产前不同剂量地塞米松暴露对新生鼠肺泡成熟的影响
陈尚勤, 郑亚兵, 李昌崇, 陈 超, 林振浪△
(温州医学院附属育英儿童医院 1新生儿科,2呼吸科,浙江 温州 325027;3复旦大学附属儿科医院新生儿科,上海 200032)
产前不同剂量地塞米松暴露对新生鼠肺泡成熟的影响
陈尚勤1, 郑亚兵1, 李昌崇2, 陈 超3, 林振浪1△
(温州医学院附属育英儿童医院1新生儿科,2呼吸科,浙江 温州 325027;3复旦大学附属儿科医院新生儿科,上海 200032)
目的探讨孕期肌注不同剂量地塞米松对新生鼠肺泡成熟的影响。方法24只SD孕大鼠分为对照组、产前1次注射组(孕19 d肌注地塞米松0.8 mg·kg-1·d-1)和产前3次注射组(孕18、19、20 d均肌注地塞米松 0.8 mg·kg-1·d-1),每组8只,取新生1、4、10、14 d鼠肺,病理切片,HE染色,观察肺泡形态学的改变,计算机图像分析。电镜检查观察产前3次注射组和对照组肺上皮细胞基底膜的连续性。结果对照组从1-14日龄鼠肺泡间隔渐变薄,肺泡面积变小,肺泡表面积/肺泡体积(S/V)增大,肺(囊)泡数渐增多,4日龄鼠肺次级隔开始长出,至14日龄隔数目渐增多。产前3次注射组的1日龄囊泡隔变薄,肺囊泡面积增大,差异非常显著(P<0.01),肺囊泡数明显减少(P<0.05);4、10、14日龄肺泡间隔明显变薄(P<0.05),肺泡明显增大,S/V较对照组小,肺囊泡数明显减少,次级隔的长出减少,相对较短,数目较少,差异非常显著(P<0.01)。产前1次注射组1日龄鼠的肺泡间隔厚度较对照组变薄,差异显著(P<0.05)。电镜检查发现,正常1、4日龄鼠外周肺上皮细胞基底膜变薄,多处不连续,上皮细胞与成纤维细胞靠近、接触。产前3次注射组肺上皮细胞基底膜的不连续现象较少,上皮间质的直接接触较少。结论产前3次注射地塞米松影响新生鼠肺泡形态发育,产前1次注射地塞米松对新生鼠肺泡形态发育影响不大。
产前暴露; 地塞米松; 肺泡; 新生大鼠
自从1972年报道产前糖皮质激素治疗(antenatal corticosteriod therapy,ACT)能促进肺表面活性物质合成和分泌,ACT广泛应用于临床预防早产儿呼吸窘迫综合征(neonatal respiratory distress syndrome,NRDS)。近40年来,NRDS发病率大为降低[1]。但是并不减低早产儿支气管肺发育不良的发生(bronchopulmonary dysplasia,BPD)[2]。而BPD 的发生与肺泡成熟障碍关系密切[3]。哺乳动物肺泡发育从孕26周开始,其关键阶段在围产期,决定了生后甚至成年后的肺形态结构及功能[4]。围产期的肺发育易受物理、化学、生物、病理等不良因素的影响,近年来随着医疗技术的进步,早产儿、超早早产儿的出生率和存活率大大提高,慢性肺疾病(chronic lung disease,CLD)成为影响早产儿生存质量的重要并发症,BPD是CLD的主要表现。
