Acrylamide Inhibition in Cookies Using Natural Antioxidants
Mohamed ABDEL-SHAFI ABDEL-SAMIE,HUANG Wei-ning,*,LI Zhen-ni,YAO Yuan,Okkyung Kim CHUNG(. State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 4, China;. Department of Food Science, Purdue University, West Lafayette IN47907, USA)
Acrylamide Inhibition in Cookies Using Natural Antioxidants
Mohamed ABDEL-SHAFI ABDEL-SAMIE1,HUANG Wei-ning1,*,LI Zhen-ni1,YAO Yuan2,Okkyung Kim CHUNG1(1. State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, China;2. Department of Food Science, Purdue University, West Lafayette IN47907, USA)
Acrylamide inhibition using natural antioxidant sources (buckwheat, cumin and black cumin), were evaluated in a cookie model system. Effects of additives on acrylamide precursors of formulated flour, antioxidant properties of flour and cookies, cookie baking properties (color, moisture and spread ratios) and acrylamide contents of cookies were examined. All addition systems to the flour increased the reducing sugars, asparagine contents and antioxidant properties, including total phenolic compounds (TPC), 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity and ABTS+· inhibition of flour and cookies. Increasing levels of additives to the cookie formulas resulted in darker cookies with lower L*, a* and b* values when compared to control cookies. Moisture contents of the cookies were increased with the addition of buckwheat and cumin, while it decreased with the addition of black cumin. Cookie spread ratios were more than the spread ratio of control cookies. The acrylamide contents of prepared cookies were lower than that of control cookies. Addition of 15% buckwheat, 15% cumin and 15% black cumin to cookie formula reduced acrylamide level by 20.2%, 67.6% and 75.9%, respectively, in comparison with the control cookie samples which contained 361.2μg/kg acrylamide. Sensory evaluation test parameters indicated that all cookies with different additives were scored as moderately liked.
acrylamide inhibition;antioxidant;buckwheat;cumin;black cumin;cookies
Acrylamide is a chemical substance used in a variety of industrial applications, including the production of polyacrylamide plastics. Many materials may contain low levels of residual acrylamide, such as tobacco smoke(INFOSANInformation, Note No. 2/2005 - Acrylamide)[1]. Acrylamide has been identified as a potential human carcinogen by the International Agency for Research on Cancer at 1994. After the announcement, acrylamide was prohibited to be used as a material to produce food storage bags or any material that would be in direct contact with food. In 2002, however, the Swedish National Food Authority and the University of Stockholm jointly found relatively high levels of acrylamide in food materials, especially carbohydrate-rich foods such as bakery products. These findings motivated producers and researchers to find new strategies to reduce acrylamide in food materials.
In food, acrylamide is formed through Maillard reaction between asparagine and reducing sugar at favorable temperatures (higher than 100℃) and low moisture conditions during heat processing (frying, roasting, baking or microwave heating)[2]. Acrylamide inhibition strategies vary according to the target product, such as cookies[3], bread[4-5]or you-tiao (a traditional Chinese, fried, twisted dough-roll)[6]. Strategies also vary according to the additives used to reduce acrylamide. For example, amino acids[7-8], dietary fiber[9], vitamins[10]and antioxidants were found to have inhibitory effects on acrylamide formation[11-12]. In the current research, antioxidant-rich sources including buckwheat, cumin, and black cumin were used to reduce acrylamide in cookies.
Buckwheat (Fagopyrium esculentum Moench) belongs to the Polygonaceae family. In some countries, such as China, Japan and Poland, buckwheat is recognized as a valuable source of functional food , and it successfully replaces rice or potatoes[13]. Natural antioxidants from buckwheat have been shown to be higher than oats and barley[14]. Six flavonoids have been isolated and identified in buckwheat grain: rutin, orientin, vitexin, quercetin, isovitexin and isoorientin. Rutin and isovitexin are the only flavonoid components of buckwheat seeds, whereas hulls contain all six identified compounds[13]. Buckwheat has increased the rutin and quercetin levels when added to wheat breads, which increases the antioxidant activity of the wheat breads[15].
Cumin (Cuminum cyminum L.) is one of the most popular spices used in many countries, including China and Arabic countries. Cumin seeds contain volatile oil (2%-5%) that is utilized in many types of flavoring compounds, perfumery, flavoring liquors and cordials. The cumin seed oil is also used as an essential ingredient in mixed soups, sausages, pickles, cheese and meat dishes, and it is also utilized for seasoning breads, cakes and candies[16-17]. The total phenolic contents (TPC) of the cumin varieties are high[17]. Cumin has high dietary fibers which is able to lower serum cholesterol and help to reduce the risk of heart attacks and colon cancer[18]. Cumin enhances the digestive enzimeatic activity because it showed higher protease, lipase and amylase activities[19]. Cumin has been reported to be an anti-spasmodic, a carminative and a digestive stimulant[20]. Cumin has been incorporated in a cookie system and prepared cookies were acceptable for consumers[21].
Black cumin (Nigella sativa L.) is unique in its nutritional profile, and black cumin s fixed and essential oils have phytochemical rich fractions[22]. The essential oil of Tunisian black cumin seeds exhibits strong ex vivo antioxidant activity and high anti-inflammatory activity. Moreover, the oil exerts antibacterial activity against Staphylococcus aureus and Escherichia coli. The anticancer and antibacterial activities of black cumin seeds may be due mainly to the action of thymoquinone and longifolene[23]. Black cumin seeds possess high antioxidant activity[24-25]. Safety assessments in animal modeling of fixed and essential oils from black cumin find that the oils are safe[22]. Black cumin seeds are used to produce seedcakes with high TPC content, high DPPH free radical scavenging abilities and a strong effect on reducing the oxidation of β-carotene. The predominant phenolic compounds in black cumin seedcakes are hydroxybenzoic, syringic and β-cumaric acids[24].
