蒙脱石对喹诺酮类抗生素的吸附平衡及动力学特征*
莫测辉,黄显东,吴小莲,李彦文,邹 星,高 鹏
(暨南大学环境工程系,广东省高校水土环境毒害性污染物防治与生物修复重点实验室,广东广州 510632)
蒙脱石对喹诺酮类抗生素的吸附平衡及动力学特征*
莫测辉†,黄显东,吴小莲,李彦文,邹 星,高 鹏
(暨南大学环境工程系,广东省高校水土环境毒害性污染物防治与生物修复重点实验室,广东广州 510632)
以蒙脱石为吸附剂进行水中2种喹诺酮类抗生素(环丙沙星和诺氟沙星)的静态吸附试验,考察初始浓度、p H值和阳离子强度对吸附性能的影响.结果表明,蒙脱石对环丙沙星和诺氟沙星的吸附过程均符合二级反应动力学方程,吸附速率常数分别为0.063和0.024 kg·mg-1·h-1.环丙沙星和诺氟沙星的吸附等温线均能较好地符合Freundlich方程,且lgKf值较大,具有较强的吸附能力.当溶液p H值小于环丙沙星和诺氟沙星的pKa2值时,具有较高吸附量;大于pKa2值时,吸附量急剧下降.环丙沙星和诺氟沙星阳离子以及兼性离子均可被蒙脱石吸附,在高p H值条件下,阴离子与蒙脱石表面络合可能是蒙脱石吸附去除环丙沙星和诺氟沙星的主要作用机制.Ca2+对吸附有重要影响,其浓度越高,环丙沙星和诺氟沙星的吸附量越低.
抗生素;喹诺酮类;水污染;蒙脱石;吸附;去除;动力学
喹诺酮类抗生素广泛用于人类医疗和动物养殖业[1],随着生产和使用数量迅速增长,大量抗生素进入环境,近年来在各种水环境中均检测出不同程度的抗生素.美国城市污水和地表水中喹诺酮类抗生素的浓度分别为2和0.12μg·L-1[2-4],而在德国医院污水中喹诺酮类抗生素的浓度高达124.5μg· L-1[5].不同环境介质中喹诺酮类抗生素的浓度虽然比较低,但可能诱导病原菌产生耐药性,对生态系统和人类健康产生严重威胁[6-7].因此,探讨抗生素污染水体的吸附处理技术具有重要的现实意义.
目前有关污泥、沉积物、土壤和纯矿物吸附抗生素的研究已有许多报道[8-11].抗生素在不同土壤和沉积物中的吸附系数(Kd)差异较大[12-14],表明抗生素在土壤上的吸附能力与土壤类型有关,土壤矿物和有机质组分可能是抗生素药物的主要吸附位点[15],其阳离子交换量被认为是影响吸附的重要因素[16].研究还发现抗生素在土壤上的吸附能力也与土壤或沉积物的p H值有关[17].
尽管对于土壤中抗生素的吸附行为已有不少研究,但有关喹诺酮类抗生素吸附机理的报道较少.铁、铝氧化物作为土壤中的重要矿物,有报道研究了其对环丙沙星的吸附行为[18-19].蒙脱石作为一种常见的矿物材料,利用其吸附去除四环素[20]、土霉素[21]以及恩诺沙星[22]有少量研究,但所研究的抗生素种类较单一,吸附去除效果也存在较大差异[23-24].为此本文通过静态吸附试验研究了蒙脱石对环丙沙星和诺氟沙星2种典型喹诺酮类抗生素的吸附动力学和吸附等温线,并探讨不同p H值、阳离子强度等环境因素对吸附去除效果的影响,为抗生素污染水体的治理提供科学依据和实用技术.
1 材料与方法
1.1 材料、试剂和仪器
供试吸附剂蒙脱石由华南理工大学提供,其粒径<2μm,比表面积为120 m2·g-1,阳离子交换容量(CEC)为783 mmol·kg-1.
诺氟沙星标准品(99.6%)和环丙沙星标准品(84.9%)购自中国药品生物制品检定所.环丙沙星和诺氟沙星均以兼性离子形态存在,环丙沙星的pKa1和pKa2分别为6.10和8.70[18],诺氟沙星的pKa1和pKa2分别为6.30和8.38[25].诺氟沙星和环丙沙星都含有胺基和羧基2种基团,当诺氟沙星溶液中的p H值低于6.10,环丙沙星溶液的p H值低于6.30时,其胺基基团均与水中H+结合而以阳离子形态存在为主;当诺氟沙星溶液中的p H值高于8.70,环丙沙星溶液的p H值高于8.38时,其羧基基团均将失去H+而以阴离子形态存在为主;当水溶液中的p H值介于两pKa值之间时,上述离子达到平衡,以兼性离子形态存在为主.