国际认可的ACT用法指产前单一疗程使用,但目前全球产科医师对高危早产孕妇每周1次的多疗程反复用药[5]的现象相当普遍,这一现象在国内也相当突出。同时ACT的不良反应近期日渐受到重视,随着 ACT重复应用增多,糖皮质激素对胎儿、新生儿的不良反应也相应增加,但尚无定论[6]。对脑发育的不良影响研究报道较多,ACT可导致发生脑瘫的危险性增加,影响智力性格的发育等;对肺研究少,而对肺发育的不良影响,尤其与BPD发生的关系正引起国内外学者的关注。国外研究发现ACT影响肺泡形态发育,甚至持续到成年,影响成年期肺功能[7]。为进一步探讨不同剂量ACT对肺泡发育的影响,我们通过对不同孕龄鼠注射不同次数的地塞米松(dexamethasone,DXM),观察新生1、4、10、14日龄鼠的肺泡结构形态发育及肺泡上皮细胞基底膜的变化,并探讨其可能机制。
材 料 和 方 法
1材料
SD孕大鼠(复旦大学医学院动物实验中心提供)共24只,分3种情况用药。
1.1产前3次注射组 随机取8只孕18 d(足月21-22 d)大鼠,于孕18、19、20 d均肌注DXM 0.8 mg·kg-1·d-1(稀释至0.5 mL)[8]。足月(21-22 d)自然分娩,每窝随机取2只,共16只;4日龄,每窝随机取2只,共16只;10日龄每窝随机取1-2只(因有4窝孕鼠产仔较少),共13只处死取肺;14 d亦每窝随机取1-2只,共12只处死取肺。
1.2产前1次注射组 随机取8只孕18 d大鼠,于孕19 d肌注DXM 0.8 mg·kg-1·d-1。孕18、20 d相应肌注0.5 mL生理盐水,后续分组似产前3次注射组。
1.3对照组 对照组孕鼠8只,于孕18、19、20 d各肌注0.5 mL生理盐水,后续分组如产前3次注射组。
2方法
2.1动物模型的建立 1、 4、10、14 d新生鼠,称重,10%水合氯醛6 mL·kg-1腹腔注射麻醉。气管插管给20 cmH2O的气道压力扩张肺泡,1 min后逐渐调低至10 cmH2O维持,同时剖开腹腔,刺破膈肌,打开胸腔,右心室穿刺留置灌注生理盐水,剪开左心房放血,待左心房流出液体变清后改用10%中性缓冲福尔马林右心灌注,持续20 min。丝线结扎气管,小心分离出气管和肺,浸入10%中性缓冲福尔马林溶液固定48 h,取出放在含50%乙醇的福尔马林中。24 h后,置于75%乙醛中,并放于4 ℃冰箱。取左肺近肺门,边缘部肺组织石蜡包埋,5 μm切片,HE染色。
2.2肺组织病理学观察 光镜下观察肺泡形态改变,IMS细胞图像分析系统的医学图像分析软件(上海申腾信息技术有限公司)作图像分析,使显微镜处于同一放大倍数(100倍)及电压,每张切片随机选择6个视野,分别测量以下指标,测量时避开大、中支气管及血管 。(1)肺泡间隔厚度:每个视野随机选6个肺泡,各测量4处间隔厚度,取平均值。(2)肺泡面积,肺泡壁周长,肺泡等效内径:反映肺泡的大小。(3)肺泡数:计数每个视野(放大100倍视野)的肺泡数,反映肺泡密度。(4)次级隔高度和次级隔数目:测量次级隔顶至基底的高度及每个视野次级隔数目,反映肺呼吸膜面积的扩大程度。(5)肺泡表面积/肺泡体积(surface area/volume,S/V):以每个视野的肺泡面积计算出等效半径,得出平均等效半径,再换算出S/V, S/V=4πr2/(4 πr3/3) =3/r,此指标反映有限的肺泡体积下呼吸膜面积。
