Synthesis and Crystal Structure of a Uranylcontaining Complex[U(CO3)3(H2O)2]·2H2O
WANG JuanL XinLI Zi-YingZHANG Yu-YanCHENG Shi-Yuan
(1School of Chemistry and Chemical Engineering,Hubei University,Wuhan 430062)
(2Beijing Research Insititute of Uranium Geology,Beijing 100029)
研究简报
Synthesis and Crystal Structure of a Uranylcontaining Complex[U(CO3)3(H2O)2]·2H2O
WANG Juan*,1LXin1LI Zi-Ying2ZHANG Yu-Yan2CHENG Shi-Yuan1
(1School of Chemistry and Chemical Engineering,Hubei University,Wuhan430062)
(2Beijing Research Insititute of Uranium Geology,Beijing100029)
A uranylcontaining compound[U(CO3)3(H2O)2]·2H2O has been synthesized under hydrothermal condition and characterized by X-ray single-crystal analysis.Crystal structural analysis indicates that this compound consists of three Cmolecules,one U6+.The results reveal that the title compound presents a 3D frame work built up by OH…O week hydrogen bonds interactions.The central uranium atom is eight-coordinated through three CO32-molecules and two H2O.The compound shows a coplanar hexagonal network structure,each hexagon containing a hexagonal hole with a water moleculet.CCDC:737313.
uranium;crystal structure;carbonate
Uranium(Ⅱ)is a common actinide found in natural media and in radioactive waste.Moreover,it is often taken as a model for other actinides found in radioactive waste.The geochemistry of uranium in oceans is mainly characterized by (1)reduction-oxidation reactions changing U between two dominant oxidation states,U4+and U6+;(2)complexation of U6+with O2to form uranyl ion and further with CO32-;and(3) adsorption of different U species onto various natural solids and solubility of U associated solids[1-5].In today′s oceans where seawater is oxic almost everywhere,U predominantly exists in the U6+state and strongly complexes with CO32-to form very soluble species,which significantly inhibits adsorption of U onto surfaces of solids such as amorphous Fe-Mn oxides,clays and organic particles.This is consistent with the absence of any vertical U concentration,[U],variations along the water column[6].In sediment pore waters where contents of O2and CO32-are decreased due to organic carbon degradation,U6+tends to be reduced to U4+by HSand/or microbial activities and U is more readily removed by stronger adsorption at a lower degree of complexation with CO32-[7-10].
In this study,[U(CO3)3(H2O)2]·2H2O were synthesi-zed by using UO2,Na2CO3as starting materials.The characterizationswere done by examining X-ray diffraction.
1 Experimental section
1.1 Materials and physical measurements
Allmaterialsand organic solventswere of analyticalgrade and were used withoutfurther purification.Distilled,deionized waterwasused throughout.All experiments were performed at the laboratory temperature.
1.2 Synthesis of the compound
UO2(0.134 3 g)was solved in hot HCl(38%),pH was adjusted to 5.4 by the addition of KOH (2 mol·L-1) solution.Na2CO3(0.110 6 g)was added into this solution.The mixture was stirred for half an hour in air.Then pH was adjusted to 6.0.The mixture was then transferred to a Teflon-lined stainless steel autoclave (25 mL)and kept at 170℃ for 6 days.After the autoclave had cooled to room temperature.The resulting solution was evaporated slowly at room-temperature.After several days,orange crystals of this compound were formed.Dry crystals disintegrated in air.
1.3 X-ray crystal structure determination
Single-crystal X-ray crystallographic analyses was performed at 293(2)K on a Bruker SMART APEX CCD sealed tube diffractometer with graphite monochromated Mo Kα(0.071 073 nm)radiation.Data collection, indexing,and initial cell refinements were carried out using SMART software[11].Frame integration and final cell refinements were carried out using SAINT software[12].Absorption corrections for each data set were applied using SADABS program[13].Structure solution, refinement,and generation of publication materials were performed using SHELXTL program[14].Anisotropic thermal parameters were used to refine all nonhydrogen atoms.Hydrogen atoms were located at their ideal positions as a riding mode.A summary of the crystallographic data and structural determination for the title compound is listed in Table 1.Selected bond lengths and bond angles are provided in Table 2 and Table 3.More details on the crystallographic studies as well as atom displacement parameters are presented in the Supporting Information.
