同种异体骨髓间充质干细胞在急性坏死性胰腺炎大鼠中的迁移和分化
陆奕 高军 吴洪玉 龚燕芳 赵航 李兆申
·论著·
同种异体骨髓间充质干细胞在急性坏死性胰腺炎大鼠中的迁移和分化
陆奕 高军 吴洪玉 龚燕芳 赵航 李兆申
目的观察同种异体来源的骨髓间充质干细胞(bone marrow mesenchymal stem cells, MSCs)在急性坏死性胰腺炎(ANP)大鼠中的迁移及分化状况。方法分离、纯化雄性SD大鼠的骨髓MSCs。按数字表法将雌性SD大鼠分为正常移植组、ANP移植组和ANP组,每组10只。采用腹腔注射L-精氨酸方法制备ANP模型。24 h后2个移植组大鼠经尾静脉输注MSCs。移植后72 h处死大鼠,取胰腺及心脏、肝脏、肾脏组织标本。胰腺组织常规病理检查并评分,采用原位杂交方法检测各组织Y染色体雄性鉴别基因sry片段的存在。结果分离的MSCs在培养3~5 d内快速分裂增殖,形成集落,传代培养至第3代,CD29+CD44+CD45-细胞群达到95%以上。ANP组大鼠胰腺组织大片坏死,大量炎细胞浸润,病理分值为(10.31±0.85)分,显著高于ANP移植组的(7.30±0.79)分(P﹤0.05)。移植组大鼠的心脏、肝脏、胰腺、肾脏均可检测到sry基因。正常移植组胰腺组织可见散在分布的sry阳性细胞;ANP移植组胰腺组织可见较多sry阳性细胞,且多聚集于损伤较严重的部位。结论炎性损伤的胰腺组织可能具有招募MSCs的能力,并能减轻大鼠胰腺局部的炎症反应。
胰腺炎,急性坏死性; 骨髓间质干细胞; 细胞运动; 细胞分化; 原位杂交
干细胞移植已经初步应用于多种人类疾病的治疗[1]。骨髓间充质干细胞(MSCs)具备很强的分化潜能,在特定条件下,可诱导分化为多种组织细胞[2-3],还可随血液循环到达其他器官组织[3], 以满足其生理更新和病理损伤修复的需要。文献报道[3-4],动员MSCs可减轻重症急性胰腺炎病情,改善其预后。本研究观察同种异体MSCs在正常大鼠与急性坏死性胰腺炎(ANP)大鼠胰腺组织中的迁移、分化情况。
材料与方法
一、MSCs的分离、培养和鉴定
