Eu离子价态对Sr2MgSi2O7:Eu发光特性的影响
吴浩怡 胡义华 陈 丽 王小涓
(广东工业大学物理与光电工程学院,广州510006)
Eu离子价态对Sr2MgSi2O7:Eu发光特性的影响
吴浩怡 胡义华*陈 丽 王小涓
(广东工业大学物理与光电工程学院,广州510006)
分别在空气、还原气体、再空气、最后再在还原气体等气氛中通过四个步骤合成了Sr1.99MgSi2O7:Eu0.01.在空气中合成的样品呈现出Eu2+和Eu3+两种价态的发光特征峰,而在还原气体中合成的样品,只呈现出Eu2+一种价态的特征发光峰,并且有长余辉和两个热致发光带.但若样品直接在还原气氛下合成,则只呈现一个热致发光带.样品在空气中合成产生空穴陷阱,此陷阱在还原合成气氛下仍被保留,并与还原气体合成条件下产生的电子陷阱有所区别,最终导致两个热致发光带.
长余辉;晶体缺陷;价态变化
1 Introduction
Afterglow refers to the light emission that persists with duration at room temperature after excitation.Because of the great commercial applications in many fields,such as traffic signs, emergency signages,textile printing,and decorations,1,2phosphors with long afterglow are drawing more and more attentions.It is reported that the afterglow phenomenon dated from the 17th century,but during last 400 years the study on the afterglow phenomenon developed slowly.The conventional long afterglow phosphors are zinc sulfides with copper and cobalt co-doping.However,there are many drawbacks of these phosphors,like the short duration of the afterglow,which limits their applications.In order to extend the afterglow duration, some radioisotopes,which are bad for human′s health,are added in the materials.In addition,the luminescent intensity of these phosphors is low,and the compound degrades in the moisture easily.In recent years,rare earth(RE)doped materials are studied widely due to the excellent optical properties of the RE ions and their potential application in solid state lighting,such as light-emitting dioxide(LED).3-6Some RE doped phosphors are found to show long afterglow,such as MAl2O4: (Eu2+,Dy3+)7,8and M2MgSi2O7:(Eu2+,Dy3+)(M=Ca,Sr).9,10The afterglow duration and the emission intensity of these phosphors are much superior to the sulfides.In addition,these phosphors are characteristic with chemical and physical stability.11Therefore,they are regarded as a new generation of the long afterglow materials.
The long afterglow phenomenon arises from the thermal detrapping of carriers,which are generated by excitation and trapped by certain defect sites.12Generally,in the Eu2+and Dy3+co-doped phosphors,Eu2+ions are considered as luminescent centers which generate the carriers while Dy3+ions induce the lattice defects.In the early studies of the long afterglow mechanism,Dy3+ions were assumed to act as hole traps in MAl2O4: (Eu2+,Dy3+)materials.13However,it involves a highly doubtful process,Eu2+to Eu+and Dy3+to Dy4+.The reduction of Eu2+and the oxidization of Dy3+are impossible under the near ultraviolet(UV)and visible excitation because of the lack of sufficient energy.14Moreover,the presence of the either Eu+or Dy4+has not been observed.More recently,Dy3+ions were proposed to act as electron traps.Autoionization creates electrons which are trapped by Dy3+to form the Dy2+,and then Eu3+is left behind.The afterglow is caused by the electrons released with thermally activated from Dy2+back to conduction band with subsequent recombination with Eu3+,accompanied by the 5d-4f emission.15If this is the case,there is no afterglow phenomenon in the Eu2+single doped phosphors.On the contrary,afterglow of Sr2MgSi2O7:Eu2+could be observed in our previous work.16This implies the existence of some defects which trap the carriers and then delay the emission,in addition to Dy3+ions.In the previous researches,the existence of hole traps and electron traps is considered in the Eu2+single doped phosphors.17,18According to the work of Aitasalo et al.,19cation vacancies and oxygen vacancies exist in the phosphors and they act as hole traps and electron traps,respectively.Consequently, they may play an important role in the generation of the afterglow in Eu2+single doped phosphors.Influence of the alterative valence of europium on the luminescent properties of Sr2MgSi2O7:(Eu2+,Dy3+)has been investigated in our group previous work.20Since Eu2+single doped phosphors also show the afterglow,the influence of the alterative valence of europium on Sr2MgSi2O7:Eu2+is studied in present work,avoiding the effect from Dy3+ions.The present work may assist to clarify the lattice defects that are responsible for the generation of afterglow in Eu2+single doped materials.