The aim of this study was to examine the effects of incorporating natural antioxidants found in plant flour (buckwheat) and powders (cumin and black cumin) into a cookie formula. These antioxidant sources are different in their chemical composition, and this study examined the relationship between the chemical composition of these antioxidant sources and acrylamide precursors (asparagines and reducing sugars) in the formulated flours. Furthermore, we investigated the effects of additives in the flours and cookies on antioxidant properties, such as TPC contents, DPPH free radical scavenging abilities and ABTS+· inhibition percentages. Cookie quality attributes, including color, moisture, diameter, thickness and spread ratios, were also analyzed. We also measured the acrylamide contents in the prepared cookies. The purpose of this study was to produce cookies with lower acrylamide contents and higher antioxidant contents while maintaining cookie quality attributes that are required by and acceptable to consumers.
1 Materials and Methods
1.1 Materials, reagents, and instruments
The following materials were used for this study: low gluten flour 11.2% protein (Great Value, 123 Shaoxin Flour Milling Company, Guandong Province, China); buckwheat flour 10.69% protein (Tian Xiang Ye Tian, Xiangyetian Food Co. Ltd., Chifeng City, Inner Mongolia Province, China); and powders including cumin and black cumin (Wuxi Teweikang Seasoning Co. Ltd., Wuxi city, Jiangsu Province, China). All other materials, including sugar, shortening, salt and baking soda, were obtained from the local markets of Wuxi city, Jiangsu province, China. Acrylamide (purity > 99%), L-Asparagine, Folin Ciocalteau reagent (2N), ABTS, DPPH, 3,5-dinitrosalycylic acid (DNSA), Glucose and Gallic acid were purchased from Sigma Aldrich Company (Germany). Other chemicals were of analytical grade and were purchased from Sinopharm Chemical Reagent Co. Ltd. (SCRC, China).
Spectrophotometer (model 721E, Shanghai Spectrum Instrument Co. Ltd., China); Agilent liquid chromatography system (Agilent, Palo Alto, CA, USA); Chroma Meter CR-400 (Konica Minolta Sensing, INC, Japan); Waters MALDI SYNAPT MS, Waters ACQUITY PDA(Waters, USA).
1.2 Determination of reducing sugar (RS) contents
Reducing sugar contents were determined using the DNSA method[26]. Flour sample (1 g) was mixed with 39 mL distilled water (first with 25 mL and then with 14 mL). The extraction was carried out for 1 h in an orbital shaker, and 2 mL of the extract was centrifuged for 5 min. The supernatant was diluted, and 2 mL of the DNSA working solution was added to 2 mL of the diluted sample in a test tube. This mixture was incubated at 100 ℃ for 4 min in boiling water bath then cooled to room temperature by running cool water. The absorbance of the developed color was read at 570 nm using a spectrophotometer . A glucose standard curve was prepared.
1.3 Asparagine contents
Samples were pretreated with the addition of 1 g of flour or 1 g of flour with additives in a 25 mL volumetric flask. A 5% solution of trichloroacetic acid was added to the flask to reach the final volume. The mixture was shaken vigorously for 2 h in a water bath and filtered through doubled filtration paper and 1 mL of the filtrate was transferred to 1.5 mL centrifuge tube. The filtrate was centrifuged for 10 min at 5000×g. The supernatant (0.5 mL) was transferred to vials for further use in the determination of asparagine[6].
For the liquid chromatography, 0.5 mL of final test sample was injected into the Agilent liquid chromatography system consisting of an Agilent 1100 quaternary pump and an UV detector set at 338 nm and 262 nm. The chromatographic separation was performed on a 5μm Hypersil ODS column (250 mm × 4.6 mm) with a gradient mixture of acetonitrile (A) and 50 mmol/L aqueous acetate buffer (B) at a flow rate of 1.0 mL/min at 25 ℃.
1.4 Cookie preparation
Cookies were produced according to the (AACC; 10-50D, 1999) method[27]with one modification. In the original method, a dextrose solution was added to the recipe to aid in developing the brown color, but the additives used in this study already had a brown color. Therefore, the color of cookies did not need to be aided, so dextrose solution was not added to the cookie formula. Single or binary additions of buckwheat, cumin and black cumin of different levels vary from 0-15 g based on 100 g flour was added to the formula. According to AACC 10-50D method[27], cookies were left at room temperature to cool down, then after 30 mins of removing the cookies from the oven, six cookies were laid edgeto-edge and measured for diameter using a scale, cookies were rotated 90℃ and re-measured for diameter again, average of the two measurements was taken and divided by 6 to get the diameter of the single cookie sample. Same number of cookies (6) was put on top of another and was measured using a caliper, then rearranged again and re-measured for thickness and average of the two values were taken and divided by 6 to get the thickness of a single cookie sample.
1.5 Physical properties of cookies
Moisture content of cookies was determined using an air oven according to the AACC method 44-15[27]. Color of cookies was determined using a Chroma Meter CR-400.
1.6 Sensory evaluation test
Cookies were presented on white, coded disposable plates with 3 different codes to 25 panelists (Arabic and Chinese), 15 males and 10 females who were asked to judge each sensory attribute. Cookies were evaluated for surface appearance, surface color, texture, taste and overall acceptability with a nine point hedonic scale according to the following scoring system: 1- extremely dislike, 5-neither like nor dislike and 9-extremely like[28].
1.7 Antioxidant properties of the flours and cookies
For the antioxidant determination, 1 g of flour and powder from finely ground cookie samples (ground in a laboratory blade mill with a 1.0 mm screen) was used. Flour and cookie powder was extracted using 20 mL of methanol at roomtemperature for 2 h with continuous shaking using an orbital shaker. Extracts were centrifuged and stored at -20 ℃ for further analysis for the determination of antioxidant properties.
1.7.1 Total phenolic compounds (TPC)
The TPC contents of extracts of flour, modified flour and cookie samples were determined using the Folin Ciocalteau method. Distilled water (4 mL) was mixed with 500μL of saturated sodium carbonate, 250μL of sample extract and 250μL of Folin Ciocalteau reagent diluted with water (1:1 as V/V). The mixture was incubated at room temperature for 25 min and then centrifuged for 10 min at 3000×g at room temperature. The absorbance of the mixture at 725 nm was determined. gallic acid was used as a standard, and the results were expressed as mg gallic acid equivalents (GAE)/100 g (mg GAE/100 g)[29].