2.3电镜检查 取0.1 cm×0.1 cm×0.1 cm的外周新鲜肺组织,2.5%戊二醛磷酸缓冲液固定2 h,0.1 mol/L磷酸漂洗液漂洗15 min×3次,1%锇酸作后固定2 h,再0.1 mol/L磷酸漂洗液漂洗15 min×3次,50%、70%、90%、90%乙醇与90%丙酮1∶1混合液、90%丙酮在4 ℃冰箱内依次脱水15 min,再100%丙酮室温脱水15 min×3次,室温包埋过夜,再37 ℃包埋2 h,固化后LKB-Ⅰ型超薄切片机切片50-60 nm,3%醋酸铀-枸橼酸铅染色,日本电子JEM—1200EX透射电镜(复旦大学附属医学院电镜室)观察3次注射组和对照组上皮细胞基底膜的连续性,并摄片。
3统计学处理
结 果
1ACT大鼠的大体表现、体重改变
与对照组相比,3次注射组的孕鼠有死产,其新生大鼠个头较小,体重偏轻,皮肤较干燥,毛色光泽度差,肤色欠红润,活动偏少,易激惹,吸吮力较弱,喂养较对照组困难,有夭折;1次注射组与对照组差异不大。3次注射组1、4日龄鼠体重较对照组非常显著下降(P<0.01,1日龄鼠下降10.3%,4日龄鼠下降23.9%);1次注射组仅1日龄的体重较对照组显著下降(P<0.05,下降幅度7.3%),见表1。
2肺泡病理组织学观察
正常鼠肺从1日龄至14日龄,次级隔长出数目渐增多,肺泡数增多,肺泡面积变小,S/V增大,肺泡间隔渐变薄,14日龄次级分隔基本结束,肺泡发育基本完成;3次注射组次级隔的长出减少,相对较短,肺泡较对照组明显增大,数目较少,呼吸膜面积较对照组小,肺泡间隔更薄,见图1A、2A、3A、4A、图1C、2C、3C、4C及表2-5。1次注射组除1日龄鼠的间隔厚度较对照组显著变薄外(P<0.05),余改变不明显,见图1B、2B、3B、4B及表2-5。
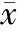
表1 各组新生鼠体重
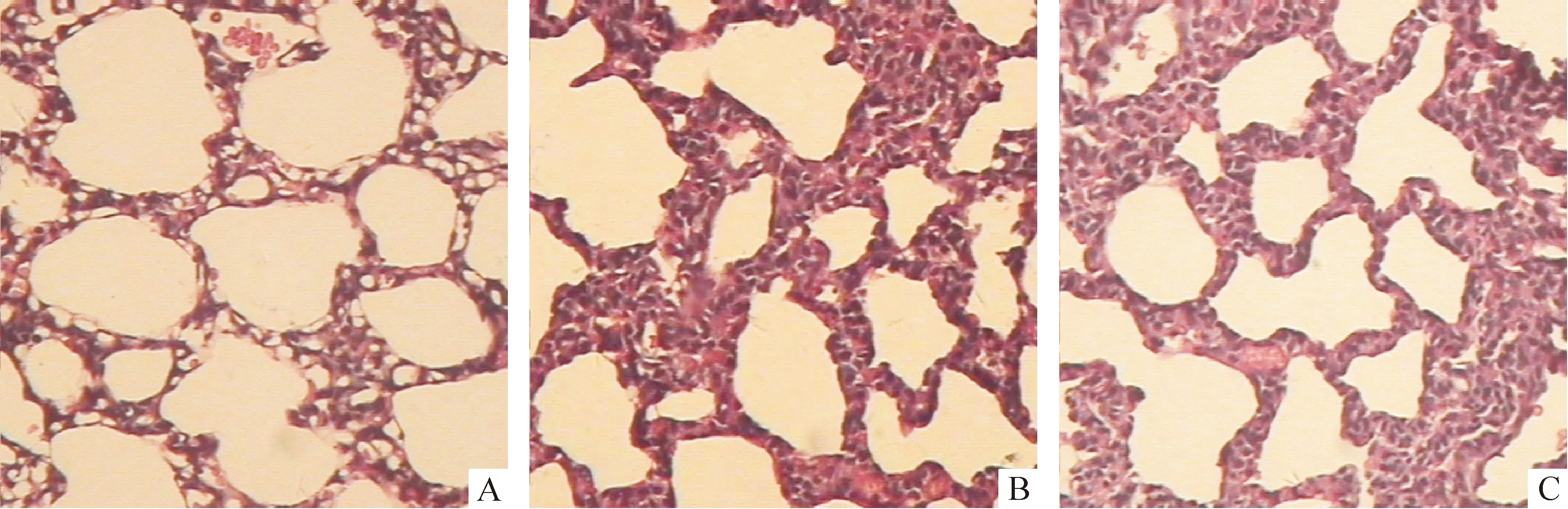
Figure 1. Effect of antenatal DXM exposure on the lung histology of 1-day-old newborn rats(×100). A: lung section from a rat in thrice-DXM group on day 1, in which the air space was larger and the intersaccular walls were thinner compared with control group on day 1(C);B: lung section from a rat in once-DXM group on day 1, in which the air space was similar to that in control group(C) and the intersaccular walls were thinner than those in the control rats on day 1(C).
