* 微波促进下苯并噻吩的傅-克酰基化反应
张变香,石高升,王琼,杨祺,康婧玲
(山西大学化学化工学院,山西太原 030006)
*微波促进下苯并噻吩的傅-克酰基化反应
张变香,石高升,王琼,杨祺,康婧玲
(山西大学化学化工学院,山西太原 030006)
进行了苯并噻吩与酸酐、乙酰氯、苯甲酰氯和草酰氯的傅-克酰基化反应,探究了微波辐射对该反应的影响.通过1H NMR、13C NMR对产物的结构进行了表征,采用高效液相色谱法测定了产物的产率与产物异构体的比例.结果表明,在苯并噻吩与草酰氯的反应中选择性地得到了3,3′-二苯并噻吩乙二酮,并且常温下12 h进行的反应在微波辐射下反应时间缩短为25 min,产率比常温反应提高了20%.
微波辐射;苯并噻吩衍生物;傅-克酰基化
苯并噻吩、噻吩衍生物是自然界中存在的含硫杂原子的环状化合物之一,不仅是重要的有机合成中间体,而且作为医药、农药、机能性材料等的基本骨架近年来被广泛利用[1-4].然而带有功能性基团的小分子苯并噻吩,作为原料因其合成步骤多产率低而使得价格昂贵.有关合成此类化合物的报道较多,主要类型有苯衍生物和噻吩衍生物的关环反应[5-12];带有卤素或羧基取代基的苯并噻吩的偶联反应[12-14].以无取代基的苯并噻吩为原料一步合成苯并噻吩衍生物的报道较少[15],寻找简便有效的合成苯并噻吩衍生物的方法具有重要的意义.
微波作用下的有机反应具有反应时间短、收率高、副反应少、操作简便及环境友好等优点,近年来在有机合成尤其是杂环化合物的合成中得到了广泛的应用[16-17].本文用微波催化和传统的加热回流两种方法对苯并噻吩的傅-克酰基化反应进行了研究,讨论了催化剂的种类和用量、反应溶剂、温度、加样方式对反应的影响.结果表明:微波催化与传统的加热回流方法相比,具有反应速度快、转化率和选择性好等优点.
1 实验部分
1.1 仪器与试剂
予华X-4数字显示显微熔点测定仪;岛津UV-265型紫外可见光谱仪;Bruker DRX-300 M Hz核磁共振仪(5 mm样品管,内标为 TM S,溶剂为CDCl3,测试温度 25℃,1H NM R和13C NM R的工作频率分别为300.40 M Hz和75.45 M Hz),P200II型高效液相色谱仪.本实验所用试剂苯并噻吩(BT)、乙酰氯(AC)、苯甲酰氯(BC)、酸酐(AA)、草酰氯(OC)均为分析纯.
1.2 常温与微波辐射下苯并噻吩的傅-克酰基化反应
在装有恒压滴液漏斗、冷凝管(带有干燥管)的100 m L三口烧瓶中放入磁搅拌子,在冰浴中依次加入苯并噻吩、溶剂、催化剂,搅拌均匀后,在10~30 min内缓慢加入酰基化试剂,滴加完毕后,恢复至室温反应,反应中通过薄层硅胶板(TLC)跟踪.反应完全后,将混合物置入冰水中,滴加盐酸酸解.反应终止后,用乙醚萃取,有机层用饱和碳酸氢钠和氯化钠溶液各洗两次,用无水硫酸镁干燥,减压蒸去有机溶剂,所得粗产物用柱层析分离(CH2Cl2∶n-Hexane=1∶1),通过液相色谱法计算转化率和2位、3位酰基化产物的比例(反应式如下式所示).

除了苯并噻吩与草酰氯的微波辐射反应,其它的微波反应的加药品方式与常温相同,加完药品后,将长颈烧瓶转入微波反应器中,设置恒定功率及反应时间,反应后处理与常温反应的方法相同.草酰氯与苯并噻吩的微波反应的加药品依次为溶剂、催化剂、草酰氯试剂,搅拌均匀后,在10~30 min内缓慢加入苯并噻吩.
1.3 产物的数据
2-乙酰基苯并噻吩:m.p.83~84 ℃(lit[18]83~84 ℃);1H NM R(CDCl3,300 M Hz)δ:2.94(s,M e,3H),7.37~7.93(m,A rH,5H);13C NMR(CDCl3,75 M Hz)δ:27.06,123.24,125.23,126.15,127.68,129.96,139.33,142.83,144.18,192.57.