100~150 g雄性SD大鼠,由第二军医大学动物实验中心提供。在无菌条件下分离出股骨。剪开骨端,用10%FBS反复冲洗髓腔,直至骨干发白。骨髓冲洗液以800 r/min离心5 min,弃上清,加入含10%FBS的DMEM+F12(Gibco BRL公司)培养液5 ml吹打均匀,按5×105/cm2密度接种于含上述培养液的培养瓶中常规培养。待细胞生长达90%融合时传代,此后约1周左右传代一次。传至第三代时收获细胞并计数,分别加入5个离心管,每管约105个细胞。3个管分别加入anti-CD29-PE/CY5(Biolegend公司)、anti-CD44-FITC(AbD Serotec公司)、anti-CD45-PE(Biolegend公司);1个管同时加入3种抗体(混合管);1管为空白管。常温下避光孵育30 min后PBS洗涤二次,重悬细胞。上流式细胞仪(美国Becton Dickinson公司)分析CD29+CD44+CD45-细胞占总体细胞比例。
二、大鼠模型的建立及MSCs移植
雌性SD大鼠,亦由第二军医大学动物实验中心提供。体重180~220 g,清洁级,适应性喂养1周。实验前12 h禁食。按数字表法将大鼠随机分为正常移植组、ANP移植组和ANP组,每组10只。参考文献[5],以腹腔注射L-精氨酸(Sigma公司)2.5 g/kg体重2次、间隔1 h方法制备急性坏死性胰腺炎(ANP)模型。造模后24 h,2个移植组大鼠经尾静脉输注三代以上的MSCs(5~7)×107个。72 h后处死,取胰腺及心脏、肝脏、肾组织,常规固定。
三、病理检查及原位杂交
胰腺组织常规病理检查,采用盲法由病理科医师阅片,按照Schmidt标准[6]进行评分。对胰腺及心、肝、肾组织,应用大鼠雄性决定基因Y染色体的sry探针(天津灏洋生物公司)行原位杂交。
结 果
一、MSCs体外培养特征
原代培养时, MSCs大量增殖形成“爆发”样集落,同时也存在散在分布的贴壁MSCs,呈现为三角形、梭形等形态,小部分细胞可呈单极、多角或不规则形状。传代培养后,散在分布的贴壁MSCs依然呈梭形、三角形、多角形等形态。起初,数个增殖活跃的MSCs稀疏排列在一起,3~5 d内,细胞大量分裂增殖,快速形成集落 (图1)。

图1 原代(a)及传代(b)培养的MSCs(×100)
二、MSCs细胞表型
原代培养细胞有多个细胞群,主要有CD29+CD44+CD45-, CD29+CD44-CD45+, CD29+CD44-CD45-等,其中CD29+CD44+CD45-群为MSCs细胞。传代培养后CD29+CD44+CD45-群的比例增加,至第3代时,达到95%以上。
