胰腺纤维化后结缔组织生长因子的表达及意义
李嘉 刘爽 孙海晨 崔叶青 李非
·论著·
胰腺纤维化后结缔组织生长因子的表达及意义
李嘉 刘爽 孙海晨 崔叶青 李非
目的观察胰腺纤维化后结缔组织生长因子(connective tissue growth factor ,CTGF)在胰腺组织内的表达,探讨其意义。方法通过高脂饲料诱导大鼠胰腺纤维化模型,16周后处死大鼠,取胰腺组织常规病理检查,天狼猩红染色和免疫组织化学染色检测胰腺纤维化组织胶原蛋白Ⅰ、α-SMA及CTGF蛋白表达。结果胰腺纤维化后,胰腺小叶和腺泡萎缩,小叶间隙增宽,间质内纤维组织明显增生;胰腺组织内胶原蛋白Ⅰ的合成较正常胰腺明显增加(1207.3±115.5比166.7±78.4,P<0.01),α-SMA表达量较正常胰腺组织增高(1500.2±255.8比57.4±23.2,P<0.01),CTGF表达较正常胰腺明显增加( 2950.5±431.9比382.2±190.8,P<0.01),且胰腺星状细胞(PSCs)大量活化。结论CTGF是胰腺纤维化的重要作用因子,其作用与PSCs活化密切相关。
胰腺; 纤维化; 结缔组织生长因子; 星形细胞
胰腺纤维化是慢性胰腺炎(CP)典型的组织病理学特征,主要表现为胰腺组织中有过量的细胞外基质(extracellular matrix,ECM)沉积[1]。结缔组织生长因子(connective tissue growth factor, CTGF)是一种富含半胱氨酸的分泌多肽, 表达于多种组织器官。大量研究表明,CTGF是重要的促组织纤维化蛋白,可经自分泌及旁分泌两种方式调节细胞增殖、迁移及ECM合成[2]。体外研究表明,在胰腺纤维化进程中起核心作用的胰腺星状细胞(PSCs)能够表达CTGF基因[3]。给予外源性CTGF会导致PSCs的增殖、迁移[4]。但在纤维化的胰腺组织内CTGF表达及其与PSCs的关系,目前尚罕见报道。本实验观察胰腺纤维化大鼠胰腺组织CTGF的表达,探讨其意义。
材料与方法
一、实验动物及分组
雄性SD大鼠12只,体重200~300 g,SPF级,购自北京维通利华公司。按数字表法随机分为对照组和纤维化组,各6只。对照组以常规饲料喂养,纤维化组动物以高脂饲料喂养[5]。大鼠饲养16周,经麻醉后腹部正中切口入腹,迅速切取胰腺组织,10%中性甲醛溶液固定,常规石蜡包埋。
二、方法
1.胰腺组织病理检查:常规HE染色,光镜下检查。
2.胶原蛋白Ⅰ表达检测:采用天狼猩红染色,具体步骤参照本实验室以往方法[5]。即切片脱蜡水化后置入天青石蓝液染6 min,蒸馏水洗3次后置入天狼猩红饱和苦味酸液染15 min,无水乙醇直接分化与脱水,光镜下观察胰腺组织中胶原蛋白沉积部位。Ⅰ 型胶原纤维呈红色,细胞核呈绿色,其余呈黄色。
3.α-SMA与CTGF蛋白检测:采用常规免疫组化染色方法。小鼠抗人α-SMA单抗购自福州迈新公司,兔抗大鼠CTGF多抗购自美国Abcam公司。细胞胞质和(或)胞膜呈棕褐色且背景清晰者为阳性,反之为阴性。