1.7.2 Free radical scavenging activity (DPPH Assay)
The antioxidant activities of the flour, flour with additives and cookie samples were measured in terms of hydrogen donating ability or free radical scavenging ability. DPPH free radicals were used to determine the scavenging activity of the methanolic extracts of both the flour and cookie samples. The methanolic extract (2 mL) was added to 2 mL of DPPH solution (200μmol/L in methanol). The mixture was vigorously shaken and then incubated at room temperature in the dark for 30 min. The absorbance of the mixture was obtained at 517 nm using a spectrophotometer[30]. The antioxidant activity was calculated according the formula.

Where ADPPHis absorbance of DPPH solution without the sample addition; Asampleis the absorbance of DPPH solution with the addition of sample extract.
1.7.3 Cation radical decolorisation (ABTS Assay)
ABTS method was used to determine the total antioxidant activity of the extracts of flour and cookie samples with and without the different additives. ABTS reagent (7 mmol/L in water) was mixed with potassium persulphate (K2S2O8) (2.45 mmol/L ) and allowed to stand for about 16 hours in dark before use. Absorbance of ABTS stock solution was adjusted to be 0.7 ± 0.02 using a sodium phosphate buffer composed of Na2HPO4and NaH2PO42.45 mmol/L. Absorbance of the ABTS reagent was reset to 0.07 every set of samples. 3 mL of working ABTS solution was mixed with 40μL of sample and mixture was left to stand for 20 minutes at dark and at room temperature. Absorbance of the mixtures was measured at 734 nm after the specified time[31]. ABTS+· inhibition percentage of the sample extract was calculated based on the formula.

Where Actrlis absorbance of control; Asampleextractis the absorbance of the sample extract.
1.8 Determination of acrylamide
1 g of cookie sample powder (finely ground using a ground mill to pass 1.0 mm screen) was defatted using 20 mL of n-hexane then solvent was removed, the powder was defatted again using the same amount of n-hexane (20 mL) then solvent was removed and evaporate under low pressure. Sample was then extracted using 10 mL methanol. The suspensions were clarified using Carrez I solution (500μL) and a Carrez II solution (500μL). Suspensions were centrifuged on 5000×g for 20 minutes, clear supernatant were transferred to a clean centrifuge tubes and residual solids were extracted again in the same procedures using 10 mL methanol but without Carrez I nor II clarification. Supernatants were combined and centrifuged at 10000×g at 10 ℃ for 10 min. For the solid phase extract (SPE) cleanup, an Oasis HLB cartridge was preconditioned with 1 mL of methanol and 1 mL of water at a rate of two drops per second using a syringe. The extract was passed through the cartridge at a rate of one drop per second using a syringe. The first ten drops were discarded to prevent sample dilution.
A LC/MS method was used to determine acrylamide contents in the cookie samples. Five microliters of the final solution was injected onto a LC column for quantification by LC-MS (Waters MALDI SYNAPT MS). The analytical separation was performed on a Waters ACQUITY UPLC with a detector (Waters ACQUITY PDA) on a 1.7μm column (BEH C18; 2.1 mm × 100 mm) with a mobile phase of 0.1% formic acid. The column temperature was 45 ℃, and the flow rate was 0.3 mL/min. The injection volume was 5 μL.
1.9 Statistical analysis
The experimental design was composed of three replicates to evaluate the experimental error. All experimental data were presented as the mean values ± SD of the replicates for each individual sample. The data were analyzed for significance using one-way analysis of variance (ANOVA) at the significance level of 0.05%. Duncan s multiple range wasused to differentiate between the mean values, and standard deviations were calculated using the same software. The data were analyzed for the statistical significance using the SPSS statistics 17.0 program. A significance level of P<0.05 was chosen.
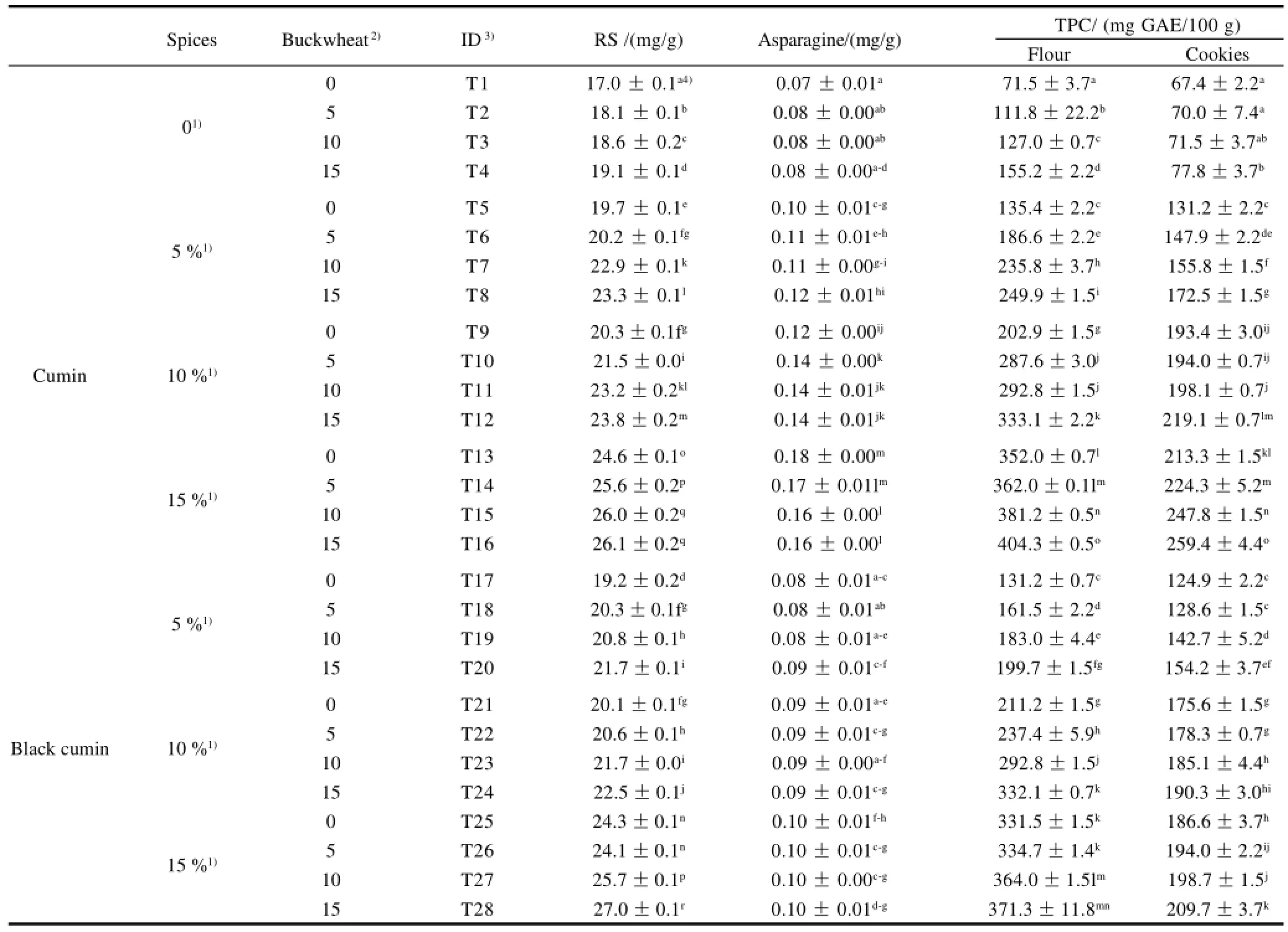
Table 1 Acrylamide precursor (reducing sugars and asparagine) contents of flour with and without additives
Twenty-eight flour and cookie formulas were designed considering three additives as follows: 1) antioxidant rich fiber cereal additive of buckwheat with addition levels of 0%, 5%, 10% and 15%; 2) antioxidant rich spice additive of cumin with the same addition levels as buckwheat; 3) antioxidant rich spice additive of black cumin with the same addition levels as buckwheat. The control cookie was the original formula without the addition of buckwheat, cumin or black cumin.
2 Results and Discussion
2.1 Contents of acrylamide precursors (Reducing sugars and asparagine) in flour