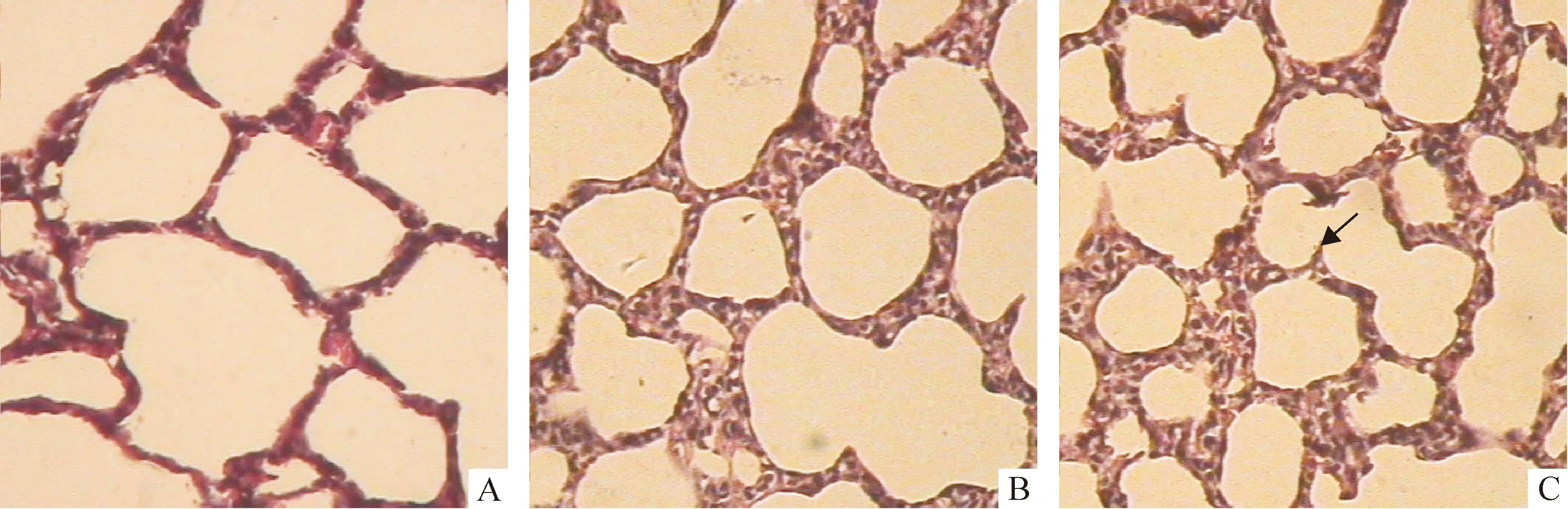
Figure 2. Effect of antenatal DXM exposure on the lung histology of 4-day-old newborn rats(×100).A:lung section from a rat in thrice-DXM group on day 4,in which there were larger air space, thinner alveolar walls and less sprouting crests compared with the control rats on day 4(C); B:lung section from a rat in once-DXM group on day 4 was similar to that in the control rats on day 4(C).Arrow designates sprouting crests.

Figure 3. Effect of antenatal DXM exposure on the lung histology of 10-day-old newborn rats(×100).A:lung section from a rat in thrice-DXM group on day 10,in which there were much larger air space, much thinner alveolar walls and apparently less and shorter secondary septa compared with the control rats on day 10(C);B:Lung section from a rat in once-DXM group on day 10 was similar to that in the control rats on day 10(C).Arrows designate secondary septa.
