胰腺癌患者血浆miR-155检测及其诊断价值
刘建强 高军 李兆申 任艳 王小玮 王卫卫 路华
·论著·
胰腺癌患者血浆miR-155检测及其诊断价值
刘建强 高军 李兆申 任艳 王小玮 王卫卫 路华
目的检测胰腺癌患者血浆miR-155表达量,评价其对胰腺癌的诊断价值。方法收集62例胰腺癌、61例慢性胰腺炎(CP)及36例正常对照者的血标本,抽提血浆RNA,应用实时PCR检测miR-155表达量,并分析其与胰腺癌临床参数的关系。应用接受者操作特征(ROC)曲线下面积(AUC)评价血浆miR-155表达量对胰腺癌的诊断价值。结果胰腺癌、CP和正常对照组的血浆miR-155表达量分别为5.41±3.14、2.59±2.49和0.77±1.17,胰腺癌血浆miR-155表达量显著高于CP及正常对照组(P<0.01 )。胰腺癌血浆miR-155表达量与患者年龄、性别、肿瘤大小等均无显著相关性,而与肿瘤TNM分期呈显著负相关(r=-0.323,P=0.01)。经ROC分析,胰腺癌对正常对照组的AUC为0.943(95%CI0.902~0.985),敏感性和特异性分别为87.1%和83.3%;胰腺癌对CP的AUC为0.762(95%CI0.678~0.846),敏感性和特异性分别为64.5%和73.8%;胰腺癌对正常对照组+CP组的AUC为0.829(95%CI0.767~0.892),敏感性和特异性分别为62.9%和84.5%。结论胰腺癌患者血浆miR-155表达量显著升高,对胰腺癌的诊断可能有一定的应用价值。
胰腺肿瘤; 微RNA; 诊断
血清CA19-9对胰腺癌的诊断敏感性为70%~80%[1],而特异性不到50%[2],这就需要发现新的胰腺癌肿瘤标志物。众多研究结果表明,microRNA(miRNA)与肿瘤的发病及预后相关,其中miR-155在胰腺癌组织中显著高表达[3]。本研究检测胰腺癌患者血浆miR-155表达量,评价其对胰腺癌的诊断价值。
资料和方法
一、临床资料
收集2008年1月至2009年12月第二军医大学附属长海医院收治的62例胰腺癌患者和61例慢性胰腺炎(CP)患者的血浆标本。所有胰腺癌患者均经手术及病理证实。CP患者通过影像学诊断,并随访半年以上无肿瘤发生者。以36例年龄匹配、无任何肿瘤病史的健康体检者作为正常对照组。
二、血浆miR-155检测
采集2 ml外周静脉血,置EDTA试管抗凝,4℃离心取上层血浆至1.5 ml Eppendorf管, 4℃ 12 000 g离心10 min后置-80℃保存。取200 μl血浆,加入5 μl作为内参的50 pmol/L的线虫miR-39(Qiagen公司),再加入20 μl 5 mol/L醋酸和750 μl TRI Regent BD(Molecular Research Center),按TRI Regent BD说明书抽提RNA。取2 μl RNA用TaqMan MicroRNA 逆转录试剂盒(Applied Biosystems公司)获取cDNA,反应条件:16℃ 30 min,42℃ 30 min,85℃ 5 min,4℃ 维持。取2 μl cDNA作为模板,用TaqMan Universal Master Mix Ⅱ试剂盒进行实时 PCR,miR-155和miR-39探针为TaqMan公司提供。PCR条件:95℃ 1 min,95℃ 15 s,60℃ 30 s,50个循环。每份标本设3个复孔。采用ABI 7500实时定量PCR仪进行检测,用SDS 2.1软件计算标本Ct值。miR-155表达量=-△△Ct=-[(Ct患者-Ct患者miR-39)-(Ct对照标本-Ct对照miR-39)]。
三、统计学处理
结 果
一、一般情况