The content of reducing sugars in the flour (with and without the addition of buckwheat, cumin and black cumin single or binary) are presented in Table 1. Reducing sugars contents in the control flour (low gluten soft wheat flour without the addition of buckwheat, cumin or black cumin) was 17 mg/g which was the lowest among all flour samples. The 15% single addition of buckwheat flour (T4), cumin powder (T13) and black cumin powder (T25) to the flour gradually increased the reducing sugar contents of the formulated flours to 19.1, 24.6 mg/g and 24.3 mg/g, respectively. Highest reducing sugars contents (27 mg/g) resulted from the binary addition of 15% black cumin and 15% buckwheat (T28). Within the binary addition of flours (buckwheat combined either cumin or black cumin), different levels of buckwheat gradually and significantly increased the reducing sugars (P<0.05).
Asparagine contents of formulated flours are presented in Table 1. There were significant differences in the asparagine contents between control flour and flours with the addition of cumin or black cumin. The asparagine content in control flour was 0.07 mg/g, which was the lowest among all flours. Buckwheat did not increase asparagine contents of formulated flours significantly (P>0.05). Single addition of black cumin increased the asparagine contents significantly (P<0.05), but asparagine contents of black cumin added samples were lower than cumin added samples. Asparagine contents was 0.1 mg/g in the 15% black cumin added flour samples.The addition of cumin to the flour resulted in higher asparagine contents. The highest asparagine content was 0.18 mg/g, which was found in flour with the single addition of 15% cumin (T13). Increased amount of reducing sugars and asparagine might have effects on acrylamide amount formed in cookie samples.
2.2 Antioxidant properties of flour

Fig.1 Effect of additives (buckwheat, cumin and black cumin) on the antioxidant properties of flour samples determined using DPPH (A) and ABTS (B)
The TPC content in the formulated flour extracts were expressed as mg GAE per 100 g of sample (mg GAE/100 g) (Table1). Significant differences (P<0.05) between the control sample and samples with the single or binary addition of buckwheat, cumin or black cumin were observed. TPC data showed that TPC content of control sample was 71.5 mg GAE/ 100 g, which was the lowest among all flour samples. The application of additives to the flour gradually and significantly increased the TPC contents (P<0.05) to 155.2, 352.0 mg GAE/100 g and 331.5 mg GAE/100 g for the single addition of 15% buckwheat (T4), 15% cumin (T13) and 15% black cumin (T25), respectively. The maximal TPC content was 404.3 mg GAE/100 g, which was found in the flour with the binary addition of 15% cumin and 15% buckwheat (T16).
Antioxidant activities reflected by DPPH free radical scavenging activities of flour samples are determined as percentages (Fig.1 A). All additives significantly increased the DPPH free radical scavenging activities (P<0.05). The DPPH free radical scavenging activity of the control flour was 9.5%, which was the lowest DPPH free radical scavenging activity value. The DPPH free radical scavenging activity of the 15% buckwheat flour extract, 15% cumin flour extract and 15% black cumin extract was 50.1%, 91.0%, and 74.1%, respectively. The maximal DPPH free radical scavenging activity was 93.6%, which was found in the extract with the 15% cumin and 15% buckwheat binary addition. It was clear that cumin had the greatest effect on the DPPH free radical scavenging activity within our additives and that could be noticed by the high values of DPPH free radical scavenging activities of flour samples extracts when cumin was used alone in 15% addition level or when it was combined with 15% of buckwheat.
To confirm the effects of our additives to improve the antioxidant capacity of the flour samples, more antioxidant assay was applied to our samples. ABTS+· inhibitions of flour are presented in Fig.1B. Same trends of the DPPH free radical scavenging activities were noticed in the ABTS+· inhibition percentages. Control sample showed the lowest ABTS+·inhibition with a score of 11.95%. All additives increased the antioxidant activity measured as ABTS+· inhibition. Buckwheat addition increased ABTS+· inhibition significantly (P<0.05) from 11.95% in control to 25.33%, 35.65%, and 40.57% with buckwheat added at 5%, 10% and 15%, respectively. Cumin also increased ABTS+· inhibition significantly (P<0.05) to be 34.99%, 51.82% and 65.72% when it was added at 5%, 10% and 15%, respectively. Combination of 15% of cumin with 15% of buckwheat showed the highest ABTS+· inhibition (90.69%). Black cumin also increased the ABTS+· inhibition of flour extract to 25.53%, 36.97% and 49.36% when 5%, 10% and 15% of black cumin were added to the flour samples, respectively. The inhibition was 71.39% when black cumin 15% was combined with 15%of buckwheat. Cumin had the maximum effect on ABTS+· inhibition when it was applied alone or combined with buckwheat.