2 Experimental
2.1 Synthesis
Sr1.99MgSi2O7:Eu2+0.01sample was synthesized via high temperature solid state reaction with four steps.The powder SrCO3(99%),MgO(99%),SiO2(99%),and Eu2O3(99.99%),with 8% (molar fraction)excess H3BO3(99%),were employed in the experiment.All the starting raw materials were analytical pure. After an appropriate weighing of each component according to nominal composition of Sr1.99MgSi2O7:Eu2+0.01,the powder mixture was milled thoroughly for 2 h,followed by a sintering process at 1250°C for 2 h in the air(step 1).Then a sample denoted as S1 was obtained.Subsequently,S1 was milled into powder and sintered at 1250°C for 2 h at reducing atmosphere(5%(volume fraction)H2+95%N2,step 2).Then a sample denoted as S2 was obtained.After that,S2 was milled into powder and sintered at 1250°C for 2 h in the air again(step 3)and then a sample denoted as S3 was obtained.At last,S3 was milled into powder and sintered at 1250°C for 2 h at reducing atmosphere again(step 4)and then a sample denoted S4 was obtained.
2.2 Characterization
The phase identification of the samples was carried out by powderX-ray diffraction (XRD)using a diffractometer (PGENERAL China)with Cu Kαirradiation(λ=0.15406 nm). The tube voltage and the tube current were 36 kV and 20 mA, respectively.The range of 2θ was from 10°to 70°.The excitation and emission spectra were measured by a F-7000 fluorescence spectrophotometer(Hitachi Japan).The decay curves and the thermoluminescence(TL)curves of all samples were recorded using a FJ-427A1 thermoluminescence dosimeter (CNNC Beijing Nuclear Instrument Factory China).Prior to the decay curve detection,each sample was excited by a fluorescence lamp for 1 min.Decay within 400 s after excitation was recorded.The temperature was maintained at 30°C during the whole detection.Before the measurement of TL,each sample was also excited by a fluorescence lamp for 1 min and then the measurement started after 15 min.The heating rate for each sample was 1 K·s-1and the range of the measurement was from room temperature to 200°C.
3 Results and discussion
3.1 Phase identification
XRD patterns of each sample was measured and the results are shown in Fig.1.As shown in the Fig.1,the phases of S1 to S4 can be indexed to the tetragonal Sr2MgSi2O7phase in the space group P421m(No.113)according to the JCPDS standard card No.75-1736.The cell parameters are a=b=0.7995 nm and c=0.5152 nm.No other structure is observed.A few of the Eu2+/ Eu3+doping has not a significant influence on the Sr2MgSi2O7phase.The oxidizing or reducing atmosphere does not affect the phase neither.

