age at menarche
Nineteen studies examined the relationship between lung cancer risk and age at menarche[21-24,26-29,31,32,34,35,39-44,46].However, Zheng et al[22], Wu-Williams et al[23], Zhou et al[26],Seow et al[28]and Matsuo et al[35]used the oldest age at menarche as the referent category, hence these 6 adjusted RRs could not be pooled with others comparing the highest versus the lowest categories, so we calculated crude RRs according to the number of cases and controls, then we used them instead. Furthermore, we excluded one study[42]because it did not provide the required data.
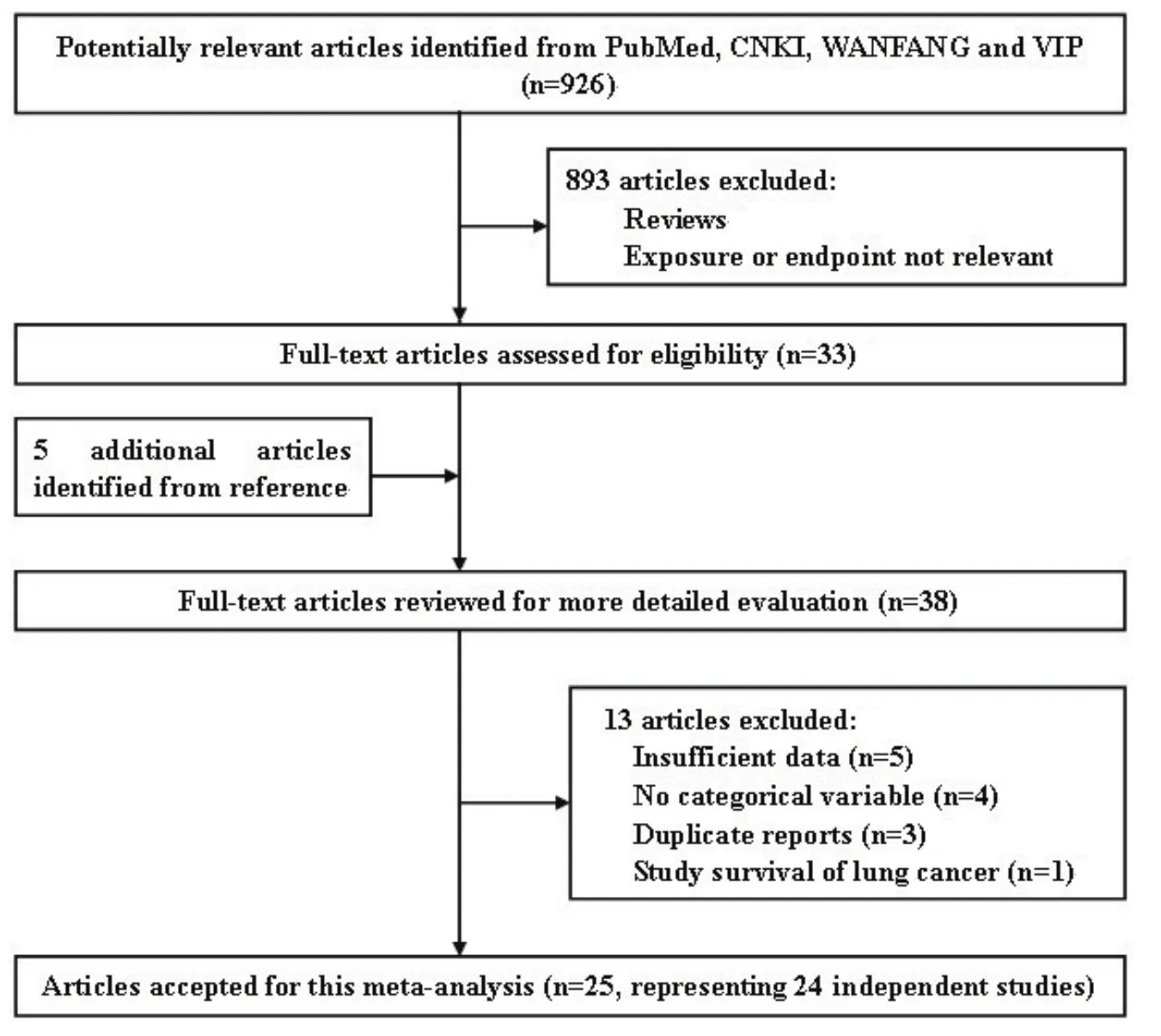
Fig 1 Flow chart of the literature search for studies of menstrual and reproductive factors and lung cancer risk
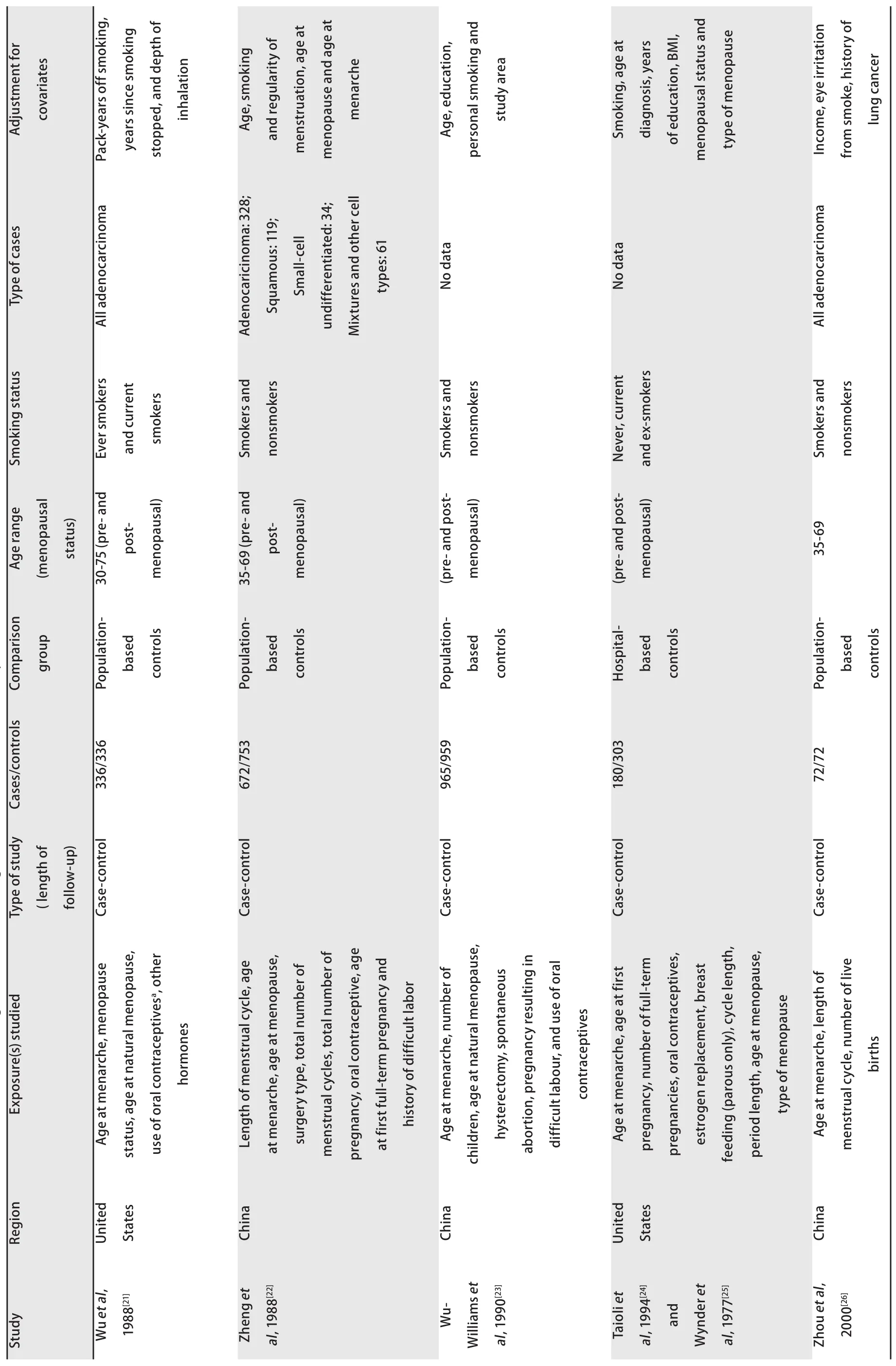
Tab1 Characteristics of observational studies addressing the association of lung cancer risk w

Tab1 Characteristics of observational studies addressing the association of lung cancer risk with

Tab1 Characteristics of observatioal studies abbressing the association of lung cancer risk with
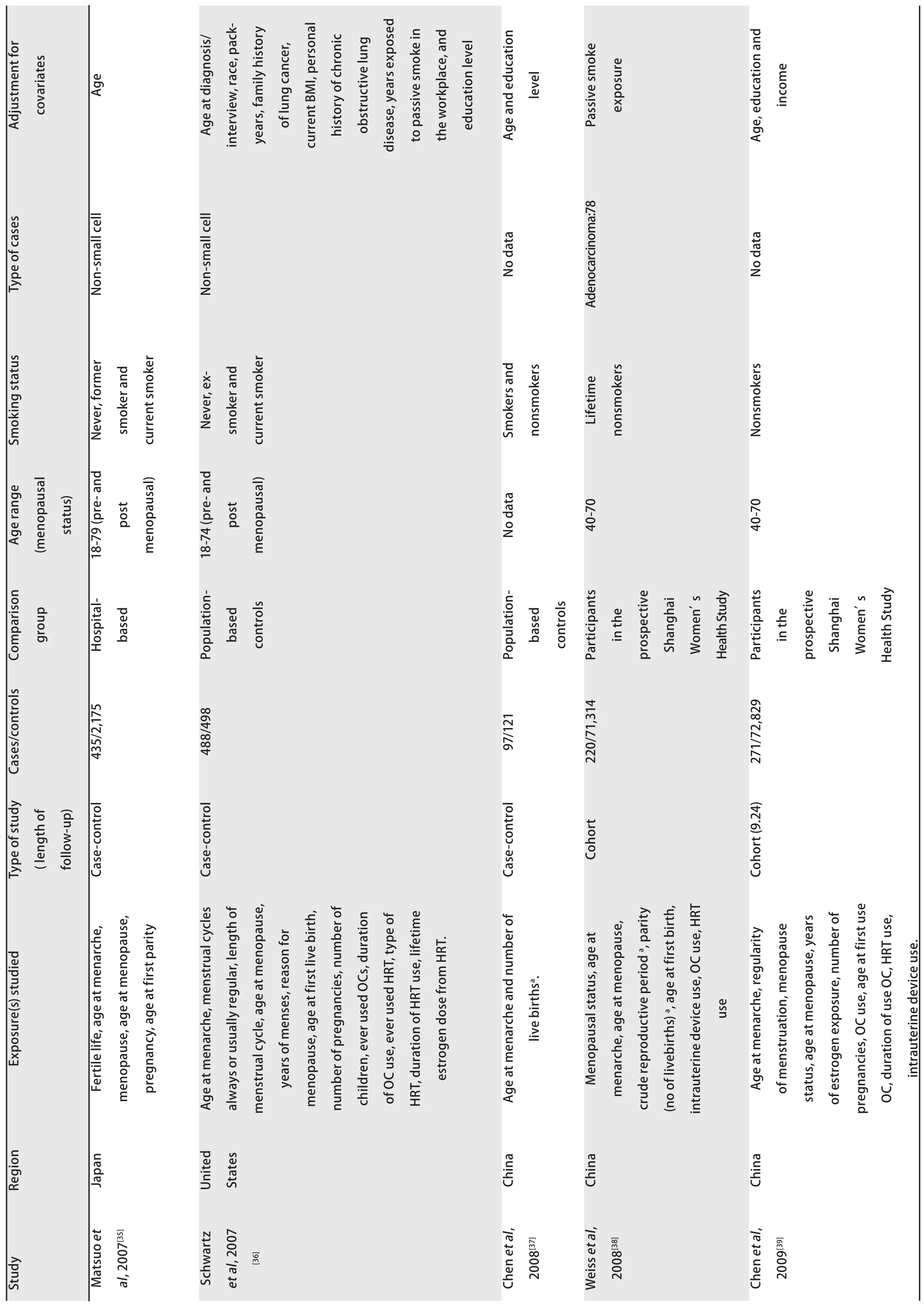