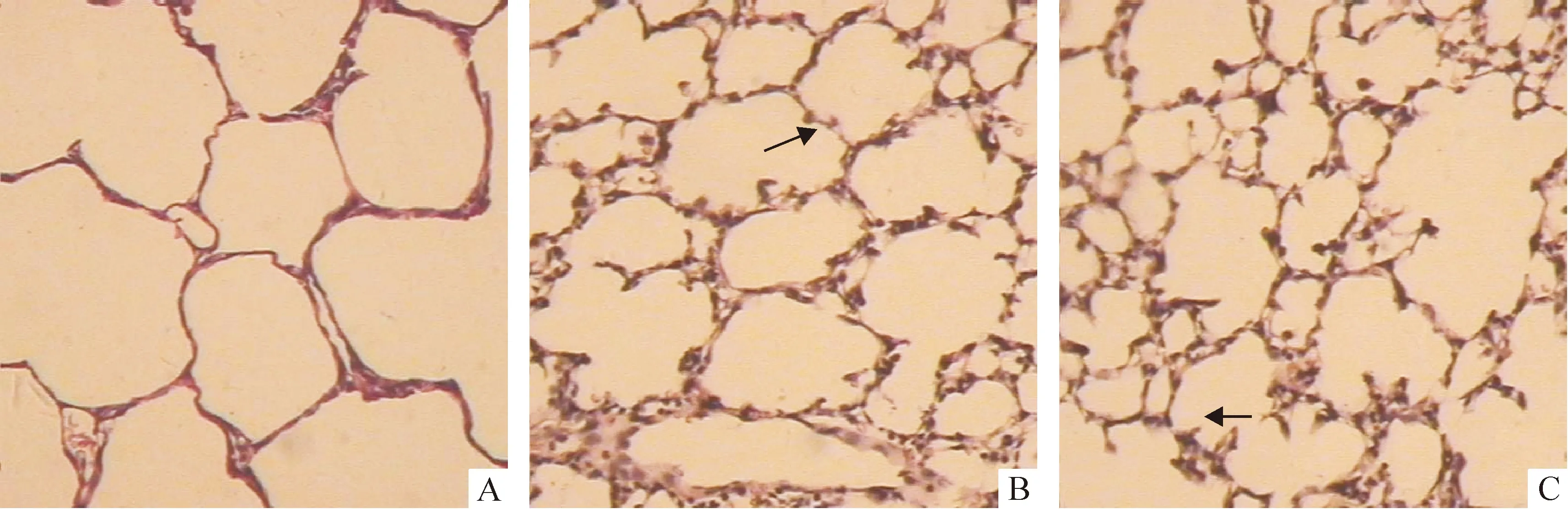
Figure 4. Effect of antenatal DXM exposure on the lung histology of 14-day-old newborn rats(×100).A: lung section from a rat in thrice-DXM group on day 14,in which there were much air space larger, thinner alveolar walls and apparently less secondary septa compared with the control rats on day 14(C) ;B: lung section from a rat in once-DXM group on day14 was similar to that in the control rats on day 14(C). Arrows designate secondary septa.
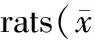
表2 1日龄鼠肺泡形态分析结果
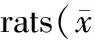
表3 4日龄鼠肺泡形态分析结果
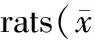
表4 10日龄鼠肺泡形态分析结果
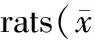
表5 14日龄鼠肺泡形态分析结果
3电镜结果
电镜下基底膜包括近细胞膜的透明层和近间质的致密层,正常1、4日龄鼠外周肺上皮细胞的BM变薄,致密层多处不连续,此现象以4日龄鼠肺更为明显,并见上皮细胞浆伸出伪足穿越这些基底膜的孔隙进入间质,上皮细胞与成纤维细胞靠近、接触,见图5B、6B。3次注射组1、4日龄鼠外周肺上皮细胞的BM的不连续现象较少,较少见伸出伪足,上皮间质的直接接触较少,见图5A、6A。
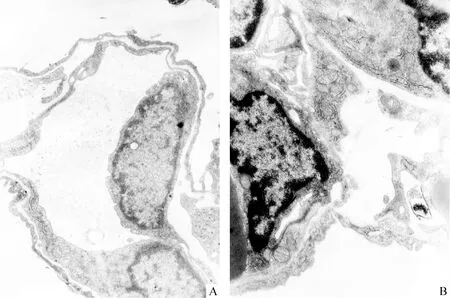
Figure 5. Transmission electron micrographs of 1-day-old rat lungs. A:from a rat in thrice-DXM group on day 1,in which the basement membrane was continuous and close contact of cells were seldom seen;B:from a rat in control group, in which the basement membrane of lung epithelial cells turned thin and discontinuous,helping the close contact between the epithelial cells and the fibroblasts.
讨 论
在产科临床重复多疗程的ACT现象相当普遍,因此带来的对新生个体近期和远期的副作用也使学术界担忧。实验报道重复ACT使胎儿宫内发育迟缓,出生体重下降[9]。我们实验观察到,产前3次注射DXM治疗的1、4 d新生鼠体重明显下降。糖皮质激素抑制蛋白合成,以及3次注射组出生时喂养较困难影响了体重增长,至10、14 d产前的外源性糖皮质激素的作用已基本消失,喂养亦较前好转,体重与对照组比较无差异。1次注射组体重改变不明显,仅1日龄体重较对照组轻度下降,14日龄体重反而略高于对照组,表明产前1次注射DXM对新生鼠影响不大,ACT对仔鼠体重的影响有剂量或重复次数的依从性。
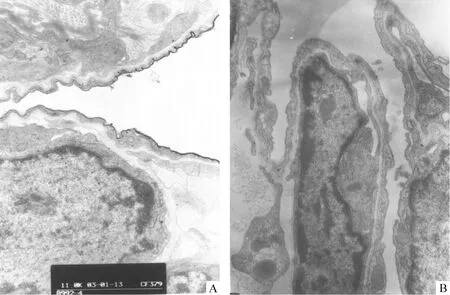
Figure 6. Transmission electron micrographs of 4-day-old rat lungs. A: from a rat in thrice-DXM group on day 4, in which the basement membrane is continuous and close contact of cells were seldom seen;B: from a rat in control group on day 4, showing the basement membrane of lung epithelial cells turned thin and discontinuous.