3-乙酰基苯并噻吩:m.p.63~65 ℃(lit[18]62~64℃);1H NM R(CDCl3,300 M Hz)δ:2.58(s,CH3,3H),7.80~7.66(m,A rH,2H),7.35~7.46(m,A rH,2H),8.19(s,A rH,1H);13C NMR(CDCl3,75 M Hz)δ:27.56,121.94,122.44,124.15,124.61,126.70,129.60,138.80,139.54,191.64.
2-苯甲酰基苯并噻吩:m.p.45~48℃(lit[18]47~48℃);1H NM R(CDCl3,300 M Hz)δ:6.82~7.03(m,A rH,5H),32~7.40(m,A rH,4H);13C NM R(CDCl3,75 M Hz)δ:122.78,123.33,124.57,125.01,126.91,127.83,128.86,131.71,136.85,137.04,139.41,142.41,189.85.
3-苯甲酰基苯并噻吩:m.p.50~53 ℃(lit[18]52~53 ℃);1H NMR(CDCl3,300 M Hz)δ:8.48(s,A rH,1H),6.88~7.05(m,A rH,5H),7.27~7.40(m,A rH,4H);13C NMR(CDCl3,75 M Hz)δ:121.78,122.45,124.53,125.03,126.91,127.83,128.67,131.71,133.83,138.14,138.52,141.86,188.71.
3,3′-二苯并噻吩乙二酮:m.p.167~169 ℃(lit[19]166~168 ℃);1H NM R(CDCl3,300 M Hz)δ:7.45~7.51(m,A rH,2H);7.89~7.92(d,J=7.2 H,A rH,1H);8.07(s,A rH,1H);8.54~8.56(d,J=7.2 Hz,A rH,1H).13C NM R(CDCl3,75 M Hz)δ:120.21,122.36,124.91,125.60,136.36,136.62,137.19,140.09,184.96.
2 结果与讨论
傅-克反应属于碳正离子对芳烃的亲电取代反应,在通常情况下,未取代的五员杂环芳烃比未取代的苯更容易进行傅-克反应,而且产物是异构体的混合物.以苯并噻吩为例,在凯库勒共振体中2、3位都存在较强的活性,容易被碳正离子进攻[20].另外,Klasinc[21]根据MO法计算了苯并噻吩环上的π电子密度,结果表明环上的电子云分布不均,3位比2位稍大(Scheme 1),因此在反应中导致异构体产物含量不同.产物的比例与催化剂的种类及用量、反应物的加入顺序、温度和溶剂有关,一般以3位酰化产物为主.
示意图1 苯并噻吩的共振式结构Scheme 1 Resonance form of the benzothiophene
2.1 非微波条件下酰基化的条件优化
以苯并噻吩和乙酰氯为底物,无水三氯化铝为催化剂,反应12 h,就反应温度、溶剂和催化剂用量对酰基化产物的产率影响进行了讨论(表1,P92).

表1 A lCl3作用下苯并噻吩(BT)和乙酰氯(AC)的酰基化反应Table 1 AlCl3 catalyzed acylation of BT with AC
由表1可知,增加无水三氯化铝的用量,酰基化产物的产率增大,但用量加到苯并噻吩的3倍时,产率有所降低.反应温度从室温15℃增加到35℃时,乙酰基苯并噻吩的产率变化不大.在反应温度相同的条件下,以二氯甲烷为溶剂时乙酰基苯并噻吩的产率较好.
2.2 酰基化试剂和催化剂对酰基化反应的影响
在上述优化的反应条件下,即苯并噻吩、酰化剂和催化剂的摩尔比为1∶1.2∶2、反应12 h、溶剂为二氯甲烷的条件下,研究了其它酰化试剂和催化剂对反应的影响(如表2).由表2可知,在苯并噻吩和乙酰氯的反应中,FeCl3、ZnCl2催化活性低于A lCl3,而FeCl3在催化苯并噻吩与苯甲酰氯的反应时,酰化产物的产率有所提高.FeCl3和A lCl3催化乙酸酐的酰基化反应的效果与乙酰氯的相当.

