以[M(DETA)2)]n+(M=Cu2+,Ni2+,Co3+)为客体的MnPS3夹层化合物的合成、结构表征与磁性研究
颜小兵陈兴国*,秦金贵INOKUCHI Makoto
(1有机高分子光电功能材料(湖北省)重点实验室,武汉大学化学与分子科学学院,武汉 430072)
(2Department of Materials Science and Engineering,Science University of Tokyo in Yamaguchi,Onoda,Yamaguchi 756-0884,Japan)
以[M(DETA)2)]n+(M=Cu2+,Ni2+,Co3+)为客体的MnPS3夹层化合物的合成、结构表征与磁性研究
颜小兵1陈兴国*,1秦金贵1INOKUCHI Makoto2
(1有机高分子光电功能材料(湖北省)重点实验室,武汉大学化学与分子科学学院,武汉 430072)
(2Department of Materials Science and Engineering,Science University of Tokyo in Yamaguchi,Onoda,Yamaguchi 756-0884,Japan)
将过渡金属配合物阳离子([M(DETA)2]n+(M=Cu2+,Ni2+,Co3+;DETA=Diethylenetriamine,二乙烯三胺)作为客体插入层状MnPS3层间得到了相应的3个夹层化合物。通过X-射线粉末衍射、元素分析和红外光谱对夹层化合物的结构进行了表征。结果表明,与主体 MnPS30.65 nm 的层间距相比较,夹层化合物(Mn0.88PS3[Cu(DETA)2]0.12)的层间距扩大了 0.32 nm,由此推测客体[Cu(DETA)2]2+在层间以平面四方的配位形式存在,而另 2 个夹层化合物(Mn0.79PS3[Ni(DETA)2]0.21和 Mn0.74PS3[Co(DETA)2]0.17)的层间距扩大了 0.48 nm,说明客体[(M(DETA)2]n+,M=Co3+,Ni2+)在主体层间以八面体配位形式存在。磁性测试结果表明过渡金属离子[(M(DETA)2]n+(M=Cu2+,Co3+)的插入能引起主体MnPS3的磁性在35~40 K发生由顺磁向亚铁磁性的转变并表现自发磁化,而客体[Ni(DETA)2]2+却使夹层化合物的反铁磁相互作用增强,抑制了自发磁化的发生。
夹层作用;层状MnPS3;二乙烯三胺;配合物;磁性
The intercalation of functional species into some layered inorganic solids is one of simple and effective approaches to build advanced functional materials,which has attracted considerable attention in recent years[1-6].Through the synergic or complementary interaction between the guest and inorganic host the intercalation nanocomposites can exhibit some interesting properties[7-12].And sometimes,they even can combine the different solid-state properties from the guest and the host to produce the multifunctional materials[13].
The transition metal phosphorus trisulfides MPS3(M=Mn,Fe,Ni,Zn,Cd etc.)are layered compounds made up of two dimensional arrays of P2S64-bridging ligand coordinated to the M2+ions[14].Among them MnPS3shows antiferromagnetism with Neel temperature of 78 K[15-16],which can undergo two types of intercalation reaction,the redox intercalation similar to that of the layered metal dichalcogenides[17]and the non-redox intercalation reaction based on exchange between Mn2+ion of the host and cationic guest[18].After non-redox intercalation some Mn2+ions will leave the intralayer position of MnPS3host and induce the dramatic change of magnetic properties[19-21].For example,Clément and co-workers[22-23]reported that the intercalation of organic guests such as stilbazolium and Me-Mepepy into thin films of MnPS3exhibited the photomagnetic effect through the structural photoisomerism by irradiation.In addition,some transition metal complexes have been chosen as the guests to be intercalated into layered MPS3to elucidate the structure and mechanism of the intercalation[24-26]and also to explore physical properties[27-31].
The transition metal complexes involving tridentate amines have attracted much attention in the past decades.Some metal complexes of diethylenetriamine(DETA)have been used as model compounds in bioinorganic systems or as building blocks in supramolecular assemblies[32].Recently,Yu and co-workers reported that[Co(DETA)2]3+as a guest into layered zirconium phosphate exhibited interesting photoluminescence in the UV-Vis spectra region[33].Here we report a series of diethylenetriamine transition metal complexes intercalated into layered MnPS3.The structural characterization and magnetic properties of these intercalation compounds are studied and the cationic guests[M(DETA)2]n+(M=Cu2+,Ni2+,Co3+)effects on magnetic properties oflayered MnPS3slab are discussed in this work.