哺乳动物的肺泡发育是由体积大、数目少、壁厚的囊泡向体积小、数目多、壁薄、次级隔长出的成熟肺泡转化的过程,呼吸膜面积成倍扩大。人类肺泡化发育于孕26周左右开始,持续到生后3岁[4],但围产期是肺泡大量发育的关键期,此期诸多不良因素,如早产、化学物质、感染等会阻碍肺泡正常发育,造成BPD。本实验观察到对照组新生大鼠随日龄增长,肺泡间隔渐变薄,次级隔萌出,数目渐增多,14日龄次级分隔基本结束,肺泡面积变小,S/V增大,肺泡数增多,肺泡基本成熟,有限肺容量下肺气体交换的表面积增加。
产前3次注射注射DXM组的新生大鼠肺泡成熟化受到抑制,肺泡间隔的变薄现象出现早,使气体通过呼吸膜的弥散速度加快,但次级隔发育较对照组明显受阻,数目减少,较短,肺泡呈现“大而少”的征象,肺泡化水平降低,阻碍了呼吸膜面积的扩大,影响了肺通/换气功能的储备,使之在机械正压通气下或/和炎症、化学等致肺损伤因素的作用下易于肺气肿或气胸的产生。有报道糖皮质激素使发育期的羊在机械通气下易产生肺气肿[10]。Satoru等[8]研究也发现类似现象:产前注射DXM的大鼠,10日龄仔鼠肺泡化明显受到抑制,表现肺泡直径变大,肺泡次级间隔发育受阻,总肺泡数减少;至13-36 d,出现追赶性肺泡化生长,先回复到不成熟状态,继而生出次级隔,但延迟的肺泡化不能代偿完全,至成年60 d治疗组肺泡直径仍大于对照组,肺泡总数少于对照组。
我们实验产前1次注射组鼠除1日龄的间隔厚度较对照组变薄外,余改变不明显,提示ACT对肺泡成熟的影响存在剂量或重复次数的依从性,单剂ACT较安全。因国外相关研究产前用DXM的剂量相差较大[8],并无定论,故本实验依据产科DXM用于孕妇最多重复5次的总剂量,按人鼠体表面积比折算成大鼠的用量分3次注射做为3次注射组[11],1次注射组相当于重复1.5次,而介于两者之间的剂量对肺泡发育的影响程度待进一步研究。
3次注射组肺泡发育停滞,呈现“大而少”的征象与早产儿BPD的发生和发展关系密切。ACT 减低了24 周至29周出生的早产儿RDS发生率,但并无改善其BPD的发生率和住院时间[12]。1999年Jobe[13]提出以肺发育受阻为特征的 “新型BPD”的说法,病理学上表现为少而大的肺泡,推测胎肺炎症,产后暴露于机械通气、吸氧等病理刺激,协同糖皮质激素、营养不良等均会致肺泡发育受抑。BPD与多剂ACT以及生后用激素的实验肺病理形态学改变相似高度提示ACT可能参与或与其它高危因素协同促进BPD的发生和发展[3],甚至影响成年后的肺功能[2,7,8]。糖皮质激素用于BPD的治疗可抑制炎症反应,但也发现类似损害肺泡化,阻碍肺发育的现象[14]。因在BPD患儿中ACT以及生后用激素的机率较高,故对ACT加重BPD的可能性值得深入研究。
肺的发育和成熟有赖糖皮质激素的作用,促皮质释放激素基因敲除的小鼠血浆糖皮质激素水平很低,出现远端肺实质解剖上成熟障碍[15]。大鼠在生后10 d内血浆皮质激素浓度出现一低谷期,恰为肺泡次级隔生成期,而过量的糖皮质激素可阻碍肺泡化。次级隔的长出,毛细血管迅速长入形成网状结构,上皮细胞、内皮细胞、间质、间质细胞间的接触和相互作用等与肺上皮细胞基底膜(BM)的重建关系密切[16],我们进一步观察了基底膜在肺泡发育中的变化。1、4日龄鼠外周肺电镜可见上皮细胞BM变薄,多处不连续,细胞浆伸出伪足穿越这些BM的孔隙进入下面的间质,与间质细胞,主要是成纤维细胞靠近、紧密接触,考虑BM的改变可能与上皮细胞的分化、增殖等生物活动有关,促进肺泡的发育。产前3次注射DXM作用下外周肺上皮细胞BM的不连续现象较少见,鲜见伸出伪足,上皮间质的直接接触较少,说明ACT使肺泡发育中的各个细胞接触、交流受阻,导致肺发育障碍。
糖皮质激素促进Ⅱ型肺泡上皮细胞成熟,但同时也减弱了Ⅱ型肺泡上皮细胞的增殖以及抑制其向Ⅰ型细胞转化,导致Ⅰ型细胞减少(后者为扩大肺泡交换膜面积的细胞),影响上皮细胞向内腔的隆起,即次级隔的长出;此外新生的次级隔必须充满毛细血管和成纤维细胞,此需相应细胞大量复制增殖,以使次级隔延长,糖皮质激素抑制细胞增殖。亦有研究认为ACT抑制了机体的抗氧化系统,使肺易受脂质氧化的损伤,而造成肺泡发育受阻[17,18]。
多中心研究表明,1个疗程的激素与每周重复使用激素比较,新生儿NRDS发生率并无显著差异[9],从正面作用角度亦无必要重复使用糖皮质激素促胎肺成熟。鉴于1次注射组DXM对新生鼠肺泡形态发育影响不大,而3次注射组DXM明显影响新生鼠肺泡形态发育,我们建议产前多疗程糖皮质激素治疗措施不常规用于有持续早产危险的孕妇,而常规1疗程的产前糖皮质激素治疗是安全的。
