Effect of magnesia on properties and microstructure of alkali-activated slag cement
Yong-hao FANG*, Jun-feng LIU, Yi-qun CHEN
College of Mechanics and Materials, Hohai University, Nanjing 210098, P. R. China
1 Introduction
Alkali-activated slag cement (AASC)is a type of new cementitious material with high strength (Yang et al. 2009; Zhao et al. 2007), high durability, and low water permeability(Douglas et al. 1992; Saud 2008; Bakhareva et al. 2003), and has been applied in different areas. It also has great potential in hydraulic structure repair. However, there is still an unsolved problem which has been hindering its wide application: the dry shrinkage of AASC paste is much greater than that of ordinary Portland cement, which may lead to cracking of paste, mortar, or concrete (Collins and Sanjayan 2000; Passuello et al. 2009).
To reduce or compensate for the shrinkage of cement paste, expansive agents have been widely and effectively used in preparing ordinary Portland cement concrete (Maltese et al.2005; Passuello et al. 2009). Of these, the CaO-type and ettringite type expansive agents are mostly used because they can cause greater expansion and the expansion is relatively easily controlled (Nagataki and Gomi 1998; Ju 2006; Maltese et al. 2005).
However, there are two problems when using the CaO-type or ettringite type expansive agent to compensate for the shrinkage of AASC: Firstly, there is not sufficient calcium hydroxide in AASC for the ettringite type expansive agent to react with and to further form ettringite. Secondly, both the CaO-type and ettringite type expansive agents release Ca2+ions as water is mixed, reacting with water glass added as the activator in the mixture and leading to flash setting of the cement. MgO-type expansive agents have also been used in concrete,especially in dam concrete (Mo et al. 2010; Gao et al. 2008). Compared with the CaO-type expansive agent, the MgO-type expansive agent has lower activity and releases Mg2+ions at a lower rate, and therefore may have less effect on the setting time of AASC. In this study,the effect of magnesia burnt at 800-950 ℃ on the properties, especially shrinkage, of AASC was studied.
2 Experimental studies
2.1 Materials
Ground granulated blast furnace slag powder, magnesia burnt at different temperatures for 2 h, water glass with a SiO2/Na2O modulus of 1.42, and river sand with a fineness modulus of 2.54 were used. The chemical compositions and some physical properties of the slag powder and magnesia burnt at 800℃ for 2 h are shown in Table 1.
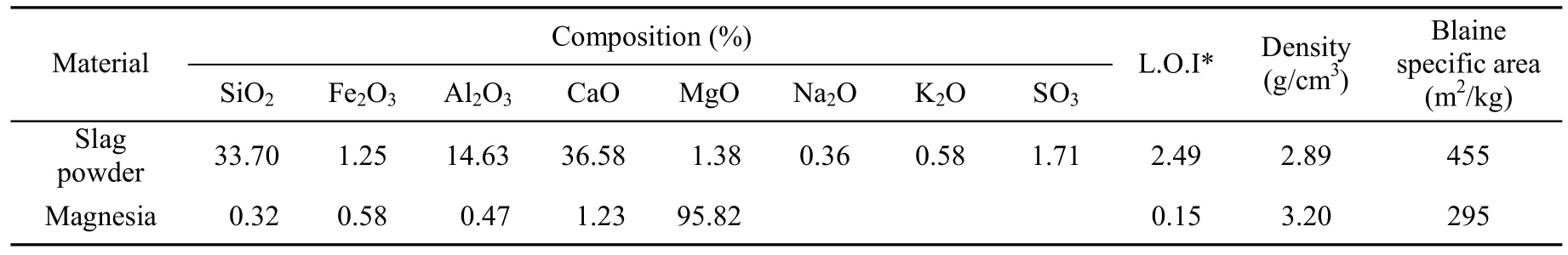
Table 1 Chemical compositions and physical properties of materials
The slag powder was pre-treated by wetting for not less than 60 d. This can prolong the setting time of AASC, as proven with a preliminary experiment, though the mechanism has not been fully understood.
2.2 Procedures
AASC was prepared with the slag powder and water glass, the amount of the latter being 5% by mass (equivalent to that of Na2O)of the former for all the specimens. The setting time and strength of the cement were tested according to Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cements (GB/T 1346-2001)and Method of Testing Cements-Determination of Strength (GB/T 17671-1999), respectively.
The shrinkage of the cement mortar specimens was tested with a method obtained by modifying the methods from Standard Test Method for Drying Shrinkage of Mortar (JC/T 603-2004). Specimens of 25 mm × 25 mm × 285 mm were cast and wet-cured at a temperature of (20 ± 1)℃ and a relative humidity of (95 ± 2)% in a curing chamber for 24 h, and the original lengths were measured soon after the specimens were de-molded. The specimens were then cured in the same condition for the next 27 d, and the wet-curing-induced shrinkages were measured at scheduled intervals. Then, the specimens were cured at a temperature of(20 ± 3)℃ and a relative humidity of (60 ± 3)% in a drying test chamber. The drying shrinkages from the 29th day to the 80th day after a 28-d wet-curing, i.e., the shrinkages during a 56-d dry-curing, were measured.