三、大鼠胰腺组织病理改变
ANP组大鼠胰腺明显充血、水肿;镜下见胰腺组织腺泡结构明显破坏,大片坏死,大量炎细胞浸润,病理分值为(10.31±0.85)分。ANP移植组胰腺充血水肿较ANP组明显改善;镜下见腺泡结构较完整,少量炎细胞浸润,出血及坏死均较ANP组明显减轻,病理分值为(7.30±0.79)分,较ANP组显著降低(P﹤0.05)。正常移植组胰腺大体及镜下均无明显改变(图2左)。
四、同种异体移植MSCs在体内的定位
正常移植组和ANP移植组大鼠的心脏、肝脏、胰腺、肾脏均可检测到sry基因,而ANP组大鼠未检测到sry基因。正常移植组胰腺内见散在的sry阳性细胞;ANP移植组大鼠胰腺内也可见sry阳性细胞,多聚集于损伤较严重部位的细胞内;ANP组大鼠胰腺内未见sry阳性细胞(图2右)。
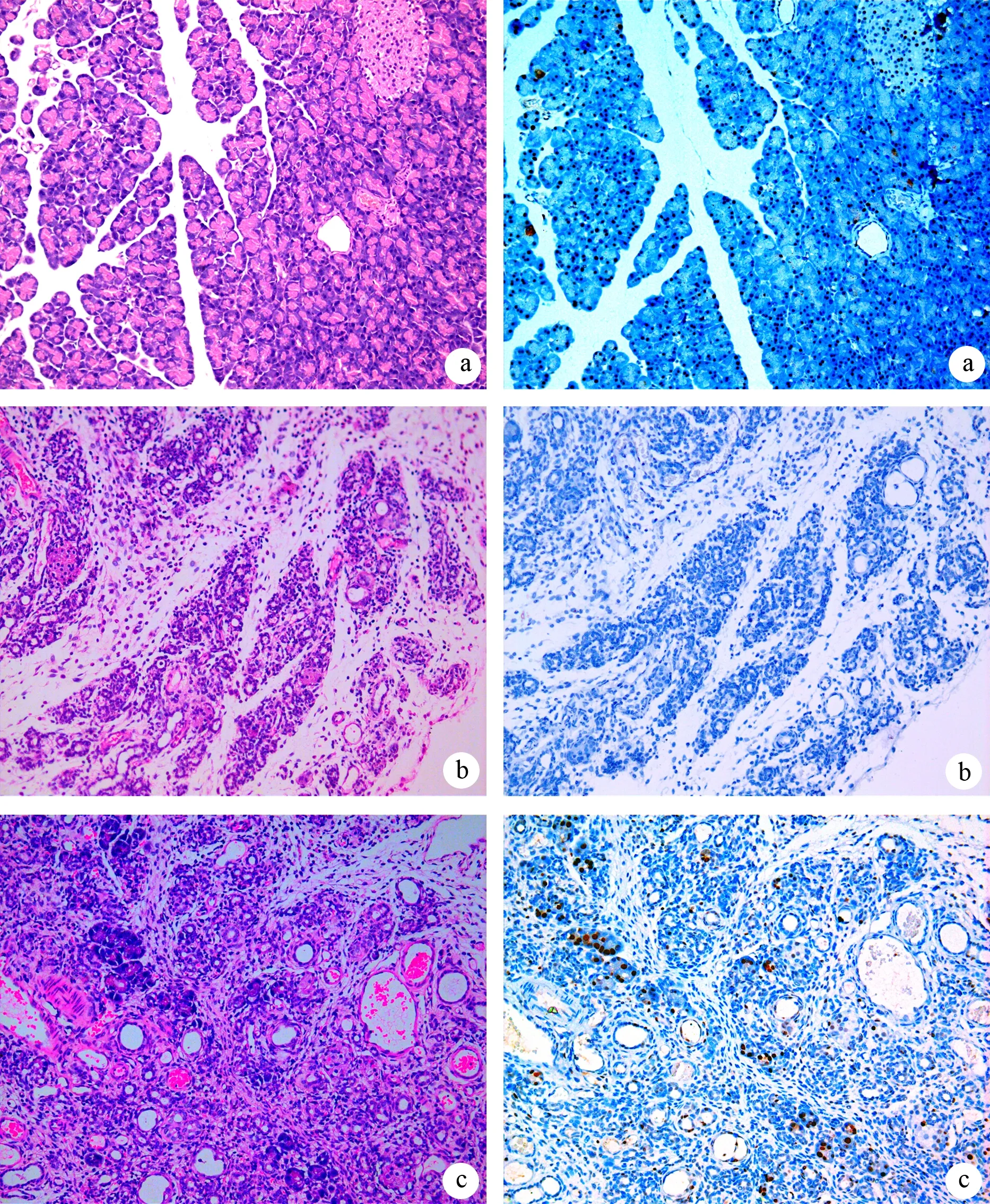
图2正常移植组(a)、ANP移植组(b)和ANP组(c)胰腺组织病理改变(左列,HE ×200)和胰腺组织sry阳性细胞(右列,原位杂交 ×200)
讨 论
生理情况下,胰腺的自我更新依赖于胰腺干细胞。Bonner-Weir等[7]切除大鼠90%胰腺后,残存的胰腺明显增殖,形成新的胰岛和胰腺外分泌组织,提示胰腺存在能分化成具有特异功能的细胞。Ishiwata等[8]在L-精氨酸诱导胰腺炎的胰腺组织内检测到nestin阳性细胞,提示胰腺成体干细胞参与了胰腺的再生。
分离、纯化人胰腺成体干细胞比较困难,而MSCs则有取材方便、创伤性小、不受伦理道德和移植免疫排斥影响等优点,并可在体外大量培养扩增。本实验结果显示,体外分离培养的大鼠MSCs可迁移至胰腺组织。在ANP大鼠,干细胞多聚集于损伤较严重的部位,推测损伤部位可能产生某种细胞因子,招募干细胞到达该部位参与组织损伤修复,与Prockop[9]的研究结果一致。移植MSCs后,ANP大鼠胰腺的炎症损伤程度明显低于对照组,表明同种异体移植的MSCs在胰腺新的微环境下转化为胰腺干细胞,参与胰腺组织重建,遏制胰腺进行性坏死。当然,MSCs在胰腺发生转分化的直接证据需要通过原位杂交与免疫组化共定位来进一步证实。
[1] Bianel P,Robey PG.Stem cells in tissue engineering.Nature,2001,414:118-121.