[1] Bonanno C,Wapner RJ. Antenatal corticosteroid treatment:what’s happened since Drs Liggins and Howie? [J].Am J Obstet Gynecol,2009,200(4):448-457.
[2] Pérez Pérez G, Navarro Merino M.Bronchopulmonary dysplasia and prematurity.Short-and long-term respiratory changes[J]. An Pediatr(Barc),2010, 72(1):79.e1-e16.
[3] Kramer BW,Lievense S,Been JV,et al. From classic to new bronchopulmonary dysplasia[J].Ned Tijdschr Geneeskd,2010,154: A1024.
[4] Smith LJ, McKay KO, van Asperen PP,et al.Normal development of the lung and premature birth[J]. Paediatr Respir Rev,2010,11(3):135-142.
[5] Koenen SV, Dunn EA, Kingdom JC, et al. Overexposure to antenatal corticosteroids:a global concern[J].J Obstet Gynaecol Can,2007, 29(11):879.
[6] Bevilacqua E,Brunelli R,Anceschi MM. Review and meta-analysis: Benefits and risks of multiple courses of antenatal corticosteroids[J]. J Matern Fetal Neonatal Med,2010, 23(4):244-260.
[7] Dalziel SR,Rea HH,Walker NK,et al. Long term effects of antenatal betamethasone on lung function:30 year follow up of a randomised controlled trial[J]. Thorax,2006,61(8):678-683.
[8] Okajima S,Matsuda T, Cho K,et al. Antenatal dexamethasone administration impairs normal postnatal lung growth in rats[J]. Pediatr Res,2001, 49(6):777-781.
[9] Mazumder P, Dutta S, Kaur J,et al. Single versus multiple courses of antenatal betamethasone and neonatal outcome: a randomized controlled trial[J]. Indian Pediatr,2008,45(8):661-667.
[10]Polk DH, Ikegai M, Jobe AH, et al. Preterm lung function after retreatment with atenatal betamethasone in preterm lambs[J]. Am J Obstet Gynecol,1997,176(2):308-315.
[11]章元沛 主编.药理学实验[M].北京:人民卫生出版社,1996.241.
[12]Manktelow BN, Lal MK, Field DJ, et al. Antenatal corticosteroids and neonatal outcomes according to gestational age: a cohort study[J]. Arch Dis Child Fetal Neonatal Ed,2010,95(2):F95-F98.