Fig.1 XRD patterns of the samples
3.2 Photoluminescence
Fig.2 is the emission and excitation spectra of the samples. As shown in Fig.2,the emission spectra of S1 and S3 consist of one emission band centering around 460 nm and several sharp lines peaking at about 580,592,and 615 nm.As we known,the Eu2+shows a broad emission band peaking at 460-470 nm under 360 nm excitation in the Sr2MgSi2O7,due to the 4f65d1to 4f7transition of the Eu2+(8S7/2-7FJ)(J=0,1,2,3, 4).10,21Therefore,it is possible that some Eu2+ions exist in the matrix,leading to the emission at 460 nm.The sharp lines in the spectra should be ascribed to the transitions within the 4f6configuration of Eu3+.The lines 580,592,and 615 nm correspond to the5D0-7F0,5D0
-7F1and5D0
-7F2transitions,respectively.The5D0-7F1is caused by the magnetic dipole transition which is sensitive to site symmetry,while the5D0-7F2is due to the electron dipole transition which is induced by the lack of the inversion symmetry at the Eu3+sites.22Therefore,emission of both Eu2+and Eu3+can be observed.Compared with S1,S3 shows stronger emission intensity,especially for the peak at 460 nm.Hence,more Eu2+may exist in S3.The excitation spectra were also measured by monitoring 460 and 615 nm emission,respectively.As can be seen,the shapes of these spectra are quite different.The emission at 460 nm exhibits an absorption band at about 360 nm,corresponding to Eu2+absorption. On the other hand,the emission at 615 nm shows a series of sharp lines,due to Eu3+absorption.
The emission and excitation spectra of S2 and S4 are shown in Fig.3.After reduction,a broad emission band peaking at about 465 nm is shown for each sample.In addition,a weak emission band at 410 nm is exhibited(Fig.3(b)).Two absorption bands at about 275 and 360 nm are observed.These are due to the transition between 4f65d1and 4f7configurations of Eu2+ions.No other emission is observed,indicating that most Eu3+ions in S1 and S3 are reduced to Eu2+.The emission of S2 and S4 peaks at an identical location with different intensities. S4 shows stronger emission intensity than S2.
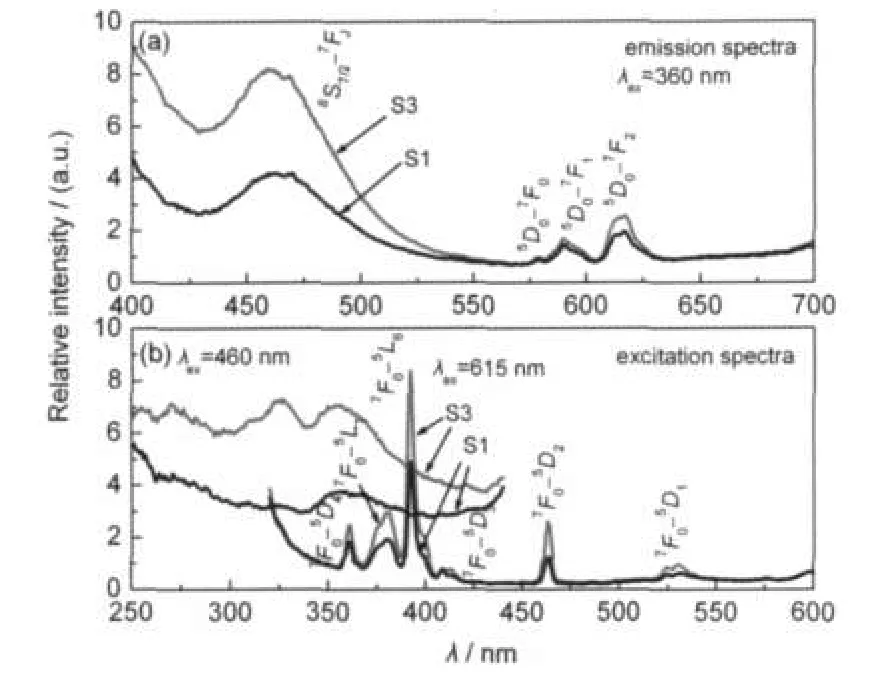
Fig.2 Excitation spectra and emission spectra of S1 and S3 prepared in air

Fig.3 (a)Excitation spectra and emission spectra of S2 and S4 prepared in the reducing atmosphere,(b)emission spectra of S2 and S4 within the range from 400 to 425 nm
Generally,if the concentration does not reach the critical quenching point,the stronger concentration of Eu2+results in the greater emission intensity.Hence the amount of Eu2+can be estimated by calculating the emisison.The integral of the emisison band of Eu2+(425-500 nm)is calculated in present work. Suppose the Eu3+is reduced to Eu2+almost in S4.Then the concentration of Eu2+in S4 can be regarded as 100%.The relative concentrations of Eu2+in S1,S2,and S3 are 1.04%,70.01%, and 2.02%,respectively.It indicates that only around 1%Eu3+is reduced to Eu2+after step 1,and around 70%Eu3+is reduced to Eu2+after step 2.After step 3,2%Eu2+can be preserved.In other word,most of Eu3+is reduced to Eu2+in reducing atmosphere and small amount of Eu2+is preserved in the oxidation synthesized atmosphere.