CCDC:737313.

Table 1 Crystallographic parameters of the title complex

Table 2 Main bond length(nm)and bond angles(°)of the title compound
Continued Table 2

Table 3 Hydrogen bond distances and bond angles for the title compound
2 Results and discussion
2.1 Description of the crystal structure
Single-crystal X-ray structural analysis reveals that in a structure unit consists of three,one ion of U6+, two coordination water molecules and two crystal water molecules(Fig.1).The U(CO3)3(H2O)2molecule is trigonal biyramid,H2O molecules at “polar”position and Cmolecules at “equator”position.The ligandmetal-ligand bite angles agree with the ideal value of O4-U1-O2 anglesis90.00°,O4-U1-O4 angles is180.00°,O2-U1-O2 angles is 120.0°.Bond O(4)-U(1) is 0.2225(11)nm.
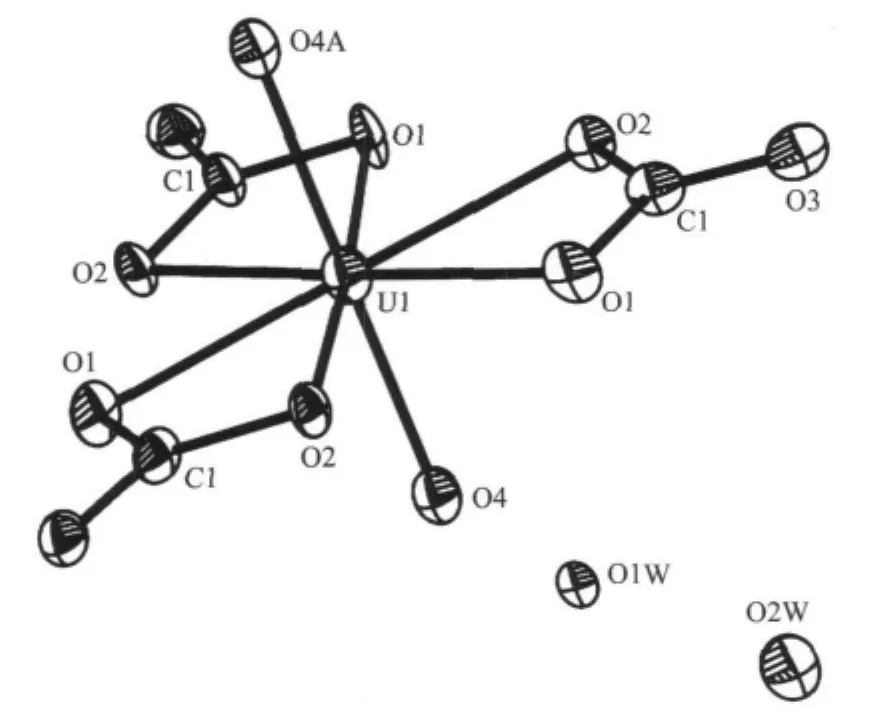
Fig.1 Molecular structure of the title compound
It is worth noting that the three Cmolecules contact with uranyl cation not by a simple static gravitationalbinding,butcoordinationbond.Meanwhile, C1,O1,O2,U1 four atoms form a quaternion ring after coordinate.All of the three quaternion rings are in the same plane.To some extent,large radius of U reduce the tension of quaternion rings,making the stability of quaternion ring.The angles of C1-O1-U1,C1-O2-U1,O1-C1-O2,O2-U1-O1,are 90.6(10)°,92.8(9)°, 119.8(16)°,63.2(4)°,respectively,which are added up to justly 366.4(39)°.The U(1)-O(2),U(1)-O(1),C(1)-O(1)and C(1)-O(2)bond distances are 0.239 6(9)nm, 0.2491(11)nm,0.1305(19)nm and 0.138(2)nm.