甲醇、乙腈为色谱纯(Sigma公司);其他化学试剂均为分析纯;实验用水为高纯水.
Shimadzu高效液相色谱仪(日本),配置有Shimadzu SIL-20A自动进样器,Shimadzu荧光检测器,柱温控制器,以及LC Solution工作站;雷磁PHS-3C精密p H计;TGL-16G型高速台式离心机;SHZ-82恒温震荡器.
1.2 实验方法
1.2.1 反应时间对吸附效果的影响
吸附试验参照OECD guideline 106批平衡方法进行[26].称取蒙脱石0.500 g置于50 m L离心管中,加入25 m L含环丙沙星和诺氟沙星浓度均为2 mg·L-1的0.01 mol·L-1CaCl2溶液(V水∶V土=50∶1).在25℃下避光恒温振荡(250 r·min-1),分别在第2,4,6,8,10,12,14,18和24 h采集样品,离心(4 000 r·min-1)10 min,取上清液过0.45μm滤膜后供HPLC分析.实验均做3个重复(下同).
蒙脱石对环丙沙星和诺氟沙星的吸附量由以下公式计算:
Cs=(C0-Ce)V/M.
式中:Cs为环丙沙星和诺氟沙星的的吸附量,mg· kg-1;C0为初始浓度,mg·L-1;Ce为上清液中浓度,mg·L-1;V为溶液体积,L;M为吸附剂质量,g.
1.2.2 初始浓度对吸附效果的影响
配制环丙沙星和诺氟沙星的初始浓度分别为1,2,3,4和5 mg·L-1,振荡12 h,参照上述方法进行实验和分析.
1.2.5 实验结果保证 为了保证实验结果的准确性,室内质控每板实验均做高、低两个浓度水平的质控,室内质控均在控,实验数据认为可靠。另外本实验室参加了国家卫计委和美国CDC串联质谱室间质评,成绩优秀。
1.2.3 p H值对吸附效果的影响
设置环丙沙星和诺氟沙星的起始浓度均为2 mg·L-1,以5%盐酸溶液或0.5 mol·L-1氢氧化钠溶液调节蒙脱石悬浊液p H值分别为4,5,6,7,8,9,10,参照上述方法进行实验和分析,并测定平衡溶液p H值.
1.2.4 阳离子强度对吸附效果的影响
设置Ca2+浓度分别为0.00,0.01,0.03,0.05和0.10 mol·L-1,参照上述方法进行实验和分析.
1.3 分析方法
色谱操作条件:色谱柱(Waters,250 mm×4.6 mm I.D.,5μm);激发波长280 nm;发射波长450 nm;柱温25℃;进样量20μL;流动相乙腈0.067 mol·L-1磷酸溶液(15/85,V/V);流速1.0 m L· min-1,每个样品运行15 min.该色谱条件下环丙沙星和诺氟沙星的保留时间分别为7.8和10.2 min.
1.4 质量控制
为控制实验过程中人为干扰,保证操作过程准确,以未含环丙沙星和诺氟沙星的处理作为空白对照,同时无蒙脱石处理作添加回收实验,环丙沙星和诺氟沙星的回收率为95%~105%,空白实验中未检出目标化合物,说明实验操作过程中无人为污染.测定浓度为0,0.01,0.02,0.05,0.1,0.2,0.5 mg· L-1的环丙沙星和诺氟沙星标准溶液,并进行线性回归分析,相关系数均大于0.999.2种喹诺酮类抗生素的检测限(S/N=3)为0.093~0.143μg·L-1.
2 结果与讨论
2.1 蒙脱石对喹诺酮类抗生素的吸附动力学
蒙脱石对环丙沙星和诺氟沙星的吸附过程可分为初始快速吸附阶段和随后的缓慢吸附阶段,在2 h后基本达到吸附平衡(见图1).在达到最大值之后幅度均有不同的微小降低,这可能与环丙沙星和诺氟沙星在开始阶段大量吸附在矿物表层,而后随着平衡过程缓慢从层间释出来有关[22],因此后面的实验设定反应时间为12 h.