1 Experimental
1.1 Materials and reagents
Pure MnPS3was synthesized by the reaction of stoichiometricamountsofhigh-purityelements(>99.9%)in an evacuated quartz tube at 650℃as described in the literature[34].It was identified by means of XRD and indexed as a monoclinic unit cell(space group C2/m),in which a=0.609 4 nm,b=1.058 9 nm,c=0.681 7 nm,β=107.23°,d=0.6496 nm.
1.2 Equipments
X-ray powder diffraction (XRD)patterns were recorded on a Shimadzu XRD-6000 X-ray diffractometer usingCu Kα radiation(λ=0.15418 nm).Fourier transform infrared (FTIR)spectra were obtained on a Nicolet SX Fourier transform spectrometer in the region of 4 000~400 cm-1on KBr pellets.Elemental analysis of carbon,hydrogen,and nitrogen was performed on a Carlorba-1106 microanalyzer.Magnetic measurements were performed on polycrystalline samples with a SQUID-magnetometer(MPMS,Quantum Design)and the magnetic susceptibility was obtained in the temperature range of 1.8~300 K with an applied magnetic field of 500 Oe.
1.3 Synthesis
1.3.1 Metal DETA complexes
General procedure for the preparation of[M(DETA)2]n+·nX-(M=Cu2+,Co3+,Ni2+;X=Cl-or Br-)[35-36]:To a solution of M2+(M=Cu,Co,Ni)ion salt in water was added twofold amounts of diethylenetriamine.The mixture was stirred at room temperature for several hours,except that the solution of[Co(DETA)2]should be subject to blowing air for the other 36 hours to make sure that Co2+ion is oxidized to Co3+ion[36].After filtration,the solution was evaporated to a small volume using rotary evaporator and the product was then precipitated from the solution by addition of ethanol.The product was filtered off and washed with ethanoland acetone.The analysis results confirmed purification of the complexes.Cu(DETA)2Br2·H2O,calculated:C,21.46;H,6.30;N,18.77.Found:C,21.46;H,5.85;N,18.36.Co(DETA)2Cl3·1.5H2O,calculated:C,24.10;H,7.33;N,21.08.Found:C,24.33;H,7.23;N,20.80.Ni(DETA)2Cl2·H2O,calculated:C,27.15;H,7.97;N,23.74.Found:C,27.19;H,7.54;N,23.61.
1.3.2 Pre-intercalation compound Mn1-xPS3K2x(H2O)y[37]
1.0 g of MnPS3and 2.0 g of KCl in 10 mL H2O were put into a 50 mL ampoule.After sealing under vacuum the mixture was stirred at 65℃for 3 days and then filtered off.The obtained solid was washed with water and methanol for 3 times,respectively.The green powder was obtained by drying under vacuum.
1.3.3 Intercalation compounds[M(DETA)2]-MnPS3
General procedure:0.15 g of pre-intercalate Mn1-xPS3K2x(H2O)yand 0.4 g of[M(DETA)2]n+·nX-was put into an ampoule containing 20 mL methanol.It was sealed under vacuum and the mixture was stirred at 65 ℃ for one month.The powder was filtered off and washed with methanol and water,and then dried undervacuum.Theanalysisresultssuggestthe formulae of the corre-sponding intercalation compounds.Mn0.88PS3[Cu(DETA)2]0.12.Calculated:C,5.55;H,1.51;N,4.85;Found:C,5.48;H,1.73;N,4.69.Mn0.74PS3[Co(DETA)2]0.17.Calculated:C,7.67;H,2.09;N,6.71.Found:C,7.75;H,2.23;N,6.58.Mn0.79PS3[Ni(DETA)2]0.21.Calculated:C,8.92;H,2.43;N,7.80.Found:C,8.91;H,2.72;N,7.63.