62例胰腺癌患者中,男42例,女20例,中位年龄62岁(36~79岁)。其中胰头癌43例,胰体尾癌19例。TNM分期Ⅰ期10例,Ⅱ期15例,Ⅲ期14例,Ⅳ期23例。61例CP患者中,男44例,女17例,中位年龄48岁(19~76岁)。36例对照者中,男20例,女16例,中位年龄49岁(25~68岁)。
二、血浆miR-155表达量
胰腺癌、CP、对照组血浆miR-155表达量分别为5.41±3.14、2.59±2.49和0.77±1.17,胰腺癌组显著高于CP组及对照组(P<0.01)。CP组血浆miRNA表达量虽高于对照组,但无统计学意义。
三、血浆miR-155水平与胰腺癌临床病理参数的关系
血浆miR-155水平与胰腺癌患者年龄、性别、CA19-9水平、肿瘤最大直径、局部淋巴结和远处转移均无显著相关性,但与T分期和临床TNM分期呈显著负相关(P<0.05,表1)。
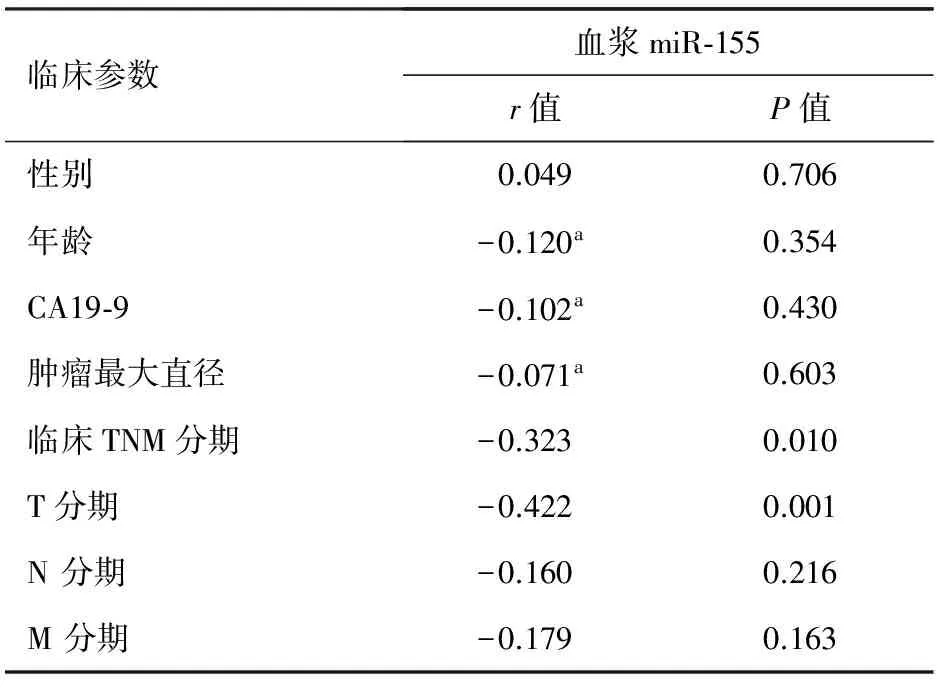
表1 血浆miRNA表达量与胰腺癌临床参数的关系
注:a:pearson相关分析,其他采用spearman秩相关分析
四、血浆miR-155表达量对胰腺癌的诊断价值
根据logistic回归模型,血浆miR-155诊断胰腺癌的AUC及诊断敏感性、特异性、准确性、阳性预测值、阴性预测值见表2。血浆miR-155表达量在鉴别胰腺癌与对照组时的敏感性和特异性较高。
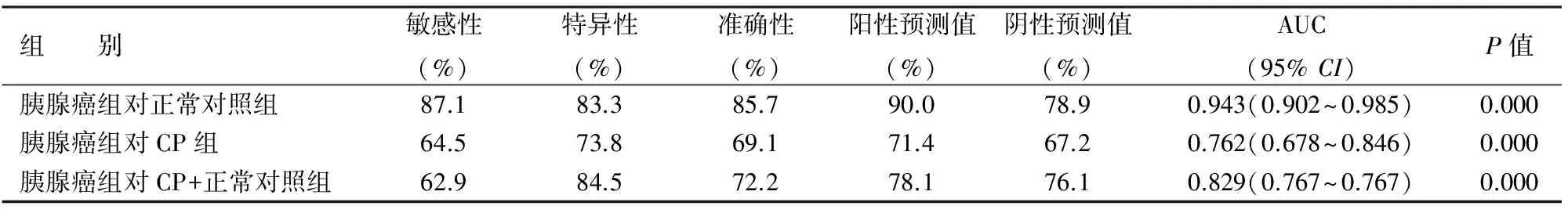
表2 血浆miR-155对胰腺癌的诊断价值
讨 论
miRNA是一类内源性的非编码调控单链小分子RNA,长度约18~25个核苷酸,通过对mRNA的调控,参与细胞增殖、分化、凋亡和代谢等过程的调节[4],目前已可预测哺乳动物约30%基因表达的miRNA调控靶点[5]。通常情况下,miRNA通过沉默基因表达而调控细胞功能,其表达异常可导致肿瘤等疾病的发生[6-7]。已经证实,miRNA在结肠癌、乳腺癌和肺癌等肿瘤中起负调控作用[8-9],在血液系统淋巴瘤和白血病中miRNA异常表达[10-11]。
MiR-155在甲状腺癌[12]、乳腺癌[2]、结肠癌[10]和胰腺导管腺癌[13-14]等肿瘤中高表达,并在肿瘤发生中起癌基因的作用[15]。研究表明,在胰腺癌早期肿瘤蛋白53诱导核蛋白1(TP53INP1)缺失,而miR-155参与了TP53INP1的转录后调控。胰腺癌细胞株转染miR-155后伴有TP53INP1缺失[16]。本结果显示,胰腺癌患者血浆miR-155表达量显著高于CP患者和对照组,CP患者血浆miR-155表达量也显著高于对照组,与正常胰腺到CP,再发展为胰腺癌的规律相符,提示miR-155具有胰腺癌的肿瘤特异性。血浆miR-155水平与胰腺癌大小、是否局部淋巴结和远处转移等临床参数之间无显著相关性,而与肿瘤的T分期和临床TNM分期呈负相关,这是否表示肿瘤浸润胰腺周边组织及血管者伴随血浆miR-155的降低还需进一步研究证实。通过logistic回归及ROC曲线分析,血浆miR-155对胰腺癌诊断的敏感性和特异性较理想,有一定的临床应用价值。
[1] Slesak B, Harlozinska-Szmyrka A, Knast W, et al. Tissue polypeptide specific antigen (TPS), a marker for differentiation between pancreatic carcinoma and chronic pancreatitis. A comparative study with CA 19-9. Cancer,2000,89: 83-88.