4.结果判断:在200倍光镜下随机全盲选取5个视野,摄片后用Image Pro-plus 6.0图像分析软件测定阳性表达区域的积分光密度值(integrated optical density,IOD)和特定区域内的平均光密度值(average optical density,AOD),评价其表达量及表达强度。
三、统计学处理
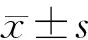
结 果
一、胰腺组织学改变
对照组大鼠胰腺组织无明显病理变化。纤维化组大鼠胰腺小叶和腺泡萎缩,小叶间隙增宽,间质内纤维组织明显增生(图1);天狼猩红染色见间质内血管、导管及胰岛周围胶原纤维增生明显,部分破坏和萎缩的腺泡结构以及胰岛被胶原纤维穿插、包绕,呈结节状纤维化(图2)。纤维化组大鼠胰腺组织胶原蛋白Ⅰ的含量为1207.3±115.5,较对照组的166.7±78.4显著增加(P<0.01)。
二、胰腺组织α-SMA表达的变化
对照组α-SMA阳性细胞仅见于小叶间隔中较大的脉管壁,而胰腺小叶内没有α-SMA表达。纤维化组腺泡周围,尤其是包绕于脉管、腺泡和胰岛周围的增生纤维组织中可见大量α-SMA阳性表达细胞,呈星形、梭形或椭圆形等形态,部分细胞可伸出较长的胞质突触,包绕在腺泡周围(图3)。纤维化组胰腺α-SMA表达量为1500.2±255.8,显著高于对照组的57.4±23.2(P<0.01)。
三、胰腺组织CTGF表达的变化
对照组胰腺在邻近小叶间隙的腺泡细胞及血管内皮细胞有CTGF的弱表达,其他区域无CTGF表达。纤维化组胰腺在纤维组织增生的区域可见大量明显表达CTGF的细胞,位于腺泡周围及增生的胶原纤维中,在胰岛细胞、腺泡细胞及血管内皮细胞也有CTGF较弱的表达(图4)。纤维化组胰腺组织内CTGF表达量为2950.5±431.9,显著高于对照组的382.2±190.8(P<0.01)。
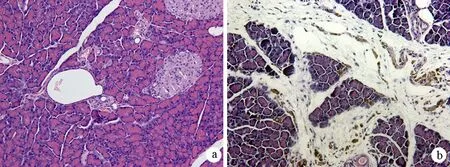
图1对照组(a)与纤维化组(b)的胰腺组织学改变(HE ×200)
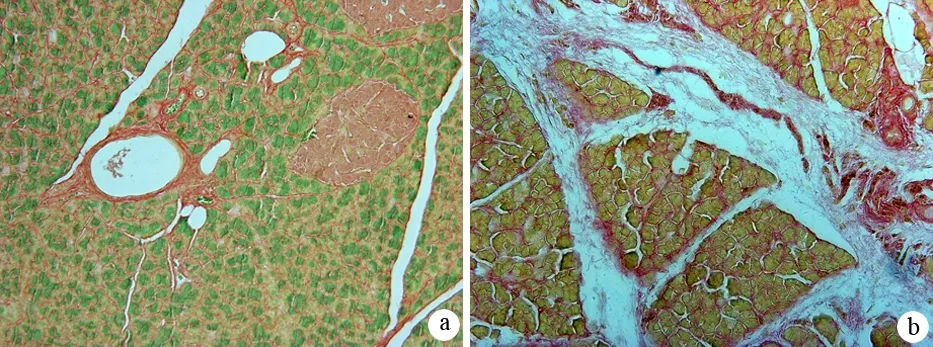
图2对照组(a)与纤维化组(b)胰腺组织胶原蛋白Ⅰ的表达(天狼猩红染色 ×200)
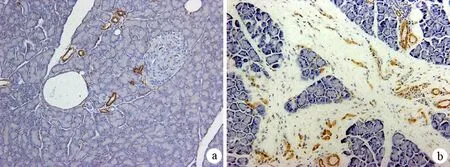
图3对照组(a)与纤维化组(b)胰腺组织α-SMA的表达(免疫组化 ×200)
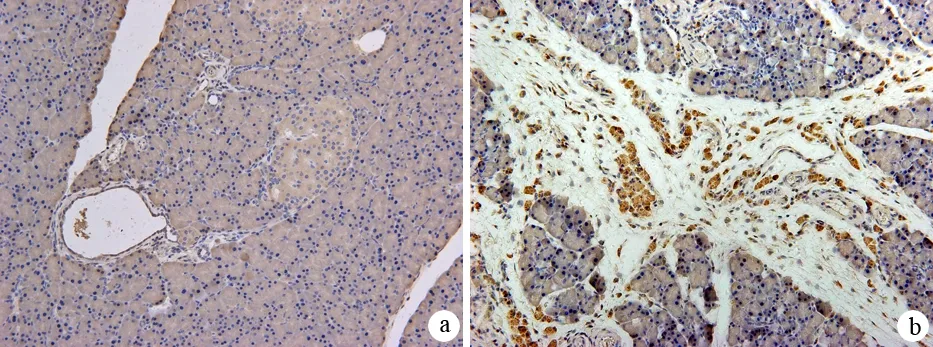
图4对照组(a)与纤维化组(b)胰腺组织CTGF的表达(免疫组化 ×200)
讨 论
胰腺纤维化是由不同原因导致的CP典型的组织病理学特征,为组织中有过量的ECM沉积,主要成分是胶原蛋白Ⅰ、Ⅲ。胰腺纤维化另一个典型的特征是纤维化的胰腺组织内有大量活化的PSCs。PSCs活化的标志是α-SMA阳性[6]。本实验结果显示,高脂饮食诱导的大鼠胰腺组织内有大量的胶原蛋白Ⅰ沉积,有大量α-SMA阳性表达的细胞,即活化的PSCs。活化的PSCs随后大量合成ECM蛋白、黏附因子以及趋化因子等物质。