3.3 Decay characteristics

Fig.4 (a)Decay curves of S1 and S3 prepared in the air,(b) decay curves of S2 and S4 prepared in the reducing atmosphere
In general,phosphors with only Eu3+doping do not show afterglow,whereas when Eu3+is reduced to Eu2+in some materials,the obvious afterglow can be observed.In the present work,we recorded the decay curve of the afterglow for each sample.The curves are exhibited in Fig.4.As shown in the Fig.4,S1 does not exhibit an obvious afterglow.When Eu3+is reduced to Eu2+via step 2,a long afterglow with high bright-ness can be obtained in S2.These results conform our prediction that phosphors with Eu3+do not show afterglow but with Eu2+show afterglow.When S2 is sintered in the air,Eu2+is oxidized to Eu3+again via step 3.However,a decay process is observed although the afterglow is neither long nor bright.As Eu3+is reduced to Eu2+again via step 4,a long afterglow is observed in S4.Compared with S2,the afterglow of S4 is stronger and the duration seems to be longer.
Since the decay process contains a rapid-decaying process and a slow-decaying process,double exponential equation which reflects the trend of the decay is used to fit the decay curvesof S2andS4.Theformof theequationisas following.23,24
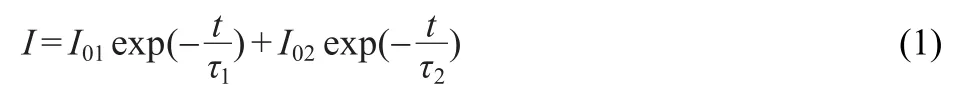
where I represents the phosphorescent intensity,I01and I02are constants,τ1and τ2are decay constants,deciding rates for the rapid and the slow exponential decay components,respectively.The fitting results of parameters of τ1and τ2are 14.2,150.1 s for S2 and 21.7,163.1 s for S4,respectively.The value of τ2for S4 is a little greater than the one for S2.It confirms longer afterglow duration of S4.
3.4 Thermoluminescence
As mentioned above,both S2 and S4 with Eu2+doping show an obvious afterglow.With Eu3+doping,S1 does not show an afterglow,whereas S3 which is oxidized from S2,exhibits a weak afterglow phenomenon.The afterglow is induced by the traps which trap the carriers and release them thermally,giving rise to the delayed luminescence after excitation.Consequently,the investigation on the traps of the phosphors may provide an appropriate explanation for the experimental phenomena. Since the afterglow is caused by the thermal detrapping of trapped carriers,temperature-dependence luminescent intensity can provide some information of traps.Consequently,TL which is physically governed by this mechanism,is introduced to investigate the traps of the samples.As shown in Fig.5(a), S1 does not show an emission significantly with the increase of temperature,indicating that this sample does not store the carriers.Hence no afterglow can be observed.Compared with S1,S3 shows a very weak TL band centering around 80°C.A few of carriers may be trapped in the traps of this sample.Then the detrapped carriers lead to a weak afterglow at room temperature.The TL curves of S2 and S4 are shown in Fig.5(b).As can be seen,two adjacent TL bands are obtained in both S2 and S4.The locations of the glow peaks are around 70 and 120°C,respectively.Because S2 and S4 are reduced from Sr1.99MgSi2O7:,their trap states may be different from those of Sr1.99MgSi2O7:1which is synthesized in the reducing atmosphere directly.In order to make a comparison,the TL curve of the direct-synthesized Sr1.99MgSi2O7:(denoted as DSSE) has been provided in Fig.5(c).This sample exhibits only one TL band peaking at about 75°C,approaching to the low temperature peaks of S2 and S4.However,the peaks at 120°C can not be observed in this sample.Moreover,compared with S2, the intensity of the peak of S4 at 70°C is stronger while the other one at 120°C is weaker,indicating the difference between S2 and S4.