图1 蒙脱石对环丙沙星和诺氟沙星的吸附平衡曲线Fig.1 Adsorption equilibration curves of ciprofloxacin and norfloxacin to montmorillonite
对吸附过程进行二级反应动力学拟合:

式中:k2为平衡速率常数,kg·mg-1·h-1;qe为平衡吸附量,mg·kg-1;q为t时间时的吸附量.结果表明,蒙脱石对环丙沙星和诺氟沙星的吸附过程均符合二级动力学方程,具有很好的线性关系(R2>0.999),平衡速率常数分别为0.063和0.024 kg· mg-1·h-1,平衡吸附量(qe)分别为86.9和84.7 mg·kg-1,所得结果均小于坡缕石[27]、累托石[28]对四环素的吸附.
2.2 蒙脱石对喹诺酮类抗生素的吸附等温线
蒙脱石对环丙沙星和诺氟沙星的吸附等温线分别依据下列方程进行拟合.
Freundlich方程:

式中:Cs为单位质量蒙脱石对环丙沙星和诺氟沙星的吸附量,mg·kg-1;Ce为平衡溶液中环丙沙星和诺氟沙星的浓度,mg·L-1;Kf和n为特征常数.方程(3)中,qe等同于方程(2)中的Cs;KL为吸附常数;qm为蒙脱石对环丙沙星和诺氟沙星的最大吸附量,mg·kg-1.
2种方程拟合所得到的吸附等温线均呈直线,表现出良好的相关性(见图2).通过拟合曲线计算得到等温吸附方程,相关参数见表1.蒙脱石对环丙沙星和诺氟沙星的lgKf值相差不大,均在2.5左右,显示了较强的吸附能力,与Nowara等利用其他粘土矿物进行实验结果相近[22].由Langmuir方程计算得到在初始浓度为2 mg·L-1时,蒙脱石对环丙沙星和诺氟沙星的最大吸附量(qm)分别为416.7和500.0 mg·kg-1,该结果远小于前人所得蒙脱石对环丙沙星的吸附量[23],也小于铁、铝氧化物[18]和针铁矿[19]对喹诺酮类抗生素的吸附量,这可能与环丙沙星和诺氟沙星的初始浓度较低有关.另外,不同研究者所用粘土矿物的理化性质如阳离子交换量、粒径、比表面积等不同也可能导致不同结果之间差异较大.
2.3 p H值对蒙脱石吸附喹诺酮类抗生素的影响
溶液中p H值对蒙脱石吸附环丙沙星和诺氟沙星的影响如图3所示.当p H值为4~8时,蒙脱石对环丙沙星和诺氟沙星的吸附量变化不大,分别为97.8~91.5和96.2~86.5 mg·kg-1.而在整个实验p H值范围内,蒙脱石的表面均带负电荷[29],所以环丙沙星和诺氟沙星的吸附量由其离子形态决定.当溶液中的p H由酸性变为中性时,环丙沙星和诺氟沙星的兼性离子比例增大,但兼性离子中的胺基基团也可以通过阳离子交换被蒙脱石吸附[14].当溶液的p H值高于pKa2值时,环丙沙星和诺氟沙星的吸附量急剧下降.这是因为在该条件下,环丙沙星和诺氟沙星均以阴离子形态存在为主,与蒙脱石的表面负电荷相排斥,这表明在高p H值下阳离子交换吸附并不起主要作用.有研究报道了水溶液中的环丙沙星可以通过与铁、铝氧化物形成表面络合物得以有效去除[18],在p H值接近pKa2值,氧氟沙星与介孔二氧化硅的吸附量仍达较大值[30].由此可知,表面络合有可能是在高p H值条件下蒙脱石吸附去除环丙沙星和诺氟沙星的主要作用机制.