2 Results and discussion
Mn1-xPS3K2x(H2O)ywas used as a pre-intercalate to react with different metal DETA complexes([M(DETA)2]n+·nX-,M=Cu2+,Co3+,Ni2+;X=Cl-or Br-)and the corresponding intercalation compounds were obtained.X-ray powder diffraction (XRD)patterns shown in Fig.1 confirm that they are the full intercalation compounds.As can be seen,the 001 reflections of Mn1-xPS3(K)2xare disappeared,instead,a series of new 00l reflections are observed.Compared with pristine MnPS3(2θ=13.60°,d=0.65 nm),the 001 reflection peak of Mn0.74PS3[Co(DETA)2]0.17and Mn0.79PS3[Ni(DETA)2]0.21is located at 2θ=7.80°,corresponding to the greatest lattice spacing of 1.13 nm.The lattice expansion of 0.48 nm indicates that cationic complexes([M(DETA)2]n+,M=Ni2+,Co3+)exists in octahedral geometry with six coordination numbers[16,18].The possible existing form and geometry of[M(DETA)2]n+,M=Ni2+and Co3+)are proposed as in Fig.2. Different from Mn0.74PS3[Co(DETA)2]0.17and Mn0.79PS3[Ni(DETA)2]0.21,the lattice spacing of Mn0.88PS3[Cu(DETA)2]0.12is only increased to 0.97 nm and the corresponding spacing expansion is about 0.32 nm (Δd=0.97~0.65 nm).This suggests that Cu2+ion adopts 4 coordination numbers to form cationic[Cu (DETA)2]2+complex at the interlayer region of MnPS3and its square geometric plane is parallel to the MnPS3layer(shown in Fig.2).In addition,the less loaded cationic[Cu(DETA)2]2+than[M(DETA)2]n+(M=Ni2+and Co3+)into interlayer space of MnPS3is also an indirect evidence to support that[Cu(DETA)2]2+is in square geometry with 4 coordination numbers.Thus,it occupies much more interlayer area than octahedral geometry with 6 coordination numbers.
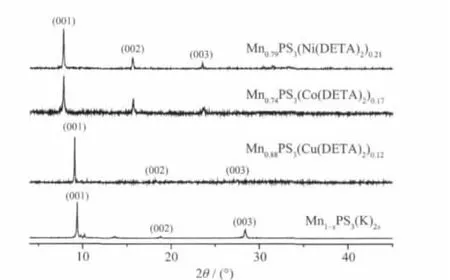
Fig.1 XRD patterns of pre-intercalate Mn1-xPS3(K)2x·yH2O,Mn0.88PS3[Cu(DETA)2]0.12,Mn0.74PS3[Co(DETA)2]0.17and Mn0.79PS3[Ni(DETA)2]0.21