CTGF是CCN家族的成员之一,C端富含半胱氨酸的分泌多肽[2],广泛分布于多种组织器官,如心脏、肺、肝、肾、胰腺、胎盘及结缔组织中。大量研究表明,CTGF是重要的促组织纤维化蛋白,主要功能为:(1)作为TGF-β的下游因子,促进肾成纤维细胞的有丝分裂[7];(2)促进多种ECM成分如蛋白多糖、胶原蛋白以及纤维连接蛋白、层黏蛋白的产生,抑制降解ECM的酶类活性,抑制ECM的降解[8];(3)促进成纤维细胞转化为表达α-SMA的成肌纤维细胞[9];(4)促进成纤维细胞向纤维连接蛋白黏附[10];(5)增加血管平滑肌细胞的迁移率[11];⑥促进血管形成[12]。
在正常胰腺组织,胰岛细胞和一些小的胰管上皮细胞可以表达CTGF,在重症急性胰腺炎的胰腺组织内,腺泡细胞也能够表达CTGF,腺泡细胞所表达的CTGF对于参与胰腺组织修复的细胞起到了旁分泌的调控作用[13]。本结果显示,胰腺纤维化组织内CTGF的表达明显高于正常胰腺组织,提示CTGF在胰腺纤维化的过程中起重要作用。进一步观察显示,CTGF显著表达于一些位于胰腺腺泡周围和小叶间隙内增生的胶原纤维中的细胞。通过与α-SMA的免疫组化染色比较,可以判断这些显著表达CTGF的细胞就是活化的PSCs。且PSCs的CTGF表达要明显高于腺泡细胞等胰腺实质细胞的表达,提示活化的PSCs可能是纤维化的胰腺组织内CTGF的主要来源。另一方面,CTGF在活化PSCs的强表达也提示PSCs活化后,CTGF对PSCs的调控作用主要是以自分泌作用为主。
[1] Etemad B,Whitcomb DC.Chronic pancreatitis:diagnosis,classification and new genetic developments.Gastroenterology,2001,120:682-707.
[2] Chen CC,Lau LF.Functions and mechanisms of action of CCN matricellular proteins.Int J Biochem Cell Biol,2009,41:771-783.
[3] Shinji T,Ujike K,Ochi K,et al.Establishment of a novel collagenase perfusion method to isolate rat pancreatic stellate cells and investigation of their gene expression of TGF-beta1,type Ⅰ collagen,and CTGF in primary culture or freshly isolated cells.Acta Med Okayama,2002,56:211-218.