X-ray diffraction (XRD)and scanning electron microscopy (SEM)analyses were conducted on the hardened cement paste specimens with and without magnesia, wet-cured for 28 d.
3 Results and discussion
3.1 Setting time
AASC is characterized by short setting time and short time intervals between initial and final setting, so the initial setting time is one of the key properties for AASC. Fig. 1 shows the initial setting time of AASC varying with the additive amount of magnesia burnt at different temperatures. AASC mortar specimens with magnesia burnt at 800, 850, 900, and 950℃ for 2 h were designated as M800, M850, M900, and M950, respectively. As shown in Fig. 1,adding magnesia accelerates the setting of AASC, and the setting time decreases with the increase of the additive amount of magnesia burnt at the same temperature. The initial setting time of AASC without magnesia was 95 min, while the value of the AASC specimen M800 added with 8%magnesia was only 10 min, much shorter than that of AASC without magnesia. The mechanism by which magnesia accelerates the setting of AASC is as follows: when mixed with water, magnesia releases Mg2+ions; though the process is not very rapid, the ions react with the water glass added as the activator in the mixture and result in flash setting of the cement, just as Ca2+ions do.

Fig. 1 Initial setting time of AASC vs. additive amount of magnesia
Comparing the setting time of AASC added with magnesia burnt at different temperatures,we can see that the setting time of AASC with a certain amount of magnesia increases with the burning temperature. The setting time for AASC added with 8% magnesia increases from 10 min for M800 to 44 min for M950. The longer setting time means a weaker acceleration effect, which results from the lower reactivity of magnesia; and the lower reactivity results from the denser crystal structure caused by a higher burning temperature.
3.2 Strength
The flexural and compressive strengths of AASC mortars with different amounts of magnesia, used for replacing slag powder of the same amount, burnt at different temperatures,are shown in Fig. 2 and Fig. 3. The results show that adding magnesia in AASC has adverse effects on both the flexural and compressive strengths. The flexural and compressive strengths of AASC decrease with the increase of the amount of magnesia, and the higher the burning temperature is, the less adverse the effect of magnesia. When magnesia, with the additive amount less than 8%, is burnt at 850 ℃ or higher, the effect on the strengths of AASC is not severe and is acceptable for use, but magnesia burnt at 800 ℃ has a severe effect evenwhen the additive amount is lower than 4%.
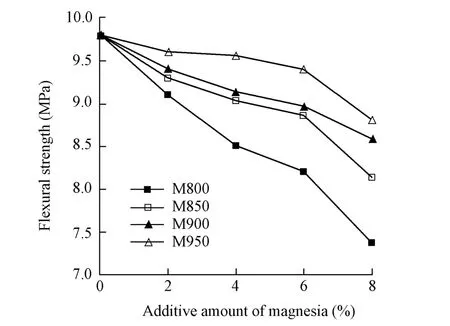
Fig. 2 Flexural strength of AASC with magnesia burnt at different temperatures

Fig. 3 Compressive strength of AASC with magnesia burnt at different temperatures
3.3 Shrinkage
The shrinkages of AASC mortars with magnesia burnt at different temperatures are shown in Fig. 4, and those with different additive amounts of magnesia burnt at 850℃ are shown in Fig. 5. The designation in the figures, AASCM8506 for instance, represents the mortar specimen of AASC with 6% magnesia burnt at 850℃ . During the first 28-d wet-curing, the AASC mortar shrunk about 0.02%, which resulted partly from the chemical and autogenous shrinkages. In the following 56-d dry-curing, the AASC mortar shrunk rapidly in the first 14 d,then evenly in the following dry-curing. The total shrinkage in the 84-d wet- and dry-curing was as high as 0.16%.
When magnesia was added, the shrinkage of AASC decreased in both the wet-curing and dry-curing periods. The two AASC specimens added with magnesia, AASCM8506 and AASCM9006, even expanded a little in the wet-curing period. The total shrinkage of the AASCM8506 mortar specimen in the 84-d wet- and dry-curing decreased to about 0.10%, a 37.7% reduction from that of AASC without magnesia. With the same additive amount of magnesia, the higher the burning temperature of magnesia is, the smaller the decrement of the shrinkage is. The reason for the phenomenon is that higher burning temperature reduces the reactivity of magnesia.