2.3 Cookies characteristics

Table 2 Color values (L*, a* and b*) and moisture content of cookies with and without additives
The effects of different additives on cookie color are determined as L*, a* and b* as shown in Table 2. The effects of buckwheat on the color values were not significant (P>0.05) whereas both cumin and black cumin significantly affected the L*, a* and b* color values (P<0.05). L* is the luminance or lightness component, which ranges from 0 to 100, and parameters a* (from green to red) and b* (from blue to yellow) are the two chromatic components, which range from -120 to 120.
Buckwheat did not affect the L* values, which may be due to the small differences between the lightness values of the buckwheat flour and the low gluten wheat flour (90.0% and 97.3%, respectively). Cumin and black cumin significantly decreased the L* values of the cookies (P<0.05). The lowest L* value was observed in the binary addition of 15% black cumin and 5% buckwheat (T26) with a score of 45.2, which was 30.9% less than the lightness score of the control cookie samples (65.3). The maximal L* value was found with the single addition of 10% buckwheat (T3) with a score of 67.7, which was not significantly different from the control cookie samples.
The addition of cumin and black cumin to the cookie formula significantly decreased a* values of the prepared cookies (P<0.05) from 8.7 in the control cookie samples to 4.8 and 4.5 in the T13 (15% cumin) and T25 (15% black cumin) additions, respectively. The lowest a* value was 4.4, which was found in T27 with the binary addition of 15% black cumin and 10% buckwheat. The a* value of T27was 49.7% lower than that of the control cookie samples, which had the highest a* value.
Buckwheat and cumin did not significantly affect the cookie b* values (P>0.05) when it was added to the cookie formula, whereas black cumin significantly decreased the b* values (P<0.05) from 35.44 in the control cookies to 20.9 in cookies prepared with the single addition of 15% black cumin (T25). The lowest b* value was observed when 15% black cumin was added to the cookie formula (T25), which had a b* value of 20.9 with a decrease of 41% when compared to that of control cookies. The b* value was the highest for T3, showing no significant difference compared with that of control.
Moisture contents of prepared cookies are presented in Table 2. Both the buckwheat and cumin addition significantly increased the cookie moisture content (P<0.05) from 5.6% in the control cookie samples to 7.4% and 6.4% for T4 (15% buckwheat) and T13 (15% cumin), respectively. The buckwheat level did not significantly affect the moisture contents (P>0.05) when it was combined with cumin and black cumin. The increased level of moisture contents with the addition of buckwheat and cumin may be due to the high fiber contents found in the buckwheat flour[32]and cumin powder[18]because higher fiber contents increase the water holding capacity. The addition of black cumin decreased the moisture content of prepared cookies from 5.6% in the control cookies to 4.2% in T25 (15% black cumin) cookies. The decrease in moisture content is related to the high spread of cookies because the high spread creates more surface area causing a higher loss of water.

Table 3 Diameters, thickness and spread ratio values of cookies with and without additives
Diameter, thickness and spread ratios of cookies are shown in Table 3. Buckwheat did not significantly affect the cookies diameter, thickness or spread ratios (P>0.05). Cumin significantly increased the cookie diameter (P<0.05) from 6.98 cm in the control to 7.08, 7.25 cm and 7.38 cm in T5 (5% cumin), T9 (10% cumin) and T13 (15% cumin), respectively. Effect of black cumin on the cookie diameter were more significant. The cookie samples with the addition of black cumin to the cookie formula had higher diameter scores when compared to both control and cumin cookie samples. For T17 (5% black cumin), T21 (10% black cumin) and T25 (15% black cumin), the cookie diameters were 7.61, 7.81 cm and 7.88 cm, respectively, which demonstrated that the increasing levels of black cumin in the cookie formula gradually and significantly increased the cookie diameters (P<0.05).
In contrast to the cookie diameter trends, cookies thickness gradually decreased with the addition of cumin and black cumin. However, the buckwheat levels did not cause significant differences in cookie thickness. The thickness of the control cookie samples was 1.27 cm. The addition of cumin to the cookie formula gradually and significantly decreased the cookie thickness (P<0.05) to 1.14, 1.16cm, and 1.13 cm with the application of T5 (5% cumin), T9 (10% cumin) and T13 (15% cumin), respectively. Black cumin incorporation into the cookie formula decreased the cookie thickness compared to control and cumin cookies. The cookie thickness gradually decreased to 1.11, 1.10 cm and 1.09 cm for the T17 (5% black cumin), T21 (10% black cumin), and T25 (15% black cumin) additions, respectively.
As a result of increased diameter and decreased thickness, spread ratios significantly increased (P<0.05) with the use of both cumin and black cumin. The control cookie samples spread ratio was 5.49. For cumin cookies, the spread ratios were increased to 6.22, 6.24 and 6.53 with the application of T5 (5% cumin), T9 (10% cumin) and T13 (15% cumin), respectively. The spread ratios of cookies with the addition of black cumin were higher than that of both control and cumin cookie samples. The cookie spread ratios were 6.86, 7.10, and 7.25 for T17 (5% black cumin), T21 (10% black cumin) and T25 (15% black cumin), respectively. The cookie spread ratio increased with the addition of cumin and black cumin powders. These effects may be attributed to the increased extension of the dough. The spread ratio may also be related to the dilution of gluten by the addition of cumin and black cumin to the cookie formula.
2.4 Sensory evaluation
Sensory evaluation scores of prepared cookies are presented in Table 4. Significant differences (P<0.05) were observed between the control cookies and cumin and black cumin added cookies, while no significant differences (P>0.05) between buckwheat added cookies and the control cookies were observed. However, sensory scores for some cookies (with single or binary additions) were comparable to the scores of the control cookies (no additives). Cookies prepared using all additives had no significant differences (P>0.05) in the texture scores when evaluated by the panelists.