[4] Karger A,Fitzner B,Brock P,et al.Molecular insights into connective tissue growth factor action in rat pancreatic stellate cells.Cellular Signalling,2008,20:1865-1872.
[5] Zhang X,Cui Y,Fang L,et al.Chronic high-fat diets induce oxide injuries and fibrogenesis of pancreatic cells in rats.Pancreas,2008,37:e31-e38.
[6] Bachem MG,Schneider E,Gross H,et al.Identification,culture and characterization of pancreatic stellate cells in rats and humans.Gastroenterology,1998,115:421-432.
[7] Kothapalli D,Grotendorst GR.CTGF modulates cell progression in camp-arrested NRK fibroblasts.J Cell Physiol,2000,182:119-126.
[8] Blaney Davidson EN,Vitters EL,Mooren FM,et al.Connective tissue growth fac2tor/CCN2 overexpression in mouse synovial lining results in transient fibrosis and cartilage damage.Arthritis Rheum,2006,54:1653-1661.
[9] Yokoi H,Mukoyama M,Sugawara A,et al.Role of connective tissue growth fac2n tor in fibronectin expression and tubulointerstitial fibrosis.Am J Physiol Renal Physiol,2002,282:F933-F942.
[10] Shi-Wen X,Stanton LA,Kennedy L,et al.CCN2 is necessary for adhesive responses to transforming growth factor-beta 1 in embryonic fibroblasts.J Biol Chem,2006,281:10715-10726.
[11] di Mola FF,Friess H,Riesle E,et al.Connective tissue growth factor is involved in pancreatic repair and tissue remodeling in human and rat acute necrotizing pancreatitis.Ann Surg,2002,235:60-67.
[12] Brigstock DR.Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61). Angiogenesis,2002,5:153-165.
[13] Yang M,Huang H,Li JZ,et al.Tyrosine phosphorylation of the LDL receptor-related protein (LRP) and activation of the ERK pathway are required for connective tissue growth factor to potentiate myofibroblast differentiation. FASEB J,2004,18:1920-1921.
2010-07-26)
(本文编辑:屠振兴)
Expressionofconnectivetissuegrowthfactorinfibroticpancreas
LIJia,LIUShuang,SUNHai-chen,CUIYe-qing,LIFei.
DepartmentofGeneralSurgery,XuanwuHospitalCapitalMedicalUniversity,Beijing100053,China
LIFei,Email:feili36@ccmu.edu.cn
ObjectiveTo observe the expression of connective tissue growth factor (CTGF) in pancreas, and discuss its significance.MethodsThe pancreatic fibrosis model was induced by high fat diets. The rats were sacrificed 16 weeks later, and the pancreatic tissue was harvested for routine pathologic examinations. Pancreatic collagen fibrosis I was determined by HE and Sirius red staining;α-SMA and CTGF expression were detected by immunohistochemistry.ResultsAfter pancreatic fibrosis, pancreatic lobules and acinar atrophy was observed, lobules gap was widened, interstitial fibrous tissue was significantly proliferated, the synthesis of pancreatic collagen fibrosis I was significantly increased when compared with normal pancreas (1500.2±255.8vs. 57.4±23.2,P<0.01), the expression of α-SMA was significantly increased when compared with normal pancreas(1500.2±255.8vs. 57.4±23.2,P<0.01), and the expression of CTGF was significantly increased when compared with normal pancreas (2950.5±431.9vs. 382.2±190.8,P<0.01), and there were abundant activated PSCs.ConclusionsCTGF participated in the regulation of pancreatic fibrosis development; the function of CTGF was closely related to PSCs activation.
Pancreatic; Fibrosis; Connective tissue growth factor; Astrocytes
10.3760/cma.j.issn.1674-1935.2011.04.015
100053 北京,首都医科大学宣武医院普通外科(李嘉、李非),外科实验室(刘爽、孙海晨、崔叶青)
李非, Email:feili36@ccmu.edu.cn