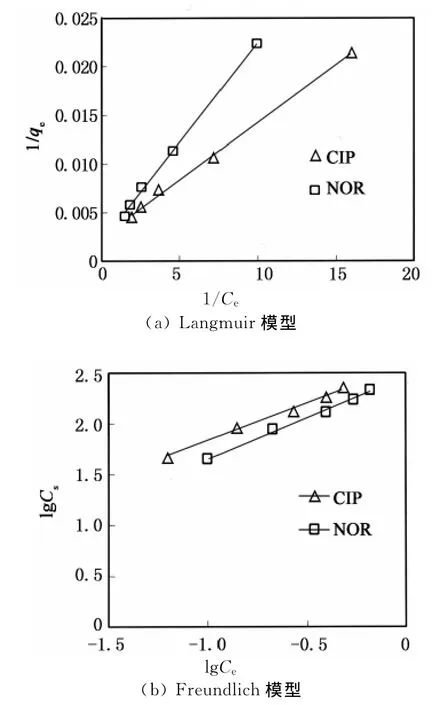
图2 蒙脱石对环丙沙星和诺氟沙星的吸附拟合曲线Fig.2 Adsorption curves of of ciprofloxacin and norfloxacin to montmorillonite

表1 吸附模型相关参数Tab.1 Parameters of the adsorption models fitted
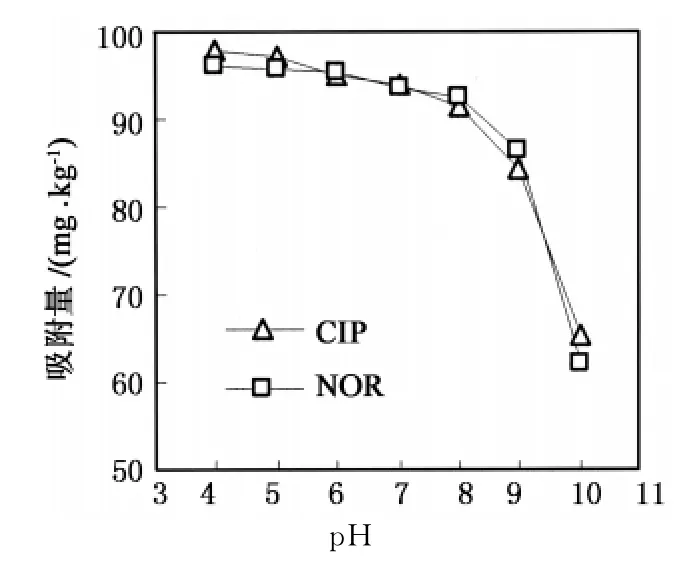
图3 p H值对吸附量的影响Fig.3 Influence of p H on adsorption capacity
2.4 阳离子强度对蒙脱石吸附喹诺酮类抗生素的影响
溶液中Ca2+的存在对蒙脱石吸附喹诺酮类抗生素有明显的抑制作用,且随着Ca2+浓度的升高,蒙脱石对环丙沙星和诺氟沙星的吸附系数Kd值(由Cs/Ce计算得到)逐渐降低(表2).这是因为蒙脱石对环丙沙星和诺氟沙星的吸附是以阳离子吸附为主,当Ca2+离子存在时,可产生(CaCl)+形态并通过电性吸附于粘土矿物表面[31],占据活性吸附位点,与环丙沙星和诺氟沙星进行竞争性吸附.因此,阳离子的存在不利于蒙脱石对环丙沙星和诺氟沙星的吸附.
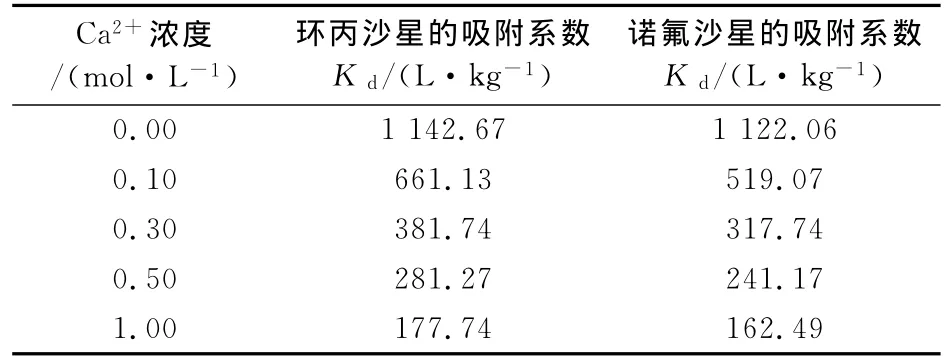
表2 不同Ca2+浓度下蒙脱石对环丙沙星和诺氟沙星的Kd值Tab.2 Kd of quinolons adsorption to montmorillonite in the presence of Ca2+
3 结 论
1)蒙脱石对环丙沙星和诺氟沙星的吸附过程均符合二级反应动力学方程,吸附速率常数分别为0.063和0.024 kg·mg-1·h-1;环丙沙星和诺氟沙星的吸附等温线均能较好地符合Freundlich方程,lgKf值较大,具有较强的吸附能力.
2)溶液p H值小于环丙沙星和诺氟沙星的pKa2值时,具有较高吸附量;大于pKa2值时,吸附量急剧下降.环丙沙星和诺氟沙星阳离子与兼性离子均可被蒙脱石吸附,在高p H值条件下,阴离子与蒙脱石表面络合可能是蒙脱石吸附去除环丙沙星和诺氟沙星的主要作用机制.Ca2+对吸附有重要影响,其浓度越高,环丙沙星和诺氟沙星的吸附量越低.