表2 酰基化试剂和催化剂对反应的影响Table 2 Effect of acylation reagentsand catalysts on the reaction
2.3 微波辐射下苯并噻吩的酰基化反应
2.3.1 苯并噻吩和乙酰氯的酰基化反应
首先进行了在微波作用下,苯并噻吩、酰化剂和催化剂的摩尔比为1∶1.2∶2,溶剂为二氯甲烷的乙酰基化反应(如表3).

表3 微波辐射下苯并噻吩(BT)和乙酰氯(AC)的酰基化反应Table 3 Acylation of BT with AC under m icrowave irradiation
由表3可知,A lCl3催化下微波照射20 min、功率为196 W,产率达到最大值86%,比常规反应条件下提高了20%.微波照射15 min、功率为260 W时,出现了黑色副产物.微波对 FeCl3催化的反应有较大影响,5 min后原料转化率为36%,产率为30%,随着微波照射时间的增加,转化率和产率都有所增大,微波照射15 m in后原料转化率都达到最大值98%.微波照射FeCl3催化下,苯并噻吩2、3位酰基化产物的比例都在0.9左右,3位产物稍稍多于2位产物,选择性上不如A lCl3催化的酰基化反应(比例在0.6左右).
图1是在FeCl3催化下苯并噻吩与乙酰氯的反应体系在常规反应10 min与微波辐射10 min后,反应混合液的液相色谱对比图,可以看出微波辐射下原料的转化率、产物的收率都有了明显提高.
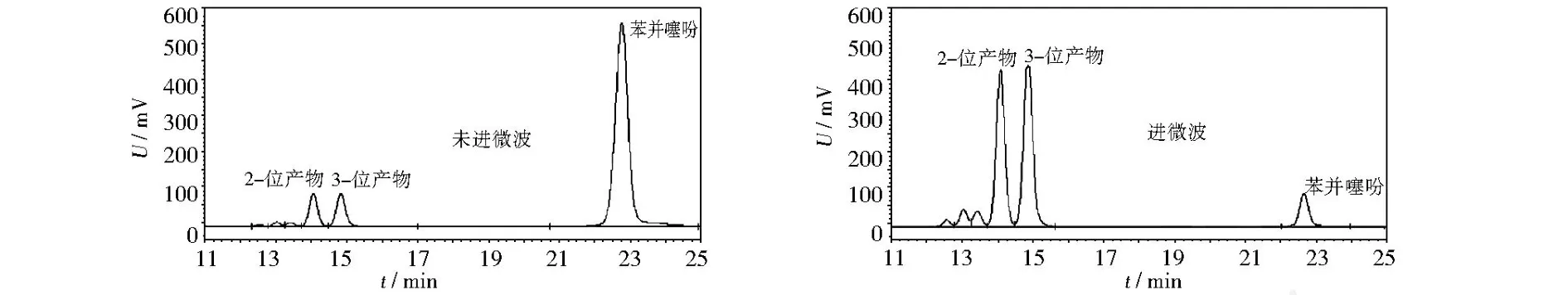
图1 微波辐射与常规条件下酰基化反应的比较Fig.1 Relative analysis of the acylation under microwave radiation and no rm al condition by using HPLC
2.3.2 微波辐射下FeCl3的用量对酰基化反应的影响
由以上结果得知,FeCl3对苯并噻吩与苯甲酰氯的酰基化反应有较好的催化效果,因此讨论了在二氯甲烷为溶剂,FeCl3的用量对酰基化反应的影响(表4).

表4 微波辐射下FeCl3催化的苯并噻吩(BT)与苯甲酰氯(BC)的反应Table 4 FeCl3 catalyzed acylation of BT with BC under microwave-irradition
由表4可知,增加催化剂FeCl3的用量,苯并噻吩的转化率和酰基化产物的产率都有所增大,当催化剂FeCl3的用量为2倍时,苯并噻吩的转化率最好;再增加催化剂的用量,转化率和产率下降,可能是过多的FeCl3包裹了FeCl3的络合物,使FeCl3的络合物不能和苯并噻吩充分反应.
2.3.3 苯并噻吩与草酰氯的酰基化反应
在以A lCl3为催化剂、微波的输出功率为260 W时,草酰氯和苯并噻吩的反应选择性地得到了3,3′-二苯并噻吩乙二酮,讨论结果如表5所示.