The characteristic IR absorption of guest molecules and the related intercalation compounds are listed in Table 1.Three intercalation compounds exhibit analogous characteristic IR absorptions to the related[M(DETA)2]n+cationic complexes in the range of 700~2000 cm-1.As an example,Fig.3 gives the IR spectra of Ni(DETA)2Cl2·H2O and Mn0.79PS3[Ni(DETA)2]0.21for comparison.The absorptions at about 1 622,1 590,1 456,1 108 and 1 069 cm-1are the characteristic vibrations for[Ni(DETA)2]2+complex,that is similar tothose results reported by some references[36].The small difference for some IR absorption vibrations between guest molecule and the related intercalation compounds can be attributed to the interlayer region environment of the host as well as the interaction of host and guest.Similar phenomenon has been observed in some other MnPS3intercalation compounds[39-40].
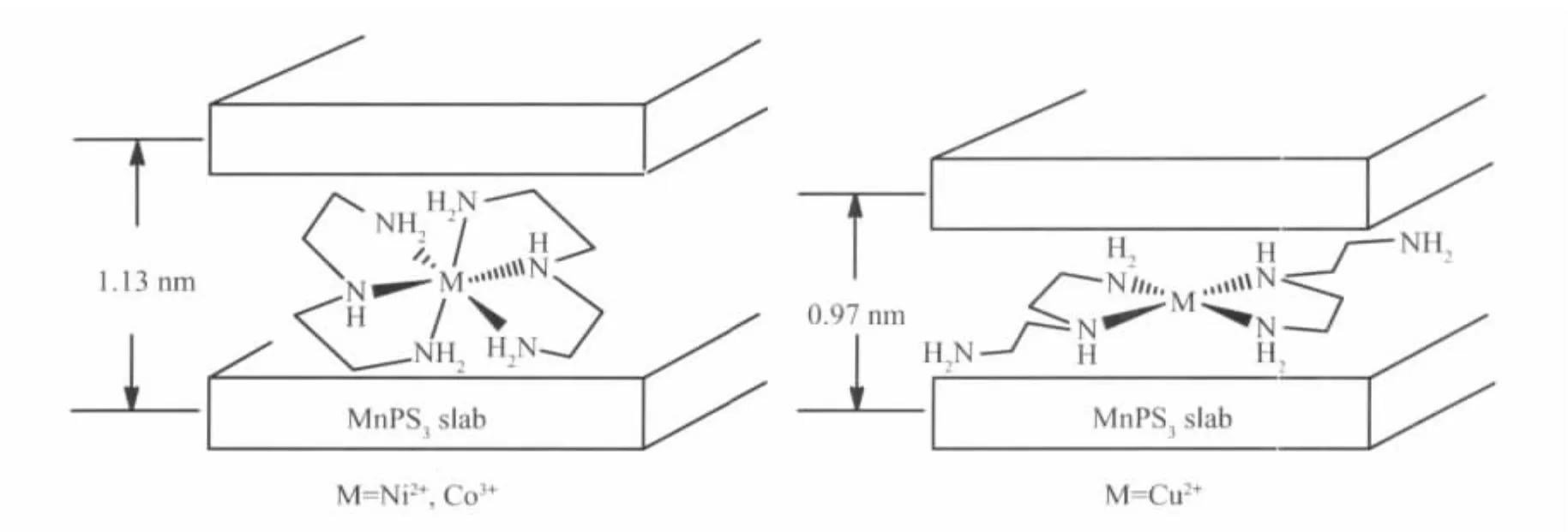
Fig.2 Possible geometry and arrangement of the cationic[M(DETA)2]n+(M=Ni2+,Co3+,Cu2+)guest in the interlayer space of MnPS3slab
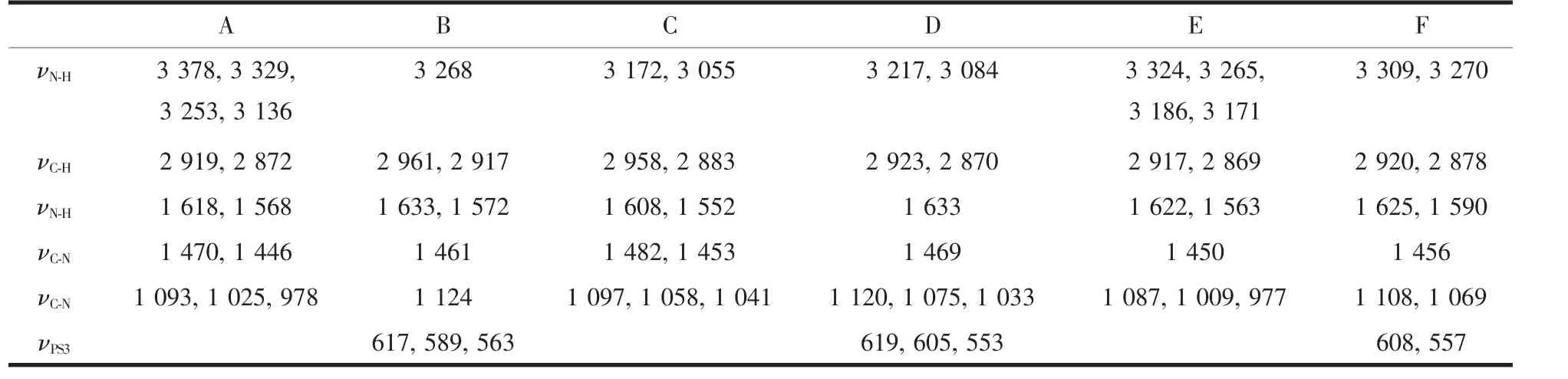
Table 1 Comparison of IR spectra of the guest molecules with the related intercalation compounds(cm-1):Mn0.88PS3[Cu(DETA)2]0.12,Mn0.74PS3[Co(DETA)2]0.17and Mn0.79PS3[Ni(DETA)2]0.21
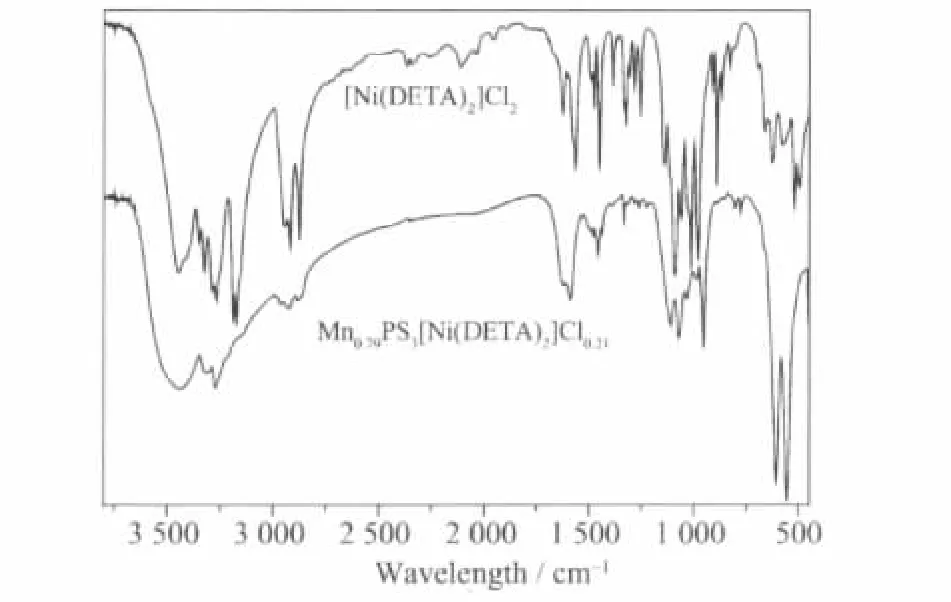
Fig.3 IR spectra of[Ni(DETA)2]Cl2and Mn0.79PS3[Ni(DETA)2]0.21
In addition,there are two or three characteristic IR absorptions occurring at around 620~550 cm-1for these intercalation compounds,which originates from the splitting of 570 cm-1of pure MnPS3.This is the evidence of the presence of intralayered Mn2+vacancies[41-42].Obviously,the inserted guest exists in the cationic form to compensate positive charge of Mn2+ions leaving from the intralayer position and also to maintain charge balance of the intercalation compound.