[1] WALSH C.Antibiotics:actions,origins,resistance[M].Washington DC:ASM Press,2003:15-20.
[2] KARTHIKEYAN K G,MEYER M T.Occurrence of antibiotics in wastewater treatment facilities in Wisconsin,USA[J].Science of the Total Environment,2006,361:196-207.
[3] RENEW J E,HUANG C H.Simultaneous analysis of fluoroquinolone,sulfonamide and trimethoprim antibiotics in wastewater using tandem solid-phase extraction and liquid chromatography electro spray mass spectrometry[J].J Chromatogr A,2004,1042:113-121.
[4] KOLPIN D W,FURLONG E T,MEYER M,etal.Pharmaceuticals,hormones,and other organic wastewater contaminants in U.S.streams,1999-2000:a national reconnaissance[J].Environ Sci Technol,2002,36:1202-1211.
[5] HARTMANN A,GOLET E M,GARTISER S,etal.Primary DNA damage but not mutagenicity correlates with ciprofloxacin concentrations in German hospital wastewaters[J].Arch Environ Contam Toxicol,1999,36:115-119.
[6] VOLMER,D A,MANSOORI B,LOCKE S J.Study of 4-quinoloneantibiotics in biological samples by short column liquid chromatography coupled with electro spray ionization tandem mass spectrometry[J].Anal Chem,1997,69(9):4143-4155.
[7] HARTMANN A,ALDER A C,KOLLER T,etal.Identification of fluoroquinolone antibiotics as the main source of genotoxicity in native hospital wastewater[J].Environ Toxicol Chem,1998,17:377-382.
[8] TERNES T A,MEISENHEIMER M,MCDOWELl D,etal.Removal of pharmaceuticals during drinking water treatment[J].Environ Sci Technol,2002,36:3855-3863.
[9] CARRASQUILLO A J,BRULAND G L,MACKAY A A,et al.Sorption of ciprofloxacin and oxytetracycline zwitterions to soils and soil minerals:influence of compound structure[J].Environ Sci Technol,2008,42(20):7634-7642.
[10]FEITOSA-FELIZZOLAA J,HANNAB K,CHIRON S.Adsorption and transformation of selected human-used macrolide antibacterial agents with iron(III)and manganese(IV)oxides[J].Environmental Pollution,2009,157(4):1317-1322.
[11]GAO Juan,JOEL A.Pedersen.Adsorption of sulfonamide antimicrobial agents to clay minerals[J].Environ Sci Technol,2005,39(24):9509-9516.
[12]RABØLLE M,SPLIID N H.Sorption and mobility of metronidazole,olaquindox,oxytetracycline and tylosin in soil[J].Chemosphere,2000,40(7):715-722.
[13]SUKUL P,LAMSHÖFT M,ZÜHLKE S,etal.Sorption and desorption of sulfadiazine in soil and soil-manure systems[J].Chemosphere,2008,73:1344-1350.
[14]CARRASQUILLO A J,BRULAND G L,MACKAY A A,et al.Sorption of ciprofloxacin and oxytetracycline zwitterions to soils and soil minerals:influence of compound structure[J].Environ Sci Technol,2008,42(20):7634-7642.
[15]TOLLS J.Sorption of veterinary pharmaceuticals in soils:a review[J].Environ Sci Technol,2001,35(17):3397-3406.
[16]VASUDEVAN D,BRYKABD G L,TORRANCE B S,etal.p H-dependent ciprofloxacin sorption to soils:interaction mechanisms and soil factors influencing sorption[J].Geoderma,2009,151:68-76.
[17]SASSMAN S A,LEE L S.Sorption of three tetracyclines by several soils:assessing the role of p H and cation exchange[J].Environ Sci Technol,2005,39:7452-7459.
[18]GU C,KARTHIKEYAN K G.Sorption of the antimicrobial ciprofloxacin to aluminum and iron hydrous oxides[J].Environ Sci Technol,2005,39:9166-9173.
[19]ZHANG H,HUANG C H.Adsorption and oxidation of fluoroquinolone antibacterial agents and structurally related amines with goethite[J].Chemosphere,2007,66:1502-1512.
[20]PAROLO M E,SAVINI M C,VALLÉS J M,etal.Tetracycline adsorption on montmorillonite:p H and ionic strength effects[J].Appl Clay Sci,2008,40:179-186.