Control cookies and buckwheat added cookies of all levels and cookies prepared with 5% and 10% cumin have the same color scores, while the addition of 15% cumin and all levels of black cumin significantly decreased the sensory evaluation scores for color. The lowest color scores were that of the highest level of black cumin (15%). Cookies with 15% cumin and 5% black cumin were still accepted for color. But cookies with the addition of 10% and 15% black cumin had decreased color scores and were disliked by the panelists.

Table 4 Sensory evaluation values of cookies
The same trends in color changes were observed in the flavor scores. The control cookies, cookies with all addition levels of buckwheat, and cookies prepared with the addition of 5% and 10% cumin had the same flavor scores without significant differences (P>0.05). In contrast, addition of 15% cumin and all levels of black cumin significantly decreased the flavor scores (P<0.05). However, the flavor of the cookies with 15% cumin and 5%, 10% and 15% of black cumin were still acceptable and moderately liked by the panelists.
The overall acceptability scores showed that, cookies with all additives were accepted by the consumers. Control cookies, cookies prepared with the addition of all buckwheat levels, and cookies prepared with the addition of 5% cumin had the same acceptability scores without significant differences. Cookies prepared with the addition of 10% and 15% cumin and with the addition of 5%, 10% and 15% black cumin had significantly decreased overall acceptability scores (P<0.05), but all the prepared cookies were scored as acceptable and moderately liked by the panelists. The overall acceptability scores ranged from 7.7 to 5.65, with the highest score for the control cookies and lowest score for the cookies with 15% black cumin.
2.5 Antioxidant properties of cookies
The TPC content in the prepared cookies extracts (with and without the single or binary addition of buckwheat, cumin and black cumin) were expressed as mg GAE per 100 g of sample (mg GAE/100 g) (Table 1). Similar trends of flour TPC were observed in the cookie samples where the control cookie samples had the lowest value of TPC content (67.4 mg GAE/100 g), and the single or binary incorporation of buckwheat, cumin and black cumin into the cookie formula significantly increased the TPC contents of cookie samples (P<0.05). The TPC contents of the cookie samples were as follows: 77.80 mg GAE/100 g, 213.3 mg GAE/100 g and 186.6 mg GAE/100 g for T4 (15% buckwheat), T13 (15% cumin) and T25 (15% blackcumin) additions, respectively. The combination of 15% cumin with 15% buckwheat in the T16 sample had the highest TPC value (259.4 mg GAE/100 g).
The DPPH free radical scavenging activities of the cookie samples are presented in Fig.2A. The same trend of the DPPH free radical scavenging activity was found in the cookie samples as was found with the flour samples. The control cookie sample had the lowest DPPH free radical scavenging activity of 43.4% while buckwheat increased the DPPH free radical scavenging activity to 61.5% in the cookie samples with the addition of 15% buckwheat. Cumin increased the DPPH free radical scavenging activity to 92.4% in the cookie samples with the addition of 15% cumin, and black cumin increased the DPPH free radical scavenging activity to 89.8% in the cookies sample with the addition of 15% black cumin. The highest DPPH free radical scavenging activity (95%) was found in the T16 sample with the binary addition of 15% cumin and 15% buckwheat. Cumin showed the highest influence effect on the DPPH free radical scavenging activity of cookie samples when it was applied alone or combined with buckwheat.
ABTS+· inhibition of cookie samples are presented in Fig.2 B. Figure showed that ABTS+· inhibition percentage was increased with the addition of buckwheat, cumin, and black cumin. The highest ABTS+· inhibition was reported when 15% of both buckwheat and cumin were combined together with an inhibition percentage of 91.19%. Buckwheat increased the ABTS+· inhibition percentage of cookie samples from 19.33% in control sample to 30.52%, 39.09% and 48% when it was added in 5%, 10% and 15%, respectively. Cumin had the highest inhibition of ABTS+· inhibition percentages values (40.24%, 66.76% and 72.04%) when it was added to the flour in 5%, 10% and 15%. The highest ABTS+· inhibition percentage (92.19%) was that of cookie sample with 15% cumin combined with 15% buckwheat. ABTS+· inhibition percentages of black cumin were also higher than that of control inhibition. 5%, 10% and 15% addition of black cumin increased the ABTS+· inhibition percentage to 28.8%, 42.67% and 51.1%, respectively.
It could be noticed that, buckwheat, cumin and black cumin significantly influenced the antioxidant activity of cookie samples which was confirmed by three methods, TPC, DPPH free radical scavenging activity and ABTS+· inhibition percentage. Highest effect of antioxidant improving was scored by cumin when it was added alone or combined with buckwheat, measured as TPC, DPPH or ABTS assay. Both buckwheat and black cumin also had higher values compared to that of control. That could be a clear conclusion that our additives influenced the antioxidant capacity of cookie samples which make cookies higher nutritional value than the control cookie.

Fig.2 Effect of additives (buckwheat, cumin and black cumin) on the antioxidant properties of cookie samples determined using DPPH assay (A) and ABTS assay(B)
2.6 Acrylamide contents
Acrylamide content was measured in prepared cookies with and without the single addition of buckwheat, cumin and black cumin or binary addition (buckwheat × cumin and buckwheat × black cumin) to the cookie formula. All additions demonstrated a strong inhibition efficiency of acrylamide formation in the cookie samples. The acrylamide content in the control cookie sample was 361.2μg/kg. The single addition of buckwheat to the cookie formula decreased the amount of acrylamide formed to 346.8, 330.4μg/kg and 288.3μg/kg when buckwheat was added at 5% (T2), 10% (T3) and 15% (T4), respectively. Adding 15% buckwheat to the cookie formula inhibited 20.2% of the acrylamide formation in the control cookie samples. Moreover, when buckwheat was combined with cumin or black cumin in the binary additions, the various levels of buckwheat resulted in different levels of acrylamide inhibition. Increasing levels of buckwheat resulted in increased levels of acrylamide inhibition.