The curves of magnetic susceptibility(χ)versus temperature(T)for three intercalation compounds are shown in Fig.4~6.As can be seen,they show similar magnetic behaviour above 50 K,where χ gradually increases with the decrease in temperature.The Curie-Weiss law can be applied to fit 1/χ-T curve well above 50 K and the Weiss constant(θ)is obtained for Mn0.88PS3[Cu(DETA)2]0.12(θ=-33 K),Mn0.74PS3[Co(DETA)2]0.17(θ=-51 K)and Mn0.79PS3[Ni(DETA)2]0.21(θ=-83 K),respectively.The negative θ value indicates that the localized magnetic interaction between the intralayer Mn2+spins is antiferromagnetic.However,the difference of negative θ value indicates that the strength of interaction for three intercalation compounds is different.For example,Mn0.79PS3[Ni(DETA)2]0.21shows the largestnegative Weiss constant(θ=-83 K)indicating that its antiferromagnetic interaction is stronger than that of the other two intercalation compounds.As the temperature further decreases to about 40 K,an obvious rise-up of the magnetic susceptibility is observed for all the intercalation compounds.This is an evidence for the occurrence of magnetic phase transition.
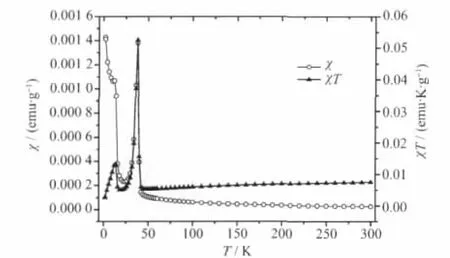
Fig.4 χ-T and χT-T curves of Mn0.88PS3[Cu(DETA)2]0.12
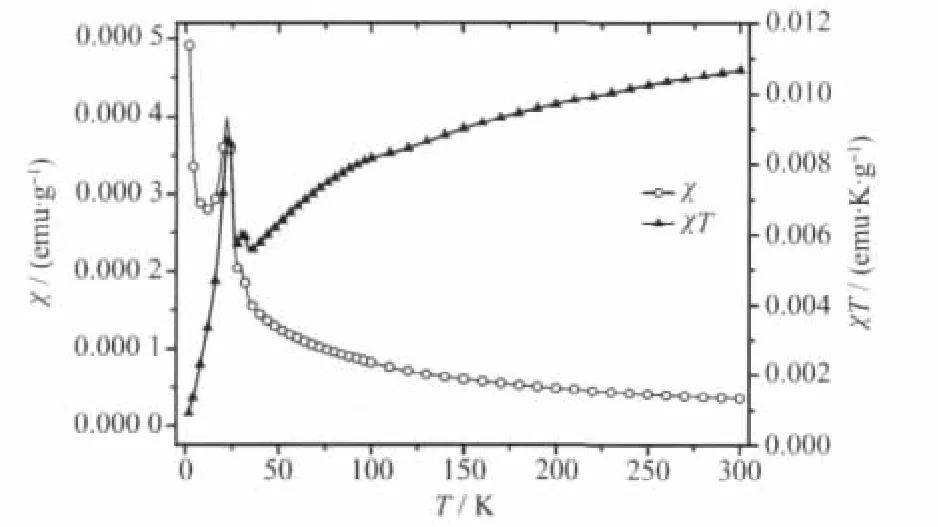
Fig.5 χ-T and χT-T curves of Mn0.74PS3[Co(DETA)2]0.17
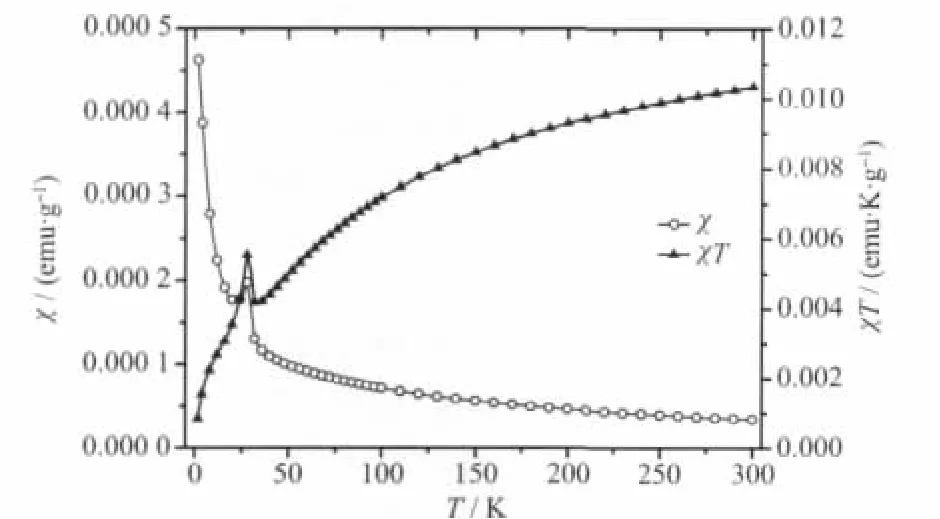
Fig.6 χ-T and χT-T curves of Mn0.79PS3[Ni(DETA)2]0.21
Fig.7~9 show the relationship of magnetization(M)versus applied magnetic field (H)measured at 1.85 K for three intercalation compounds.It can be seen that Mn0.88PS3[Cu(DETA)2]0.12and Mn0.74PS3[Co(DETA)2]0.17show the hysteresis with remnant magnetization of 251 and 57 emu·mol-1and coercive field of 200 and 300 Oe,respectively.This confirms that both of them are low-temperature ferrimagnets.However,Mn0.79PS3[Ni(DETA)2]0.21does not show obvious hysteresis loop,indicating that spontaneous magnetization does not occur at low temperature.

Fig.7 M-H curve of Mn0.88PS3[Cu(DETA)2]0.12at 1.85 K
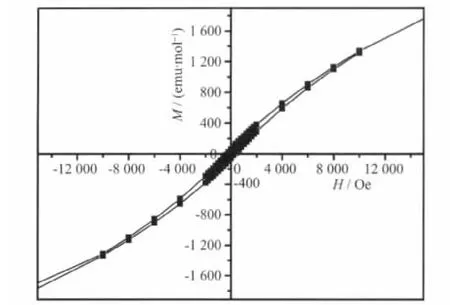