[21]KULSHRESTHA P,GIESE R F,AGA D S.Investigating the molecular interactions of oxytetracycline in clay and organic matter:insights on factors affecting its mobility in soil[J].Environ Sci Technol,2004,38:4097-4105.
[22]NOWARA A,BURHENNE J,SPITELLER M.Binding of fluoroquinolone carboxylic acid derivatives to clay minerals[J].J Agric Food Chem,1997,45(4):1459-1463.
[23]WU Qing-feng,LI Zhao-hui,HONG Han-lie,etal.Adsorption and intercalation of ciprofloxacin on montmorillonite[J].Applied Clay Science,2010,50(2):204-211.
[24]WANG C J,LI Z H,JIANG W T,etal.Cation exchange interaction between antibiotic ciprofloxacin and montmorillonite[J].Journal of Hazardous Materials,2010,83:309-314.
[25]ROSS D L,RILEY C M.Aqueous solubilities of some various substituted quinolone antimicrobials[J].Int J Pharm,1990,63:237-250.
[26]OECD.OECD guidelines for testing of chemicals,test guideline 106:adsorption/desorption using a batch equilibrium method[M].Revised Draft Document,Paris:OECD,2000:1-45.
[27]CHANG P H,LI Z H,YU T L,etal.Sorptive removal of tetracycline from water by palygorskite[J].Journal of Hazardous Materials,2009,165:148-155.
[28]CHANG P H,JEAN J S,JIANG W T,etal.Mechanism of tetracycline sorption on rectorite[J].Colloids Surf A:Physicochem Eng Aspects,2009,339:94-99.
[29]SAKA E E,GÜLER C.The effect of electrolyte concentration,ion species and p H on the zeta potential and electrokinetic charge density of montmorillonite[J].Clay Miner,2006,41:853-861.
[30]GOYNE K W,CHOROVER J,KUBICKI J D,etal.Sorption of the antibiotic ofloxacin to mesoporous and nonporous alumina and silica[J].J Colloid Interface Sci,2005,283:160-170.
[31]SPOSITO G.Effect of chloride ions on sodium-calcium and sodium-magnesium exchange on montmorillonite[J].Soil Sci Soc Am J,1991,55:965-967.
Adsorption Equilibrium and Kinetics of Quinolone Antibiotics on Montmorillonite
MO Ce-hui†,HUANG Xian-dong,WU Xiao-lian,LI Yan-wen,ZOU Xing,GAO Peng
(Department of Environment Engineering,Jinan Univ,Key Laboratory of Water/Soil Toxic Pollutants Control and Bioremediation,Department of Education of Guangdong Province,Guangzhou,Guangdong 510632,China)
A static adsorption experiment was carried out to investigate the influence of initial concentration,p H,and cationic strength on adsorption of two kinds of quinolone antibiotics(ciprofloxacin and norfloxacin)on montmorillonite.The results showed that the adsorption process of ciprofloxacin and norfloxacin were consistent with pseudo-second-order kinetics,and the adsorption rate constants were 0.063 and 0.024 kg·mg-1·h-1respectively.Adsorption isotherms were well described by Freundlich equation,and the high lgKfshowed strong adsorption ability.When the solution p H was less than thepKa2of ciprofloxacin and norfloxacin,the adsorption capacity was higher.The adsorption capacity decreased sharply when the solution p H was above thepKa2.Both cationic and zwitterionic forms of ciprofloxacin and norfloxacin could be adsorbed significantly.At higher p H,the complexation of anion on the montmorillonite surface might become the main mechanism.The concentration of Ca2+had a great influence on adsorption. The higher the concentration,the lower the adsorption capacity of ciprofloxacin and norfloxacin.
antibiotics;quinolones;water pollution;montmorillonite;adsorption;removal;kinetics
X506
A
1674-2974(2011)06-0064-05*
2010-10-22
国家自然科学基金资助项目(30671208,40773062);中央高校基本科研业务费专项资金资助项目(21610410,21609709);广东省自然科学基金重点资助项目(07117909);广东省科技计划资助项目(2005B20801002,2006B20601003,2010B020311006);广东省高校高层次人才项目;广州市科技计划资助项目(10A82070466);东莞市科技计划资助项目(2007108101110);惠州市科技研究计划资助项目(2009B010001009)
莫测辉(1965-),男,广西柳州人,暨南大学教授
†通讯联系人,E-mail:tchmo@jnu.edu.cn