Cumin also decreased the formation of acrylamide to 285.2, 199.1μg/kg and 117.0μg/kg when cumin was added at 5% (T5), 10% (T9) and 15% (T13), respectively. The maximal addition of cumin (15% in T13) inhibited 67.6% of theformed acrylamide in the control cookie samples. The combination of 15% buckwheat with 15% cumin (T16) decreased the acrylamide level to 66.7μg/kg, (81.5% inhibition of the acrylamide formation) when compared to the control cookie samples.
Black cumin scored the highest acrylamide inhibition among the additives. The addition of 5%, 10% and 15% of black cumin to the cookie formula lowered the acrylamide contents of the cookies to only 140.6, 104.7μg/kg and 87.2 μg/kg, respectively. The addition of 15% black cumin to the cookie formula reduced the acrylamide formation by 75.9% when compared to the control cookie samples. The combination of 15% buckwheat with 15% black cumin (T28) reduced acrylamide by 89.5%when compared to the control cookie samples, and this resulted in the lowest acrylamide content of 38.0μg/kg.

Table 5 Effect of additives (buckwheat, cumin and black cumin) on the acrylamide contents of prepared cookies
Increasing the addition level of buckwheat, cumin and black cumin decreased the acrylamide contents in the prepared cookies, despite the increased acrylamide precursors in the formulated flours. This may be due to the high antioxidant activity of the formulated flours with the addition of buckwheat, cumin and black cumin. These results were similar to the findings reported by Zhang et al.[11]who found that the antioxidant of bamboo leaves and extract of green tea effectively reduces the formation of acrylamide. Zhang et al.[11]reported that antioxidant compounds may block the oxidation of 3-aminopropionamide a transient intermediate formed via decarboxylating reaction of asparagine and is an important and direct precursor contributing to the formation of acrylamide . The reaction explained by a quinine-amine interaction between antioxidants and the direct precursor of acrylamide (3-APA). The oxidation of the polyphenols to quinones subsequently reacted with a key intermediate such as 3-APA which may reduce the production of acrylamide.
3 Conclusions
Acrylamide formation in food, such as bakery products, is a critical point that should be controlled because of its carcinogenic effects. Reduction of acrylamide in food should be accomplished while maintaining the nutritional and sensory qualities of the end products. In this study, we attempted to inhibit the formation of acrylamide while preparing cookies with acceptable quality attributes for consumers from different cultures. We used antioxidant-rich plant materials (buckwheat, cumin and black cumin) to inhibit acrylamide. We were able to decrease the acrylamide contents from 361.2μg/kg in the control cookie samples to 288.3, 117.0 μg/kg and 87.2μg/kg with a single addition of 15% buckwheat, 15% cumin and 15% black cumin, respectively. Furthermore, the combination of 15% buckwheat with 15% black cumin decreased the acrylamide content to 38.0μg/kg in the cookie sample. At the same time, the cookie antioxidant properties were increased with these additives resulting in high nutritional values in the final product. While the quality attributes, including color, moisture and spread ratios, were affected, but all the cookies were acceptable to the panelists. This indicates the potential acceptability of the prepared cookies in this study to consumers.
[1] DEARFIELD K L, ABERNATHY C O, OTTLEY M S, et al. Acrylamide: its metabolism, developmental and reproductive effects, genotoxicity, and carcinogenicity[J]. Mutation Research-Reviews in Gene and Toxic Research, 1988, 195: 45-77.
[2] ZHANG Yu, FANG Haoran, ZHANG Ying. Study on formation of acrylamide in asparagine-sugar microwave heating systems using UPLCMS/MS analytical method[J]. Food Chemistry, 2008, 108(2): 542-550.
[3] GOKMEN V, A AR O , KOKSEL H, et al. Effects of dough formula and baking conditions on acrylamide and hydroxymethylfurfural formation in cookies[J]. Food Chemistry, 2007, 104: 1136-1142.
[4] MUSTAFA A, FINK M, KAMAL-ELDIN A, et al. Interaction effects of fermentation time and added asparagine and glycine on acrylamide content in yeast-leavened bread[J]. Food Chemistry, 2009, 112: 767-774.
[5] CAPUANO E, FERRIGNO A, ACAMPA I, et al. Effect of flour type on Maillard reaction and acrylamide formation during toasting of bread crisp model systems and mitigation strategies[J]. Food Research International, 2009, 42: 1295-1302.
[6] HUANG Weining, YU Shengdi, ZOU Qibo, et al. Effects of frying conditions and yeast fermentation on the acrylamide content in you-tiao, a traditional Chinese, fried, twisted dough-roll[J]. Food Research International, 2008, 41: 918-923.
[7] KIM C T, HWANG E S, LEE H J. Reducing acrylamide in fried snack products by adding amino acids[J]. Journal of Food Science, 2005, 70: C354-C358.
[8] BECALSKI A, LAU B P Y, LEWIS D, et al. Acrylamide in french fries: influence of free amino acids and sugars[J]. Journal of Agricultural and Food Chemistry, 2004, 52: 3801-3806.
[9] RYDBERG P, ERIKSSON S, TAREKE E, et al. Investigations of factors that influence the acrylamide content of heated foodstuffs[J]. Journal of Agricultural and Food Chemistry, 2003, 51: 7012-7018.
[10] ZENG Xiaohui, CHENG Kawing, JIANG Yue, et al. Inhibition of acrylamide formation by vitamins in model reactions and fried potato strips[J]. Food Chemistry, 2009, 116: 34-39.
[11] ZHANG Yu, ZHANG Ying. Effect of natural antioxidants on kinetic behavior of acrylamide formation and elimination in low-moisture asparagine-glucose model system[J]. Journal of Food Engineering, 2008, 85: 105-115.
[12] LEVINE R A, SMITH R E. Sources of variability of acrylamide levels in a cracker model[J]. Journal of Agricultural and Food Chemistry, 2005, 53: 4410-4416.
[13] DIETRYCH S D, OLESZEK W. Effect of processing on the flavonoid content in buckwheat (Fagopyrum esculentum Moench) grain[J]. Journal of Agricultural and Food Chemistry, 1999, 47: 4384-4387.
[14] HOLASOVA M, FIEDLEROVA V, SMRCINOVA H, et al. Buckwheat: the source of antioxidant activity in functional foods[J]. Food Research International, 2002, 35: 207-211.