Tab1 Charavteristics of observational studies addressing the association of lung canver risk with

Tab1 Characteristics of observational studies addressing the association of lung canver risk with

Tab1 Charavteristics of observational studies addressing the association of lung cancer risk with mens
Study-specific RRs for the oldest age at menarche as compared with the youngest age ranged from 0.345 to 3.158,and the pooled RR was 0.93 (95%CI: 0.79-1.10)(Fig 2). The RR for case-control study was 0.92 (95%CI: 0.71, 1.17),and the RR for cohort study was 0.96 (95%CI: 0.79-1.18).Between-study heterogeneity was high (I2=65.2%), but in subgroup analysis according to geographic region we found a significant decreased risk of lung cancer associated with older age at menarche in North America women (RR=0.83;95%CI: 0.73-0.94).
Length of menstrual cycle
Fig 3 represents a forest plot of the effect size distribution for the seven literatures[22,24,26-29,31]that studied on length of menstrual cycle. Zheng et al[22]and Zhou et al[26]used the longest length of menstrual cycle as the referent category, so we calculated and used crude RRs instead. Study-specific RRs for the longest versus the shortest length of menstrual cycle ranged from 0.46 to 0.91. The combined RR suggested a significant inverse association with a 28% decreased risk of lung cancer and low between-study heterogeneity.
Number of pregnancies
Associations of lung caner risk with number of pregnancies were suggested in seven studies[24,29,31,35,36,39,40],among which two crude RRs[35,36]were calculated according to the number of cases and controls. Study-specific RRs for highest number of pregnancies as compared with the lowest ranged from 0.69 to 1.683. The pooled RR was 1.10 (95%CI:0.91-1.34)(Fig 4), with low heterogeneity among the studies.
Parity
Eighteen studies[23,26-28,30-34,36-38,40-42,44-46]provided information on parity, of which a crude RR[36]was calculated based on the number of cases and controls, with studyspecific RRs for highest number of live births in comparison with the lowest ranging from 0.39 to 4.744, and the summary RR was 0.91 (95%CI: 0.75-1.10; I2=75.8%)(Fig 5). In the eighteen studies, half studies[27,33,34,36,38,40,41,45,46]used the nulliparous women as the reference group, the pooled RR was 0.93 (95%CI: 0.68-1.27; I2=82.4%)(Fig 6), whereas only three studies[31,42,44]used a parous comparison group (1-2 children) so we did not estimate the risk, others were not clearly classified.
Age at first live birth
Risk estimates for oldest versus youngest age at first live birth were reported in 11 studies[28,31,32,34,35,38,40,42,44-46],including two crude RRs[34,42]instead, and ranged from 0.49 to 1.50. The combined RR was 1.03 (95%CI: 0.88-1.21)(Fig 7), with moderate heterogeneity across studies.

Fig 2 Random-effects meta-analysis of lung cancer relative risk (RR) associated with age at menarche(highest vs lowest category)
Age at menopause
Sixteen studies[21-24,27-29,31,32,35,39-42,44,46]reported the relationship between age at menopause and lung cancer risk,including five crude RRs[24,32,35,44,46]calculated according to the number of cases and controls. However, two studies were excluded because one[27]used not yet present as the referent category and the other[42]did not provided required data. Study-specific RRs for the oldest age at menopause as compared with the youngest age ranged from 0.39 to 1.7. The pooled RR was 0.91 (95%CI: 0.70-1.17)(Fig 8), with high heterogeneity across studies.