Fig.5 (a)TLcurves of S1 and S3 prepared in the air,(b)TL curves of S2 and S4 prepared in the reducing atmosphere,(c)TL curves of DSSE prepared in the reducing atmosphere directly
The trap depth of S2,S4 and the DSSE sample can be estimated according to the TL curves.Since two TL bands are overlapping in one curve,the estimating methods based on the total half width of the band are not available.Trap depths corresponding to the band at about 70°C can be estimated by a method based on the low temperature at half width of the band. The form of the equation is as following.25,26

3.5 Discussion
The emission of Eu2+can be observed in the emission spectra of S1 and S3.As we known,the Eu3+can be easily reduced to Eu2+in most matrices if materials are prepared in the reducing atmospheres.However,no reducing atmosphere is involved during the preparation of S1 and S3.When the compounds are prepared in air condition at a high temperature,theabnormal reduction of Eu3+to Eu2+has been reported in early researches.27-30Four conditions seem to be necessary for the reduction of Eu3+to Eu2+in solid state compounds when compounds prepared at high temperature in the air have been proposed.They are:(1)no oxidizing ion present in the host,(2) the trivalent Eu3+ions replace the divalent cations in the matrix,(3)the substituted cation has similar radius to that of the divalent Eu2+ion(0.127 nm),and(4)an appropriate structure, which is composed of the tetrahedral anion groups in the matrix.31Generally,three sites available for the incorporating Eu3+in the Sr2MgSi2O7matrix are Sr2+site,Mg2+site,and Si4+site. The ionic radii of Mg2+(0.072 nm)and Si4+(0.026 nm)are small.But for Sr2+(0.126 nm),it is comparable in size to Eu3+(0.109 nm).So the Eu3+ions probably occupy the Sr2+sites.According to above,the first three conditions are fulfilled in S1 and S3.According to the work of Ochi,32the Sr2MgSi2O7contains a Si2O7double pyramid composed by two tetrahedral structures(SiO4tetrahedron).Therefore,the condition(4)is also confirmed.It is possible that Eu2+exists in the Sr2MgSi2O7matrix even prepared in the air.The mechanism of the reduction of Eu3+to Eu2+in the air has also been proposed.In order to keep charge balance,two Eu3+ions occupy two Sr2+sites and then one Sr2+vacancy is created by charge compensation in vicinity.Then the Sr2+vacancy acts as a donor of electrons and two Eu3+ions at the Sr2+sites become acceptors.Consequently, two electrons in each vacancy will transfer to two adjacent Eu3+ions and then Eu2+ions are formed.22,23If this is the case,Sr2+vacancies play an important role in the reduction of the Eu3+to Eu2+in air.The integral calculation of emission indicates that the concentration of Eu2+in S3 is about twice as in S1.It is possible that more Eu2+ions are preserved during step 3 because the tetrahedral anion groups can not be destroyed completely.