Fig.8 M-H curve of Mn0.74PS3[Co(DETA)2]0.17at 1.85 K
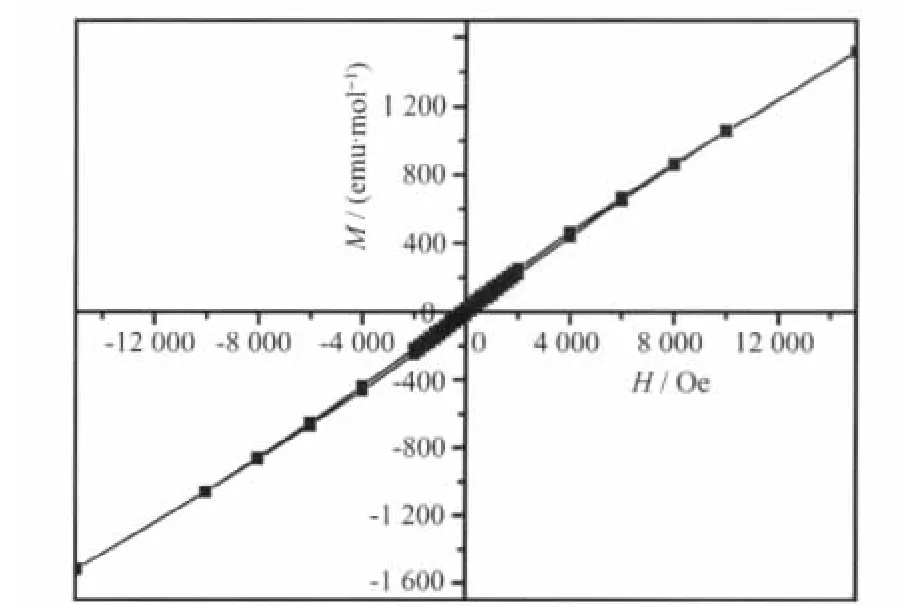
Fig.9 M-H curve of Mn0.79PS3[Ni(DETA)2]0.21at 1.85 K
The origin of magnetism for MnPS3intercalation compounds had been investigated by O′Hare and Clément et al.[43].It was proposed that the ferrimagnetism arises from parts of Mn2+ions leaving MnPS3lattice to form the ordered Mn2+vacancies during the ionexchange process.According to this proposition it can be inferred that the insertion of cationic[M(DETA)2]n+makes Mn2+ions leave the MnPS3lattice and result inthe incomplete com pensation of antiferromagnetically coupled Mn2+spins. Among them,intercalation of[M(DETA)2]n+(M=Cu2+,Co3+)into MnPS3induces spontaneous magnetization at low temperature.However,the intercalation of[Ni(DETA)2]2+seems to strengthen antiferromagnetic interaction of the intralayer Mn2+spins that suppresses the occurrence of low-temperature spontaneous magnetization.
3 Conclusions
Via the insertion of cationic complexes[M(DETA)2]n+(M=Cu2+,Co3+,Ni2+)into layered MnPS3three intercalation compounds are obtained.XRD results confirm the occurrence of full intercalation and infrared spectra support the presence of cationic complex guests located at the interlayer space of MnPS3.The magnetic measurements indicate that the intercalation of cationic complexes with different metal ions can influence the magnetic properties of the layered MnPS3slab.
[1]Yi T,Clément R,Haut C,et al.Adv.Mater.,2005,17:335-338
[2]Fogg M,Green V M,O′Hare D.Chem.Mater.,1999,11:216-217
[3]Yamamoto T,Umemura Y,Sato O,et al.Chem.Mater.,2004,16:1195-1201
[4]Furube A,Shiozawa T,Ishikawa A,et al.J.Phys.Chem.B,2002,106:3065-3072
[5]Cariati E,Macchi R,Roberto D,et al.Chem.Mater.,2007,19:3704-3711
[6]Bhambhani A,Kumar C V.Adv.Mater.,2006,18:939-942
[7]Leaustic A,Riviere E,Clément R.Chem.Mater.,2003,15:4784-4789
[8]Panda H S,Srivastava R,Bahadur D.J.Phys.Chem.C,2009,113:9560-9567