[2] Pittenger MF,Martin BJ.Mesenchymal stem cells and their potential as cardiac therapeutics.Circ Res,2004,95:9-20.
[3] Cui HF,Bai ZL.Protective effects of transplanted and mobilized bone marrow stem cells on mice with severe acute pancreatitis.World J Gastroenterol,2003,9:2274-2277.
[4] 江学良,李兆申,崔慧斐.骨髓间充质干细胞在胰腺生理更新和病理再生中的作用.世界华人消化杂志,2006,14:398-404.
[5] Tani S,Itoh H,Okabayashi Y,et al.New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats.Dig Dis Sci,1990,35:367-374.
[6] Schmidt J,Rattner DW,Lewandrowski K,et al.A better model of acute pancreatitis for evaluating therapy.Ann Surg,1992,215:44-56.
[7] Bonner-Weir S,Taneja M,Weir GC,et al.In vitro cultivation of human islets from expanded ductal tissue.Proc Natl Acad Sci USA,2000,97:7999-8004.
[8] Ishiwata T,Kudo M,Onda M,et al.Defined localization of nestin expressing cells in L-arginine induced acute pancreatitis.Pancreas,2006,32:360-368.
[9] Prockop DJ.Marrow stromal cells as stem cells for nonhematopoietic tissues.Science,1997,276:71-74.
2010-02-05)
(本文编辑:屠振兴)
Migrationanddifferentiationofintravenouslytransplantedbonemarrowmesenchymalstemcellsinratswithacutenecrotizingpancreatitis
LUYi,GAOJun,WUHong-yu,GONGYan-fang,ZHAOHang,LIZhao-shen.
DepartmentofGastroenterology,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
LIZhao-shen,Email:zhsli@81890.net
ObjectiveTo observe whether the intravenously transplanted homologous bone marrow mesenchymal stem cells (MSCs) can migrate to the impaired pancreas tissue in the rats with acute necrotizing pancreatitis (ANP).MethodsMSCs were isolated from bone marrow of male Sprague-Dawley (SD) rats and adhered to the culture plate in vitro. Female rats were divided randomly into 3 groups: normal transplantation group, ANP transplantation group, ANP group with 10 rats in each group. ANP was induced by intraperitoneal injections with L-arginine. Both transplantation group
MSCs infusion through tail vein. 72 h later, the rats were sacrificed, the pancreas, heart, liver and kidney tissues were harvested, and the morphological changes were examined and scored, the characteristics of migration of MSCs to pancreas were detected with the expression of sry gene of Y chromosome by using chromogenic in situ hybridization (CISH).ResultsRapid proliferation occurred in isolated MSCs after culture for 3~5 days and colonies were formed. After 3 generations, CD29+CD44+CD45-cells accounted for over 95% of all the cells. There ware massive tissue necrosis, inflammatory cells infiltration in the pancreas of ANP group, the pathological score was 10.31±0.85, which were significantly higher than that in ANP transplantation group (7.30±0.79,P﹤0.05). Sry gene could be detected in the pancreas, heart, liver and kidney tissues. In addition, scattered distributed sry positive cells were observed in the normally transplanted pancreatic tissue, lots of sry positive cells were observed in the ANP transplanted pancreatic tissue, and they were located in the most injured areas.ConclusionsThe inflammatory pancreatic tissue has the ability of recruiting MSCs in vivo, which can alleviate local inflammation.
Pancreatitis, acute necrotizing; Bone marrow mesenchymal stem cells; Cell movement; Cell differentiation; In situ hybridization
10.3760/cma.j.issn.1674-1935.2011.01.014
国家自然科学基金(30670943)
200433 上海,第二军医大学长海医院消化内科(陆奕、高军、吴洪玉、龚燕芳、李兆申);上海市第六人民医院内科(陆奕);上海市第一人民医院消化内科(赵航)
李兆申,Email:zhsli@81890.net