Table 1 Estimated results of the trap depths
As shown in Fig.3(b),a weak emission band is observed in both S2 and S4.The similar phenomenon appears in SrAl2O4: (Eu2+,Dy3+).Clabau et al.12proposed a model to explain persistent luminescence,refering this emission.According to the model,a small amount of Eu3+exists in the matrices of S2 and S4 because it is impossible to reduce all Eu2+to Eu3+.Moreover,the oxidization process(steps 1 and 3)raises the probability of the existence of Eu3+.Fig.6 is drawn according to the mechanism proposed by Clabau et al.12As shown in the figure, under UV excitation,electrons of Eu2+are promoted from the occupied 4f level to the empty 5d level,and from valence band (VB)to the top of unoccupied 4f levels of residual Eu3+via charge transfer(CT).The electrons promoted to 5d levels can be trapped by oxygen vacancies,which are created due to the reducing atmosphere in the synthesis process.Meanwhile, holes created in VB can be trapped at hole traps.Because of these trapping process,Eu2+is oxidized to Eu3+while the residual Eu3+is reduced to Eu2+.The thermal energy at ambient temperature causes the detrapping of the trapped electrons.The detrapped electrons transfer to the 5d levels of Eu3+,leading to the 4f65d1→4f7blue afterglow.In the present work,the TL band in S2,S4,and DSSE at 70°C may be caused by this electron-detrapping process since the samples are prepared in the reducing atmosphere and then have oxygen vacancies.The TL band at 120°C appears in S2 and S4 but not DSSE.Owing to S2 and S4 are originated from the oxidized S1 and S3,the trap corresponding to 120°C TL band may be caused by oxidizing process of S1 and S3 and the effect is preserved to S2 and S4, respectively.Sr2+vacancy is the possible defect in S1 and S3 because two Eu3+ions occupy two Sr2+sites,one Sr2+vacancy is created for charge compensation.33However,trap depth of Sr2+vacancy is reported to be 0.15 eV above the VB top in SrAl2O4.12Owing to the similar trap depth of Eu2+doped SrAl2O4and Sr2MgSi2O7(about 0.6-0.7 eV for electron trap), Sr2+vacancy is shallower than oxygen vacancy in Sr2MgSi2O7. So the 120°C TL band should not attribute to the Sr2+vacancy. Since S1 and S3 are synthesized in the air,some O2-may exist in the matrices as Frenkel defects.Similarity is performed in CaWO4.34After UV excitation,electrons are transferred from the matrix to the residual Eu3+,then the preserved holes migrate in VB.Subsequently they may be attracted by traps induced by O2-,the detrapping of holes leads to the CT of Eu2+→Eu3+(410 nm emission).This is the possible reason for the generation of 120°C TL band.However,these hole traps may not have a significant contribution to the afterglow because the probability of CT is low,and the necessary energy for the detrapping is high.Compared with S2,this TL band of in S4 is weaker.Since S4 undergoes reduction twice(steps 2 and 4), more Eu3+ions are reduced to Eu2+ions.This on one hand,declines the possibility of the CT,on the other hand,disfavors the existence of O2-,resulting in the low concentration of hole traps.That is why S4 shows a weaker 120°C TL band.From the analysis of TL we find that the trap depth corresponding to 70°C TL band is around 0.65-0.67 eV.Hence the decay processes of S2 and S4 should be similar.However,the decay curves shows that S4 possesses a longer afterglow duration than S2,contrary to the presumption.Since hole traps exsit in the matrix,they trap holes then reduce the recombination of electrons and holes.If the 120°C TL band is responsible for hole traps,more holes are captured in S2,reducing the recombination probability.This may be the reason for the shorter afterglow duration of S2 although it possesses a deeper trap than S4.