[9]Wontcheu J,Bensch W,Wilkening M,et al.J.Am.Chem.Soc.,2008,130:288-299
[10]Wei M,Pu M,Guo J,et al.Chem.Mater.,2008,20:5169-5180
[11]Ay A N,Zümreoglu-Karan B,Temel A,et al.Inorg.Chem.,2009,48:8871-8877
[12]Coradin T,Clément R,Lacroix P G,et al.Chem.Mater.,1996,8:2153-2158
[13]Lacroix P G,Clément R,Nakatani K,et al.Science,1994,263:658-660
[14]Klingen W,Ott R,Hahn H.Z.Anorg.Allg.Chem.,1973,396:271-278
[15]Prouzet E,Ouvrard G,Brec R.Mater.Res.Bull.,1986,21:195-200
[16]Brec R.Solid State Ionics.,1986,22:3-30
[17]Johnson J W.In Intercalation Chemistry,Eds.Whittingham M S,Jacobson A J,New York:Academic,1982.267
[18]Clément R.J.Chem.Soc.Chem.Commun.,1980,14:647-648
[19]Zhang X,Su X,Chen X,et al.Micropor.Mesopor.Mater.,2008,108:95-102
[20]Chen X,Li Z,Zhou H,et al.Polymer,2007,48:3256-3261
[21]Zhou H,Su X,Zhang X,et al.Mater.Res.Bull.,2006,41:2161-2167
[22]Bénard S,Léaustic A,Rivière E,et al.Chem.Mater.,2001,13:3709-3716
[23]Léaustic A,Sour A,Rivière E,et al.C.R.Acad.Sci.Paris,Chimie/Chemistry,2001,4:91-95
[24]Clerc D G,Cleary D A.J.Phys.Chem.Solids,1995,56:69-78
[25]Glueck D S,Brough A R,Mountford P,et al.Inorg.Chem.,1993,32:1893-1902
[26]Evans J S O,O′Hare D,Clément R.J.Am.Chem.Soc.,1995,117:4595-4606
[27]Clément R.J.Am.Chem.Soc.,1981,103:6998-7000
[28]Floquet S,Salunke S,Boillot M L,et al.Chem.Mater.,2002,14:4164-4171
[29]Floquet S,Clément R,Boillot M L.New.J.Chem.,2004,28:535-541
[30]Yang K,Xu S,Chen X,et al.J.Solid State Chem.,2004,77:4300-4304
[31]Zhang X,Zhou H,Su X,et al.J.Alloys Compds.,2007,432:247-252
[32]Ward M D Ed.Comprehensive Coordination Chemistry IIFrom Biology to Nanotechnology,Vol.9:Applications of Coordination Chemistry.Oxford:Elsevier,2004.
[33]Du Y,Pan Q,Li J,et al.Inorg.Chem.,2007,46:5847-5851
[34]Taylor B I,Steger J,Wold A.J.Solid State Chem.,1973,7:461-467
[35]Stephens F S.J.Chem.Soc.A,1969:2233-2240
[36]Keene F R,Searle G H.Inorg.Chem.,1972,11:148-156