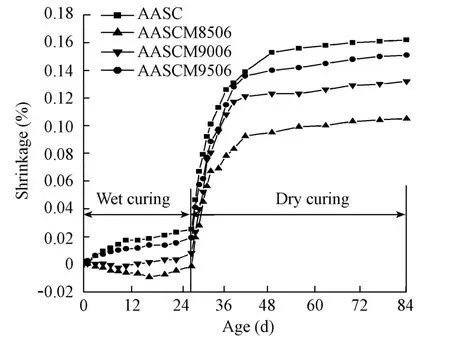
Fig. 4 Shrinkage of AASC with 6%magnesia burnt at different temperatures
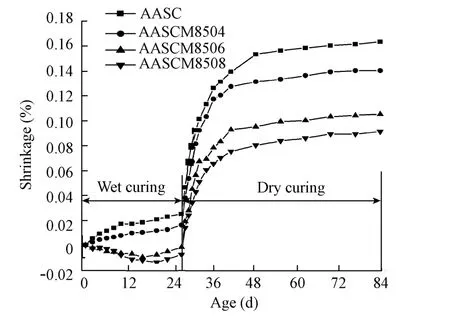
Fig. 5 Shrinkage of AASC with different additive amounts of magnesia burnt at 850℃
As shown in Fig. 5, the shrinkage-reducing effect of magnesia increases with its additive amount. The total shrinkages of AASCM8504, AASCM8506, and AASCM8508 specimens in the 28-d wet-curing were 0.016 5%, -0.001 8%, and -0.007 5%, respectively, and those in the 84-d wet- and dry-curing were 0.140%, 0.105%, and 0.091%, respectively. Although the higher additive amount of magnesia can decrease shrinkage, it is not wise to add magnesia with an additive amount more than 8%, because it has an adverse effect on the strength of AASC, as shown in Section 3.2.
3.4 XRD and SEM analyses
Fig. 6 shows the XRD patterns of the hardened AASC paste without magnesia and the AASCM8508 paste, wet-cured for 28 d. There are strong MgO peaks in the pattern of the AASCM8508 paste,indicating that most of the magnesia added has not hydrated yet at the 28th day.There are also weak Mg(OH)2peaks in the pattern. The formation of Mg(OH)2peaks in the 28-d wet-curing may help to explain the effect of shrinkage reduction in the wet-curing period, and the larger proportion of remaining magnesia demonstrates the potential of shrinkage reduction in the subsequent curing (Chatterji 1995).
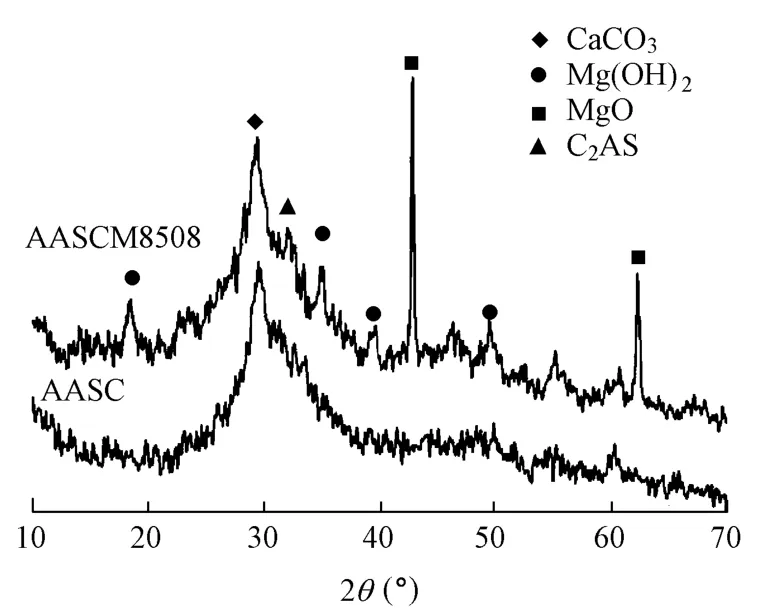
Fig. 6 XRD patterns of AASC paste without magnesia and AASCM8508 paste (2θ is diffraction angle)
Figs. 7(a)and 7(b)are the SEM images of the AASC paste without magnesia and the AASCM8508 paste, wet-cured for 28 d. The structures of the two specimens are very compact,and both specimens are composed of fine gel particles. The particles are larger in the AASCM8508 paste than in the AASC paste. There are also crystalline MgO particles with a size of about 1.0 μm in the AASCM8508 paste, and thin hexagonal platelets of Mg(OH)2embedded in the gel particles. The result conforms with that from the XRD analysis in this study, and also with the result reported by Gao et al. (2009), which indicates that only a small fraction of magnesia hydrated after 90 d when the AASCM8508 paste was cured at 20℃.