OC use
Eight studies[24,29,33,34,36,39,42,44]reported the risk estimates for ever versus never OC use. What's more, the studies by Wu et al[21]and Seow et al[28]provided 2 RRs depending on duration of use, which we pooled to acquire overall RRs for ever use of 0.604 (95%CI: 0.273-1.336) for Wu et al[21]and 0.854 (95%CI: 0.615-1.184) for Seow et al[41]. The pooled RR of lung cancer for ever users of OC as compared with never users was 0.97 (95%CI: 0.89-1.06)(Fig 9), with modern heterogeneity among the studies.
HRT use
Risk estimates for ever versus never HRT use were reported in eight studies[24,29,33,34,36,39,41,44], but one study[29]was excluded because OC use was included in HRT use. In addition, the studies by Wu et al[21], Baik et al[42]and Brinton et al[46]represented 2 RRs depending on duration of use,which we calculated to obtain overall RRs for ever use of 1.074 (95%CI: 0.749-1.539) for Wu et al[21], 0.948 (95%CI:0.859-1.046) for Baik et al[42]and 0.934 (95%CI: 0.874-0.998)for Brinton et al[46]. The pooled RR of lung cancer for ever users versus never users of HRT was 0.99 (95%CI:0.91-1.08)(Fig 10), with modern heterogeneity among the studies.
Sensitivity analysis
A single study involved in the meta-analysis was omitted at a time to reflect the influence of the individual data set to the pooled RRs, and the corresponding combined RRs were not materially altered (Fig 11), suggesting that our results were stable and reliable.
Publication bias
The Begg's funnel plot was conducted to assess the publication bias of studies (Fig 12). Then, the Egger's test was performed to provide statistical evidence of funnel plot symmetry. The P values for Egger's test were greater than 0.05 for all exposure variables with the exception of age at menopause (P=0.028; Tab 2) and HRT use (P=0.041; Tab 2),the results indicated publication bias for age at menopause and HRT use.