As viewed from the packing diagram along c axis, U(CO3)3(H2O)2molecules combined with two coordination water molecules by hydrogen bond interactions. The two-dimensional structure was further connected by hydrogen-bonding interactions between the hydroxide atoms from coordination water molecules and oxygen atoms from Cwith bond distances:O1W-H…O1 bond distance is 0.268 9 nm,O1W-H…O2 is 0.2715 nm,O2W-H…O1 is 0.2871 nm,O2W-H…O3 is 0.298 5 nm.The extensive hydrogen bonding increases the stability of the structure.
Observed along the c axis compound showed a coplanar hexagonal network structure,the hexagon′s vertexes are uranium molecules,each hexagon containing a hexagonal hole.The distance of O1…C1 in the hole is 0.865 1 nm.This structure may have potential application value for the screening of match size of the molecules or molecular recognition.
3 Conclusion
In summary,we have synthesized a new uranium compounds,the crystal structure have been obtained. Unusual Quaternion rings were found in complex.In addition,strong hydrogen bonds play important roles in constructing the supermolecular frameworks.
Acknowledgements:WANG Juan thanks WEN Li-Li and WANG Cheng-Gang from Huazhong Normal University for their comments and suggestions.Financial support from the Beijing Research Insititute of Uranium Geology is greatly appreciated.
[1]Langmuir D.Geochim.Cosmochim.Acta,1978,42:547-569
[2]Turner D R,Whiteld M,Dickson A G.Geochim.Cosmo-Chim. Acta,1981,45:855-881
[3]Djogic R,Sipos L,Branica M.Limnol.Oceanogr.,1986,31: 1122-1131
[4]Russell A D,Emerson S,Nelson B K,et al.Geochim.Cosmochim.Acta,1994,58:671-681
[5]Clark D L,Hobart D E,Neu M P.Chem.Rev.,1995,95:25-48
[6]Chen J H,Edwards R L,Wasserburg G.J.Earth Planet.Sci Sci.Lett.,1986:241-251
[7]Anderson R F.Uranium,1987,3:145-164
[8]Anderson R F,Fleisher M Q,Lehuray A P.Geochim.Cosmochim.Acta,1989,53:2215-2224
[9]Klinkhammer G P,Palmer M R.Geochim.Cosmochim.Acta, 1991,55:1799-1806
[10]Lovely D R,Phillips E J P,Gorby Y A,et al.Nature,1991, 350:413-416
[11]SMART,Version5.624;BrükerAXS,Inc.:Madison,WI,2002.
[12]SAINT,Version6.36A;BrükerAXS,Inc.:Madison,WI,2002.
[13]Sheldrick G.SADABS,Version 2.10;University of Göttingen, Göttingen,Germany,2003.
[14]Sheldrick G M.SHELXTL SHELXTL-97,Program for Crystal StructureRefinement,University ofGöttingen,Germany,1997.
[U(CO3)3(H2O)2]·2H2O配合物的合成及晶体结构
王 娟*,1吕 鑫1李子颖2张玉燕2程时远1
(1湖北大学化学化工学院,武汉 430062)
(2核工业部北京地质研究院,北京 100029)
铀;晶体结构;碳酸根
O614.62
A
1001-461(2010)02-0351-04
2009-05-18。收修改稿日期:2009-11-14。
王 娟,女,44岁,副教授;研究方向:配位化学及材料化学。
湖北省自然科学基金资助项目。
*通讯联系人。E-mail:wangjuan_hd@163.com
- 无机化学学报的其它文章
- Synthesis,Crystal Structure and Antitumor Activity of a New Trinuclear Ni(Ⅱ)Complex Ni3(C14H8N3O5)2(C5H5N)4
- Synthesis,Crystal Structure and Luminescent Property of[CdCl(HL)(dpp)(H2O)]n·nH2O
- 一种新奇的d-f异双核配合物[Fe(phen)3]2[FeCe(tiron)3]·6H2O的水热合成、晶体结构和磁性
- Synthesis and Crystal Structure of a Two-Dimensional Hofmann-Type Bimetallic Complex Cu(DMF)2[Pt(CN)4]
- α-Fe2O3空心球的水热法制备及其对苯酚的吸附性能
- TiO2纳米管阵列薄膜制备及生长机理的研究