[13]Jobe AH,The new BPD:an arrest of lung development[J].Pediatr Res,1999,46(6):641-643.
[14]Grier DG, Halliday HL.Effects of glucocorticoids on fetal and neonatal lung development[J]. Treat Respir Med,2004,3(5):295-306.
[15]Muglia LJ,Bae DS,Brown TT,et al.Proliferation and differentiation defects during lung development in corticotropin releasing hormone- deficient mice[J]. Am J Respir Cell Mol Biol,1999, 20(2): 181-188.
[16]Burri PH.Structural aspects of postnatal lung development-alveolar formation and growth[J]. Biol Neonate,2006,89(4):313-322.
[17]Verhaeghe J, van Bree R, van Herck E. Oxidative stress after antenatal betamethasone: acute downregulation of glutathione peroxidase-3[J]. Early Hum Dev,2009,85(12):767-771.
[18]陈佰义,姜 莉,侯显明.实验性肺纤维化大鼠肺泡巨噬细胞脂质过氧化先于其细胞因子的释放[J]. 中国病理生理杂志,2000,16(11):1228-1230.
Antenatalexposuretodifferentdosesofdexamethasoneleadstodelayedalveolarizationinnewbornrats
CHEN Shang-qin1, ZHENG Ya-bing1, LI Chang-chong2, CHEN Chao3, LIN Zhen-lang1
(1NewbornDivision,2RespiratoryDivision,TheAffiliatedYuyingChildren’sHospital,WenzhouMedicalCollege,Wenzhou325027,China;3NewbornDivision,AffiliatedChildren’sHospital,FudanUniversity,Shanghai200032,China.E-mail:linzhenlang@hotmail.com)
AIM: To evaluate the effects of prenatal dexamethasone(DXM) exposure on growth and lung maturation in rats.METHODSTwenty-four pregnant Sprague-Dawley(SD) rats were divided into 3 groups: 8 pregnant rats in antenatal thrice-DXM group were injected intramuscularly with DXM(0.8 mg·kg-1·day-1) for 3 consecutive days(on gestational day 18, 19 and 20); 8 pregnant rats in antenatal once-DXM group were injected intramuscularly with DXM on gestational day 19. The control rats
equivalent volume of isotonic saline at the same time points. The lungs of the newborn rats on day 1, 4, 10 and 14 were examined. The tissue sections were prepared with HE staining for morphological investigation under light microscope. The data analysis was performed by means of a digital image analysis system, including thickness of interalveolar walls, alveolar(saccules) areas, the number of air spaces, the number and length of secondary septa, and the surface densities of the air spaces. Transmission electronic microscope was used to observe the basement membrane of the lung epithelial cells.RESULTSIn control group, and thickness of interalveolar walls, smaller alveolar(saccules) area,increased surface area/volume and number of air spaces were observed from day 1 to day 14. On day 4, an appreciable number of sprouting crests appeared, then the number and length of secondary septa increased until day 14. In antenatal thrice-DXM group, the air spaces were larger, and the thickness of intersaccular walls were thinner as compared to saccules of the control rats on day 1(P<0.01,P<0.05). There were thinner inter-air space septa(P<0.05), larger and fewer air spaces, and a reduction in the number of sprouting secondary septa on day 4, 10 and 14 than those in the control rats with significant differences(P<0.01). In antenatal once-DXA group, intersaccular walls were thinner than those in the control rats with significant differences(P<0.05). The results of electron microscopy revealed that the basement membrane of lung epithelial cells turned thin and discontinuous on day 1 and day 4 in the control rats, resulting in the close contact between epithelial cells and fibroblasts. In antenatal thrice-DXN group, the discontinuous basement membrane and close contact of the cells were seldom seen.CONCLUSIONAntenatal thrice-injection of DXM applied on gestational day 18, 19 and 20 impairs the alveoli formation in newborn rats. Antenatal once-injection of DXM has no remarkable effect on postnatal alveolarization.
Prenatal exposure; Dexamethasone; Pulmonary alveoli; Newborn rats
R363
A
10.3969/j.issn.1000-4718.2011.02-025
1000-4718(2011)02-0343-07
2010-09-15
2010-11-08
△通讯作者 Tel:0577-88816447;E-mail: linzhenlang@hotmail.com