Fig 3 Fixed-effects meta-analysis of lung cancer RR associated with length of menstrual cycle (highest vs lowest category)

Fig 4 Fixed-effects meta-analysis of lung cancer RR associated with number of pregnancies (highest vs lowest category)

Fig 5 Random-effects meta-analysis of lung cancer RR associated with parity (highest vs lowest category)

Fig 6 Random-effects meta-analysis of lung cancer RR associated with parity (highest vs nulliparous catrgory)
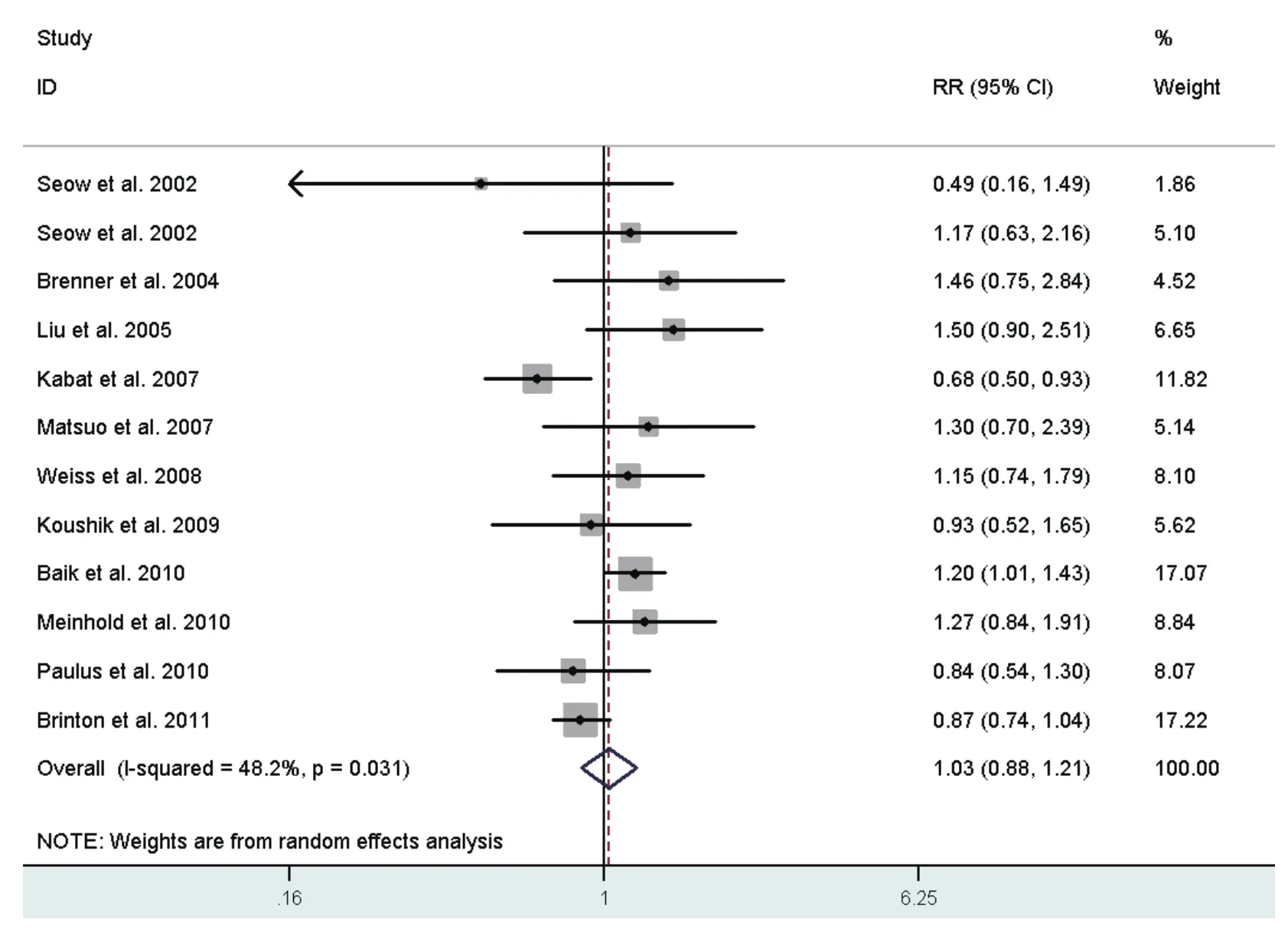
Fig 7 Random-effects meta-analysis of lung cancer RR associated with age at first live birth (highest vs lowest category)

Fig 8 Random-effects meta-analysis of lung cancer RR associated with age at menopause (highest vs lowest category)

Fig 9 Random-effects meta-analysis of lung cancer RR associated with OC use (ever vs never)

Fig 10 Random-effects meta-analysis of lung cancer RR associated with HRT use (ever vs never)

Tab 2 Summary of meta-analysis results
Discussion
Several menstrual and reproductive factors have been suggested related to lung cancer risk, however, many of these results are inconsistent. There is a common point that women are more likely to be diagnosed with lung adenocarcinoma and non-small cell lung cancer (NSCLC). Our meta-analysis identified that decreased lung cancer risks were prone to present in women with longer length of menstrual cycle. We also found that age at menarche of North America women was inversely associated with lung cancer risk. Other six factors did not appear to be strongly associated with risk of this tumor. In summary, these findings support the hypothesis that estrogen exposure has an effect on the risk of lung cancer in women.