Fig. 7 SEM images of AASC paste without magnesia and AASCM8508 paste
4 Conclusions
(1)Adding 4%-8% lightly-burnt magnesia in AASC may shorten the setting time, and slightly reduce the flexural and compressive strengths of the cement. The effect decreases with the increase of the burning temperature. The additive amount of magnesia in AASC should not exceed 8%. Otherwise the flexural and compressive strengths of the cement may severely decrease.
(2)Adding magnesia in AASC can remarkably reduce the shrinkage of the AASC paste.The shrinkage-reducing effect increases with the additive amount of magnesia, and decreases with the increase of the burning temperature of magnesia.
(3)Magnesia may slightly decrease the compactness of the hardened AASC paste, and it cannot fully hydrate in the 28-d wet-curing, which implies that it has a potential effect on long-term shrinkage reduction.
Bakhareva, T., Sanjayana, J. G., and Cheng, Y. B. 2003. Resistance of alkali-activated slag concrete to acid attack. Cement and Concrete Research, 33(10), 1607-1611. [doi:10.1016/S0008-8846(03)00125-X]
Chatterji, S. 1995. Mechanism of expansion of concrete due to the presence of dead-burnt CaO and MgO.Cement and Concrete Research, 25(1), 51-56. [doi:10.1016/0008-8846(94)00111-B]
Collins, F., and Sanjayan, J. G. 2000. Cracking tendency of alkali-activated slag concrete subjected to restrained shrinkage. Cement and Concrete Research, 30(5), 791-798. [doi:10.1016/S0008-8846(00)00243-X]
Douglas, E., Bilodeau, A., and Malhotra, V. M. 1992. Properties and durability of alkali-activated slag concrete. ACI Materials Journal, 89(5), 509-516.
Gao, P. W., Lu, X. L., Geng, F., Li, X. Y., Hou, J., Lin, H., and Shi, N. N. 2008. Production of MgO-type expansive agent in dam concrete by use of industrial by-products. Building and Environment, 43(4),453-457. [doi:10.1016/j.buildenv.2007.01.037]
Gao, P. W., Lu, X. L., and Tang, M. S. 2009. Shrinkage and expansive strain of concrete with fly ash and expansive agent. Journal of Wuhan University of Technology (Materials Science Edition), 24(1), 150-153.[doi:10.1007/s11595-009-1150-4]
Ju, J. Y. 2006. Application of UEA expansive agent and UEA compensate concrete in bridge structure.Highway, (11), 81-85. (in Chinese)
Maltese, C., Pistolesi, C., Lolli, A., Bravo, A., Cerulli, T., and Salvioni, D. 2005. Combined effect of expansive and shrinkage reducing admixtures to obtain stable and durable mortars. Cement and Concrete Research, 35(12), 2244-2251. [doi:10.1016/j.cemconres.2004.11.021]
Mo, L. W., Deng, M., and Tang, M. S. 2010. Effects of calcination condition on expansion property of MgO-type expansive agent used in cement-based materials. Cement and Concrete Research, 40(3),437-446. [doi:10.1016/j.cemconres.2009.09.025]
Nagataki, S., and Gomi, H. 1998. Expansive admixtures (mainly ettringite). Cement and Concrete Composite,20(2-3), 163-170. [doi:10.1016/S0958-9465(97)00064-4]
Passuello, A., Moriconi, G., and Shah, S. P. 2009. Cracking behavior of concrete with shrinkage reducing admixtures and PVA fi bers. Cement and Concrete Composites, 31(10), 699-704. [doi:10.1016/j.cemconcomp.2009.08.004]
Saud, A. O. 2008. Durability of concrete incorporating GGBS activated by water-glass. Construction and Building Materials, 22(10), 2059-2067. [doi:10.1021/jm0605785]
Yang, K. H., Song, J. K., Lee, K. S., and Ashour, A. F. 2009. Flow and compressive strength of alkali-activated mortars. ACI Materials Journal, 106(1), 50-58.
Zhao, F. Q., Ni, W., Wang, H. J., and Liu, H. J. 2007. Activated fly ash/slag blended cement. Resources Conservation and Recycling, 52(2), 303-313. [doi:10.1016/j.resconrec.2007.04.002]
 Water Science and Engineering2011年4期
Water Science and Engineering2011年4期
- Water Science and Engineering的其它文章
- Method of coupling 1-D unsaturated flow with 3-D saturated flow on large scale
- Temperature control and cracking prevention in coastal thin-wall concrete structures
- Two-dimensional physical habitat modeling of effects of habitat structures on urban stream restoration
- Experimental study on total dissolved gas supersaturation in water
- Hydrodynamic effects of reconnecting lake group with Yangtze River in China
- Sediment transport following water transfer from Yangtze River to Taihu Basin