Fig.6 Mechanism model proposed based on the work of Clabau et al.12
In addition,the weak TL band illustrates that some oxygen vacancies are preserved in S3 even it is prepared in the air. That is why S3 exhibits a weak decay.However,some phenomena are still unclear and the problems are needed to be addressed:(1)A hole trap is proposed in the present work and they trap hole of Eu3+after CT of S2 and S4.S1 and S3,in which Eu3+should have a high concentration,do not show an obvious TL band that is responsible for this hole trapping process.(2)In order to explain the 120°C TL band,Frenkel O2-defects are proposed.More experiments should be carried out to further confirm its existence.(3)As we known,Dy3+can enhance the afterglow of Eu2+doped phosphors.Since some Eu3+ions still exist in the matrices of S2 and S4,their role and influence on the afterglow should be established in future work.
4 Conclusions
The redox process of the europium ions in Sr2MgSi2O7matrix indicates that samples prepared in the air show both Eu2+and Eu3+emission while samples prepared in the reducing atmosphere only show Eu2+emission.The more redox process enhances emission intensity of the samples no matter with oxidation or reduction.The samples reduced from oxidization show long afterglow and two TL bands(at about 70 and 120 °C),while only one TL band(at about 70°C)is observed if the sample is prepared in the reducing atmosphere directly.This is due to the existence of Eu3+.The 70°C band is responsible for electron traps for Eu2+while the other at 120°C is responsible for hole traps for the residual Eu3+.The afterglow mechanism of the phosphors with single Eu2+doping may be that the electrons from Sr2+vacancies transfer to Eu3+ions and then form the Eu2+ions even without reducing atmosphere.Oxygen vacancies and O2-act as electron and hole traps,respectively.
(1) Chen,Y.;Cheng,X.;Liu,M.;Qi,Z.;Shi,C.J.Lumin.2009, 129,531.
(2)Teng,X.;Liu,Y.;Liu,Y.;Hu,Y.;He,H.;Zhuang,W.J.Lumin. 2010,130,851.
(3)Yao,G.Q.;Feng,Y.E.;Duan,J.F.;Lin,J.H.Acta Phys.-Chim. Sin.2003,19,226.[姚光庆,冯艳娥,段洁菲,林建华.物理化学学报,2003,19,226.]
(4) Cao,F.B.;Tian,Y.W.;Chen,Y.J.;Xiao,L.J.;Liu,Y.Y.Acta Phys.-Chim.Sin.2009,25,299. [曹发斌,田彦文,陈永杰,肖林久,刘云义.物理化学学报,2009,25,299.]
(5) Luo,X.X.;Cao,W.H.;Sun,F.Chin.Sci.Bull.2008,53, 1010.[罗昔贤,曹望和,孙 菲.科学通报,2008,53,1010.]
(6) Zeng,Q.H.;Zhang,X.G.;Liang,H.B.;Gong,M.L.J.Chin. Rare Earth Soc.2011,29,8.[曾琦华,张信果,梁宏斌,龚孟濂.中国稀土学报,2011,29,8.]
(7)Murayama,Y.;Takeuchi,N.;Aoki,Y.;Matsuzawa,T. Phosphorescent Phosphor.US Patent 5424006,1995-6-13.
(8) Lü,X.;Sun,M.;Zhang,J.;Wang,T.J.Rare Earth 2010,28, 150.
(9) Xiao,Z.;Xiao,Z.LongAfterglow Silicate Luminescent Materials and Its Manufacturing Method.US Patent 6093346, 2000-7-25.
(10) Fei,Q.;Chang,C.;Mao,D.J.Alloy.Compd.2005,390,133.
(11) Xu,Y.;Chen,D.Ceram.Int.2008,34,2117.
(12) Clabau,F.;Rocquefelte,X.;Jobic,S.;Deniard,P.;Whangbo,M. H.;Garcia,A.;Mercier,T.L.Chem.Mater.2005,17,3904.
(13)Matsuzawa,T.;Aoki,Y.;Takeuchi,N.;Murayama,Y. J.Electrochem.Soc.1996,143,2670.
(14)Aitasalo,T.;Hölsä,J.;Jungner,H.;Lastusaari,M.;Niittykoski, J.J.Lumin.2001,94-95,59.