表5 微波作用下苯并噻吩(BT)和草酰氯(OC)的酰基化的反应Table 5 Acylation of BT with OC under microwave-irraditiona
由表5可知,以CH2Cl2为溶剂,苯并噻吩和草酰氯在微波中进行反应,苯并噻吩的转化率和3,3′-二苯并噻吩乙二酮的产率较高.因为CH2Cl2能溶解A lCl3复合物,有利于碳正离子的生成,选择性地得到碳正离子亲电取代产物;CH2ClCH2Cl虽然转化率较高,但得到的副产物较多,无论苯并噻吩与草酰氯的摩尔比为1∶2.2或是2∶1,亲电取代产物的产率大约都在40%;CS2对A lCl3复合物溶解性较差,碳正离子亲电取代反应相对较缓慢,苯并噻吩的转化率较低.以FeCl3为催化剂,CS2为溶剂时几乎没有发生反应;CH2Cl2为溶剂时,产率较低.
根据Friedel-Crafts反应机理,苯并噻吩与草酰氯在A lCl3作用下的反应符合碳正离子亲电取代反应历程,催化剂首先和草酰氯生成复合物,形成碳正离子进攻苯并噻吩环上电子云密度较高的3位,得到3,3′-二苯并噻吩乙二酮(Scheme 2).这一结果类似于[Emim]Cl-A lCl3离子液体催化草酰氯与蒽的反应[22],与A lCl3催化下草酰氯和萘的反应主要生成二萘甲酮有所不同[23].

示意图2 3,3′-二苯并噻吩乙二酮的合成机理Scheme 2 Synthetic mechanism of 3,3′-dibenzothiophene enthyldione
[1] Bvolton J L,Thateher G R J,Liu H.Chemical Modification Modulates Estrogenic Activity,Oxidative Reactivity,and Metabolic Stability in 4′F-DMA,a New Benzothiophene Selective Estrogen Recep tor Modulato r[J].Chem Res Toxicol,2006,19(6):779-787.
[2] Jordan V C.Tamoxifen:A Most Unlikely Pioneering Medicine[J].Nat Rev Drug Discov,2003,2(3):205-213.
[3] Rossouw J E,Anderson GL,Prentice R L,et a l.Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women.Principal Results from the Women’s Health Initiative Randomized Controlled Trial[J].J Am M ed Assoc,2002,288(3):321-333.
[4] Katritzky A R,Bobrov S,Khashab N,et al.Benzotriazolyl Mediated 1,2-Shifts of Electron-Rich Heterocycles[J].J Org Chem,2004,69(12):4269-4271.
[5] Kobayashi K,Horiuchi M,Fukamachi S,et al.An Efficient Synthesis of 3-aryl-2-aryl(ormethyl)sulfanylbenzo[b]Thiophenes Via Cyclization of Aryl 2-aryl(ormethyl)Sulfanylmethylsulfanylphenyl Ketones[J].Tetrahedron,2009,65(46):9633-9636.
[6] Mehta S,Waldo T P,Larock R C.Competition Studies in Alkyne Electrophilic Cyclization Reactions[J].J Org Chem,2008,74(3):1141-1147.
[7] Yoshida S,Yorimitsu H,Oshima K.Synthesis of Benzo[b]Thiophenes by Cyclization of Arylketene Dithioacetal Monoxides under Pummerer-like Conditions[J].Org Lett,2007,9(26):5573-5576.
[8] Jeong H J,Yoon U Y,Jang S H,et al.A New Zn/TiCl4/LiA lH4 Mediated App roach to 2-A ryl-or 2-A lkyl-Substituted Benzothiophenes via Intramolecular Cyclization[J].Synlett,2007,9:1407-1410.
[9] Nakamura I,Sato T,Yamamoto Y.Gold-Catalyzed Intramolecular Carbothiolation of Alkynes:Synthesis of 2,3-Disubstituted Benzothiophenes from(α-A lkoxy A lkyl)(o rtho-A lkynyl Phenyl)Sulfides[J].Angew Chem,Int.Ed.2006,45(27):4473-4475.
[10] Hessian K O,Flynn B L.Iodine-Induced Reaction Cascades fo r the Rapid Construction of Variously Substituted Benzothiophenes[J].Org lett,2003,5(23),4377-4380.