[15] LIN Liyun, LIU Hsiuman, YU Yawen, et al. Quality and antioxidant property of buckwheat enhanced wheat bread[J]. Food Chemistry, 2009, 112: 987-991.
[16] BEHERA S, NAGARAJAN S, JAGAN M R. Microwave heating and conventional roasting of cumin seeds (Cuminum cyminum L.) and effect on chemical composition of volatiles[J]. Food Chemistry, 2004, 87: 25-29.
[17] THIPPESWAMY N B, NAIDU K A. Antioxidant potency of cumin varieties-cumin, black cumin and bitter cumin-on antioxidant systems [J]. European Food Research and Technology, 2005, 220: 472-476.
[18] SOWBHAGYA H B, SUMA P F, MAHADEVAMMA S, et al. Spent residue from cumin: a potential source of dietary fiber[J]. Food Chemistry, 2007, 104: 1220-1225.
[19] MILAN K S M, DHOLAKIA H, TIKU P K, et al. Enhancement of digestive enzymatic activity by cumin (Cuminum cyminum L.) and role of spent cumin as a bionutrient[J]. Food Chemistry, 2008, 110: 678-683.
[20] SRINIVASAN K. Spices as influencers of body metabolism: an overview of three decades of research[J]. Food Research International, 2005, 38: 77-86.
[21] ABDEL-SAMIE M A M, HUANG W N, OKKYUNG C, et al. Effects of cumin and ginger as antioxidants on dough mixing property and cookie quality[J]. Cereal Chemistry, 2010, 87: 454-460.
[22] TAUSEEF S M, BUTT M S, ANJUM F M. Safety assessment of black cumin fixed and essential oil in normal Sprague Dawley rats: serological and hematological indices[J]. Food and Chemistry Toxicology, 2009, 47: 2768-2775.
[23] BOURGOU S, PICHETTE A, MARZOUK B, et al. Bioactivities of black cumin essential oil and its main terpenes from Tunisia[J]. South African Journal of Botany, 2009, 76: 210-216.
[24] MARIOD A A, IBRAHIM R M, ISMAIL M, et al. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake[J]. Food Chemistry, 2009, 116: 306-312.
[25] SALIH B, SIPAHI T, DONMEZ E O. Ancient nigella seeds from BoyalI Hoyok in north-central Turkey[J]. Journal of Ethnopharmacology, 2009, 124: 416-420.
[26] MILLER G L. Use of dinitrosalicylic acid reagent for determination of reducing sugar[J]. Analytical Chemistry, 1959, 31: 426-428.
[27] American Association of Cereal Chemists. Approved methods of analysis, methods 10-50D, 44-15[S]. St Paul, MN 2002.
[28] HOODA S, JOOD S. Organoleptic and nutritional evaluation of wheat biscuits supplemented with untreated and treated fenugreek flour[J]. Food Chemistry, 2005, 90: 427-435.
[29] EMMONS C L, PETERSON D M, PAUL G L. Antioxidant capacity of oat (Avena sativa L.) extracts. 2. in vitro antioxidant activity and contents of phenolic and tocol antioxidants[J]. Journal of Agricultural and Food Chemistry, 1999, 47: 4894-4898.
[30] TEPE B, SOKMEN M, ASKIN A H, et al. in vitro antioxidant activities of the methanol extracts of four Helichrysum species from Turkey[J]. Food Chemistry, 2005, 90: 685-689.
[31] HU C, KITTS D D. Studies on the antioxidant activity of echinacea root extract[J]. Journal of Agricultural and Food Chemistry, 2000, 48: 1466-1472.
[32] STEADMAN K J, BURGOON M S, LEWIS B A, et al. Buckwheat seed milling fractions: description, macronutrient composition and dietary fiber[J]. Journal of Cereal Science, 2001, 33: 271-278.
天然抗氧化剂抑制曲奇中丙烯酰胺含量的研究
Mohamed ABDEL-SHAFI ABDEL-SAMIE1,黄卫宁1,*,李珍妮1,姚 远2,Okkyung Kim CHUNG1
(1. 江南大学 食品科学与技术国家重点实验室,江苏 无锡 214122;2. 普渡大学食品科学系,美国 印第安纳州 西拉法叶 IN47907)
采用天然抗氧化剂荞麦、孜然和黑种草籽抑制曲奇中丙烯酰胺的生成。研究这3种配料对面粉中丙烯酰胺前体的含量、面粉和曲奇抗氧化的特性、曲奇烘焙特性和丙烯酰胺含量的影响,结果表明:这3种配料组合都可以增加面粉中还原糖和天门冬酰胺的含量,也可以增加面粉和曲奇的抗氧化特性(包括总酚类物质、DPPH自由基清除能力和ABTS+·的抑制能力)。当所添加的天然抗氧化剂含量增加时,曲奇颜色变深,表现为更低的L*,a*和b*值;荞麦和孜然的引入会增加曲奇中的水分含量,而黑种草籽则相反。所有天然抗氧化剂组合都可以增加曲奇的延展率。未添加天然抗氧化剂的曲奇中丙烯酰胺的含量为361.2μg/kg,而单独添加15%荞麦、15%孜然和15%黑种草籽使得曲奇中丙烯酰胺的含量分别降低了20.2%、67.6%和75.9%。感官分析结果表明:富含天然抗氧化剂的曲奇都可以被人们所喜欢。
丙烯酰胺抑制剂;抗氧化性;荞麦;孜然;黑种草籽;曲奇
TS213.2
A
1002-6630(2011)07-0129-12
2010-12-20
美国农业部国际合作项目[A-(86269)];加拿大农业部国际交流与合作项目(CCSIC-Food-00107);国家“863”计划项目(2007AA100401);国家自然科学基金项目(20576046)
Mohamed Abdel-Shafi Abdel-Samie(1982—),男,博士研究生,研究方向为烘焙科学、功能配料和食品添加剂。E-mail:mampowered@yahoo.com
*通信作者:黄卫宁(1963—),男,教授,博士,研究方向为食品烘焙与发酵技术、谷物食品化学。E-mail:wnhuang@jiangnan.edu.cn