(15) Dorenbos,P.Phys.Stat.Sol.B 2005,242,R7.
(16)Wu,H.;Hu,Y.;Wang,Y.;Zeng,B.;Mou,Z.;Deng,L.;Xie,W. J.Alloy.Compd.2009,486,549.
(17)Meng,X.;Wang,Y.;Jin,H.;Sun,L.J.Lumin.2007,122-123, 385.
(18) Sun,J.;Liu,Z.;Du,H.J.Rare Earth 2011,29,101.
(19) Aitasalo,T.;Hölsä,J.;Jungner,H.;Lastusaari,M.;Niittykoski, J.J.Phys.Chem.B 2006,110,4589.
(20) Chen,X.;Hu,Y.;Wang,Y.J.Nanosci.Nanotechnol.2010,10, 1.
(21) Chen,Y.;Liu,B.;Kirm,M.;Qi,Z.;Shi,C.;True,M.;Vielhauer, S.;Zimmerer,G.J.Lumin.2006,118,70.
(22) Shi,Q.;Zhang,J.;Cai,C.;Cong,L.;Wang,T.Mater.Sci.Eng. B 2008,149,82.
(23)Kubo,H.;Aizawa,H.;Katsumata,T.;Komuro,S.;Morikawa,T. J.Cryst.Growth 2005,275,e1767.
(24)Zhu,Y.;Zheng,M.;Zeng,J.;Xiao,Y.;Liu,Y.Mater.Chem. Phys.2009,113,721.
(25) Grossweiner,L.I.J.Appl.Phys.1953,24,1306.
(26) Chen,R.J.Mater.Sci.1976,11,1521.
(27) Peng,M.;Pei,Z.;Hong,G.;Su,Q.Chem.Phys.Lett.2003, 371,1.
(28) Peng,M.;Qiu,J.;Ynag,L.;Zhao,C.Opt.Mater.2004,27,591.
(29) Peng,M.;Hong,G.J.Lumin.2007,127,735.
(30) Pei,Z.;Zeng,Q.;Su,Q.J.Phys.Chem.Solids 2000,61,9.
(31) Pei,Z.;Su,Q.;Zhang,J.J.Alloy.Compd.1993,198,51.
(32) Ochi,Y.Mater.Res.Bull.2006,41,1825.
(33)Wang,Y.;Wang,L.J.Appl.Phys.2007,101,053108.
(34) Shao,Z.;Zhang,Q.;Liu,T.;Chen,J.Nucl.Instrum.Meth.B 2008,266,797.
November 19,2010;Revised:March 25,2011;Published on Web:April 11,2011.
Effect of Europium Valence on the Luminescent Properties of Sr2MgSi2O7:Eu
WU Hao-Yi HU Yi-Hua*CHEN Li WANG Xiao-Juan
(School of Physics and Optoelectronic Engineering,Guangdong University of Technology,Guangzhou 510006,P.R.China)
Sr1.99MgSi2O7:Eu0.01samples were prepared in four steps under air,a reducing atmosphere,air again,and a reducing atmosphere again.The samples prepared in air showed both Eu2+and Eu3+emission while the samples prepared in a reducing atmosphere showed Eu2+emission with a long afterglow and two thermoluminescence(TL)bands.However,only one TL band was observed for the sample prepared directly in the reducing atmosphere.Hole traps were created during the synthesis in air and were preserved during the reducing synthesis.These hole traps are different from the electron traps created by a reducing atmosphere.The hole traps and the electron traps result in two TL bands.
Long afterglow;Lattice defect;Valence change
O644
*Corresponding author.Email:huyh@gdut.edu.cn;Tel:+86-20-39322262;Fax:+86-20-39322265.
The project was supported by the National Natural Science Foundation of China(21071034,20871033).
国家自然科学基金(21071034,20871033)资助项目