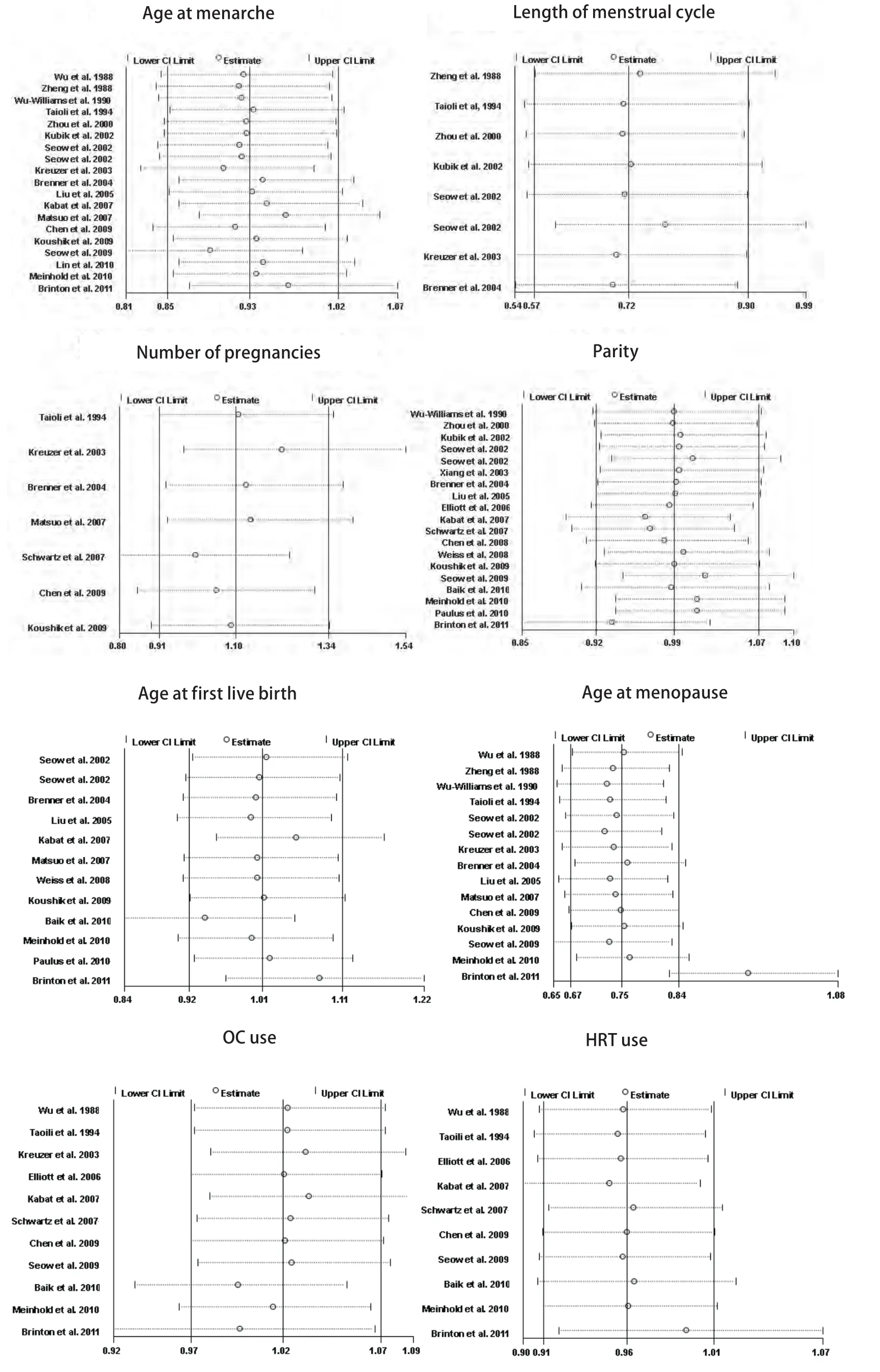
Fig 11 Results of the sensitivity analysis

Fig 12 Begg's funnel plots with pseudo 95% CIs for lung cancer risk associated with age at menarche (A) and length of menstrual cycle (B)
Shorter length of menstrual cycle indicated an overall increase in the period of unopposed estrogen exposure,and younger age at menarche implied more menstrual cycles over the lifetime and hence longer periods of estrogen exposure in total. Women who undergo shorter length of menstrual cycle and younger age at menarche may have an increased risk of lung cancer, possibly due to more cumulative exposure to endogenous estrogen, which may be involved in the etiology of this disease. There are several lines of evidence that estrogens may promote lung tumorigenesis: 1) estrogens can exert their biological effect through two ER subtypes, ERα and ERβ, particularly ERβ,which promotes estrogen-dependent growth of lung cancer cells[50,51]; 2) hydroxylated estrogen metabolites can undergo redox cycling to generate free radicals, which cause DNA damage and lead to carcinogenic mutations[52]; 3) estrogens can directly stimulate the transcription of estrogenresponsive genes in the nucleus of lung cells and can also transactivate growth-factor-signaling pathways, such as the epidermal growth factor receptor (EGFR) pathway, which was involved in NSCLC growth, protection from apoptosis,and angiogenesis[53-55]; moreover, 4) estradiol (E2) can enhance the expression of midkine (MK) protein and E2 increased MK mRNA expression in lung adenocarcinoma cells. Both estrogen and MK can induce NSCLC epithelialmesenchymal transition, which plays an important step in the migration of lung tumor cells[56].