[11] Yang SM,Shie JJ,Fang JM,et al.Synthesisof Polysubstituted Benzothiophenes and Sulfur-Containing Polycyclic Aromatic Compounds via Samarium Diiodide Promoted Three-Component Coup ling Reactions of Thiophene-2-carboxylate[J].J Org Chem,2002,67(15):5208-5215.
[12] Mitsudo K,Thansandote P,Wilhelm T,et al.Selectively Substituted Thiophenes and Indoles by a Tandem Palladium-Catalyzed Multicomponent Reaction[J].Org Lett,2006,8(18):3939-3942.
[13] David E,Perrin J,Lmaire M,et al.Efficient Access to 2-A ryl-3-Substituted Benzo[b]thiophenes[J].J Org Chem,2005,70(9):3569-3573.
[14] Maehara A,Surugi H,Satoh T,et al.Regioselective C-H Functionalization Directed by a Removable Carboxyl Group:Palladium-Catalyzed Vinylation at the Unusual Position of Indole and Related Heteroaromatic Rings[J].O rg Lett,2008,10(6):1159-1162.
[15] Sarbani P,Bindu P,Dubey P K,et al.Transition-metal/Lew is Acid Free Synthesis of Acyl Benzothiophenes via C-CBond Forming Reaction[J].Beilstein J Org Chem,2007,3(35):5397-5403.
[16] Kappe CO.Microwave Dielectric Heating in Synthetic Organic Chemistry[J].Chem Soc Rev,2008,37(6):1127-1139.
[17] Kappe C O.Controlled Microwave Heating in Modern Organic Synthesis[J].Angew Chem Int Ed,2004,43(46):6250-6284.
[18] Yuldashev KH YU.Acylation of Benzothiophene in the Presence of Small Amounts of Ferric Chloride[J].J Chem Heterocycl Com pounds,1987,14(8):831-833.
[19] Campaigne,E Neiss,E SBenzo[b]thiophene derivatives.V III.Benzo[b]thiophene-3-earboxaldehyde and derivatives[J].J Heterocycl Chem,1966,3(1):46-50.
[20] Farrar M W,Levine R.Condensations Effected by Acidic Catalysts.IV.The Acylation of Substituted and Condensed Thiophenes and Furans[J].J Am Chem Soc,1950,72(10):4433-4436.
[21] Klasinc L,Pop E,Trinajstic N,et a l.The Oretical Studies of Positional Isomers Obtained by Annelation of Benzene and 5-membered Ring Heterocyclics Containing Nitrogen,Oxygen,o r Sulphur[J].Tetrahedron,28:3465-3474.
[22] 陈敏,张春燕,袁新华,等.[Emim]Cl-A lCl3离子液体催化蒽与草酰氯的Friedel-Crafts酰基化反应[J].化学试剂,2007,29(10):628-630.
[23] 伍林,易德莲,秦晓蓉,等.萘与草酰氯反应机理的探讨[J].武汉科技大学学报:自然科学版,2005,28(2):166-168.
Microwave-Enhanced Friedel-Craftsacylation of Benzothiophene
ZHANGBian-xiang,SH IGao-sheng,WANG Qiong,YANG Qi,KANG Jing-ling
(School of Chemistry and Chemical Engineering,Shanxi University,Taiyuan030006,China)
The Friedel-Crafts acylation reactions of benzothiophene were investigatel,and the effect of microwave radiation on the reaction was discussed.The structures of products were characterized by1H NM R and13C NMR.The yield and C-2/C-3 isomer ratio of the products were determined by high performance liquid chromatography(HPLC).The effects of various conditions on the reaction were investigated by HPLC analysis.In the result,we obtained selectively 3,3′-dibenzothiophene ethyldione in the reaction of benzothiophene and oxalyl chlo ride,and found that compared with the normal temperature reaction for 12 hours,microwave radiation reaction needed 25 minutes,and yield could increased by 20%.
microwave radiation;benzothiophene derivatives;Friedel-Crafts acylation
O626
A
0253-2395(2011)01-0090-06*
2010-09-13;
2010-11-13
留学回国人员科研启动基金(2006);山西省自然科学基金(2007011022)
张变香(1968-),女,山西太谷人,博士,副教授,从事有机合成和杂原子化学研究.E-mail:zbxthh@sxu.edu.cn