[2] Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res,2005,65:7065-7070.
[3] Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA,2007,297:1901-1908.
[4] Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell,2004,116: 281-297.
[5] Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet,2008,9:102-114.
[6] Slack FJ, Weidhaas JB. MicroRNA in cancer prognosis. N Engl J Med,2008,359:2720-2722.
[7] Visone R, Croce CM. MiRNAs and cancer. Am J Pathol,2009,174:1131-1138.
[8] Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature,2005,435:834-838.
[9] Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA,2006,103:2257-2261.
[10] Calin GA, Liu CG, Sevignani C, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci USA,2004,101:11755-11760.
[11] Lawrie CH, Soneji S, Marafioti T, et al. MicroRNA expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int J Cancer,2007,121:1156-1161.
[12] Nikiforova MN, Tseng GC, Steward D, et al. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab,2008,93:1600-1608.
[13] Lee EJ, Gusev Y, Jiang J, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer,2007,120:1046-1054.
[14] Szafranska AE, Davison TS, John J, et al. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene,2007,26:4442-4452.
[15] Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer,2006,6:259-269.
[16] Gironella M, Seux M, Xie MJ, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci USA,2007,104:16170-16175.
(本文编辑:吕芳萍)
DiagnosticvalueofplasmamiR55forpancreaticcancer
LIUJian-qiang,GAOJun,LIZhao-shen,RENYan,WANGXiao-wei,WANGWei-wei,LUHua.
DepartmentofGastroenterology,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
LIZhao-shen,lizhaoshen@yahoo.com
ObjectiveTo detect the level of plasma miR-155 in patients with pancreatic cancer and evaluate its diagnostic value for pancreatic cancer.MethodsSixty-two cases of pancreatic cancer patients, 61 patients of chronic pancreatitis and 36 normal controls were included in the study. miR-155 in the total RNA extracted from the plasma was measured using real time PCR. The diagnostic parameters and relationship of the miR-155 with clinical characteristics in pancreatic cancer patients were analyzed.ResultsThe relative abundance of plasma miR-155 in pancreatic cancer, chronic pancreatitis and normal controls were 5.41±3.14,2.59±2.49 and 0.77±1.17, and the value was significantly higher in pancreatic cancer group compared with those of chronic pancreatitis and normal group (P<0.01). No significant correlation between the level of plasma miR-155 and age, sex, tumor size in pancreatic cancer patients was found. But there was a negative correlation between the level of plasma miR-155 and TNM staging (r=-0.323,P=0.01). For discriminating pancreatic cancer from normal control, the AUC ROC of miR-155 was 0.943 (95%CI0.902-0.985), and the sensitivity and specificity were87.1% and 83.3%, respectively. For discriminating pancreatic cancer from chronic pancreatitis, the AUC ROC of miR-155 was 0.762 (95%CI0.678-0.846), and the sensitivity and specificity were 64.5% and 73.8%, respectively. For discriminating pancreatic cancer from chronic pancreatitis and normal, the AUC-ROC of miR-155 was 0.829(95%CI0.767-0.892), and the sensitivity and specificity were 62.9% and 84.5%, respectively.ConclusionsPlasma miR-155 was
共同第一作者:高军
significantly increased in patients with pancreatic cancer, and can be used for diagnosis of pancreatic cancer.
Pancreatic neoplasms; MicroRNAs; Diagnosis
10.3760/cma.j.issn.1674-1935.2011.02.001
国家科技支撑计划资助项目(2006BAI02A12)
200433 上海,第二军医大学附属长海医院消化内科
李兆申,Email:lizhaoshen@yahoo.com
2010-12-29)