Furthermore, Henningson et al found that short length of menstrual cycle (<27 days) were significantly more common with increasing number of variant A2 alleles[57]. The A2 allele was thought to enhance the transcriptional activity of the CYP17 gene leading to elevated levels of estrogen[58-60], which may increase the risk of lung cancer.
OC use and HRT use, as surrogates of exogenous sex hormonal exposure, seemed not to have a strong impact on lung cancer risk. This may suggest that endogenous and exogenous sex hormone play different roles in lung tumorigenesis, yet further researches using larger study populations are needed to confirm this assumption.
Heterogeneity is often a concern in a meta-analysis. Some evidence of heterogeneity was observed throughout our study. This was partially owing to the following facts: the studies we included focused on different types of design,most of them were case-control studies; studies we used were conducted in different geographic regions, mostly Asia and North America, where people share little in the field of genetic background, lifestyle, and lung cancer incidence;and the ranges of exposure variables in most studies were inconsistent. On this occasion, subgroup analysis was carried out to explain the heterogeneity. As a result, we found that differences in geographic region might contribute to the heterogeneity between studies.
Egger's test suggested little evidence of publication bias in our meta-analysis. We cannot preclude the possibility, as with any meta-analysis, that other unpublished studies may have been missed during our literature search. Meanwhile,we could hardly found the articles written in authors'mother tongue. Moreover, studies with null effects were less published than those with positive ones, which made it different for us to obtain.
Potential limitations of our meta-analysis should be considered. First, our analysis was limited by the inconsistent categorization of the exposure variables,especially those with more than two strata. However, all adjusted RRs were estimated on the basis of the highest versus the lowest category of the exposure variables, and the wide ranges of exposure variables probably reduced this bias. Second, residual confounders were always concerned in observational studies. Although we used the reported multivariable adjusted RRs where available, we still could not exclude the probability that other unmeasured factors have influenced the real relationship. Nonetheless, our study had a noteworthy strength. As individual study had insufficient statistical power, our meta-analysis of 24 studies involving a large number of participants enhanced the power to detect significant associations and provided more reliable estimates.Moreover, our results are consistent with the hypothesis that estrogen exposure may increase the risk of lung cancer in women, but the mechanisms involved are likely to be complex. It is clear that further studies, both mechanistic and epidemiologic, are warranted in this area. Our findings provide further evidence on the public health with respect to the lung cancer prevention in women.
Conclusion
On balance, older age at menarche in North America women (RR=0.83; 95%CI: 0.73-0.94) was associated with a significant decreased risk of lung cancer. Longer length of menstrual cycle was also associated with decreased lung cancer risk (RR=0.72; 95%CI: 0.57-0.90). The other six exposures were not significantly associated. More investigations in large and well-designed studies are needed to extend these findings and to clarify the underlying mechanisms.
Acknowledgements
This study was supported by grants No.81102194 from National Natural Science Foundation of China,No.LS2010168 from Liaoning Provincial Department of Education, and grant No.00726 from China Medical Board.The authors are most grateful to all the participants in this study.
Conflict of interest
No competing financial interests exist for any of the authors.