[37]Clément R,Garnier O,Jegoudez J.Inorg.Chem.,1986,25:1404-1409
[38]Junk P C,Steed J W.Inorg.Chim.Acta,2007,360:1661-1668
[39]Joy P A,Vasudevan S.J.Am.Chem.Soc.,1992,114:7792-7801
[40]Qin J,Yang C,Yakushi K,et al.Solid State Commun.,1996,100:427-431
[41]Mathey Y,Clément R,Sourisseau C,et al.Inorg.Chem.,1980,19:2773-2779
[42]Sourisseau C,Forgerit J P,Mathey Y.J.Solid State Chem.,1983,49:134-149
[43]Evans J S O,O′Hare D,Clément R,et al.Adv.Mater.,1995,7:735-739
Intercalation of Cationic[M(DETA)2)]n+(M=Cu2+,Ni2+,Co3+)into Layered MnPS3:Synthesis,Characterization and Magnetic Properties
YAN Xiao-Bing1CHEN Xing-Guo*,1QIN Jin-Gui1INOKUCHI Makoto2
(1Department of Chemistry andHubei Key Laboratory on Organic and Polymeric Opto-electronic Materials,Wuhan University,Wuhan 430072)
(2Department of Materials Science and Engineering,Science University of Tokyo in Yamaguchi,Onoda,Yamaguchi 756-0884,Japan)
Three intercalation compounds based on the insertion of cationic complex guests([M(DETA)2]n+(M=Cu2+,Ni2+,Co3+;DETA=Diethylenetriamine)into layered MnPS3have been synthesized and characterized with X-ray powder diffraction,elemental analysis and infrared spectroscopy.Compared with pristine MnPS3the lattice spacing of Mn0.88PS3[Cu(DETA)2]0.12is expanded by about 0.32 nm suggesting that cationic[Cu(DETA)2]2+guest exists in square geometry at the interlayer space of MnPS3.But 0.48 nm of the lattice expansion for Mn0.79PS3[Ni(DETA)2]0.21and Mn0.74PS3[Co(DETA)2]0.17indicates that[M(DETA)2]n+(M=Co3+,Ni2+)adopts the octahedral geometry.The magnetic measurements indicate that the intercalation of[M(DETA)2]n+(M=Cu2+,Co3+)can induce the magnetic phase transition of MnPS3slab from paramagnetism to ferrimagnetism at low temperature,but the insertion of[Ni(DETA)2]2+strengthens antiferromagntic interaction of the spins that suppresses the occurrence of spontaneous magnetization.
intercalation;layered MnPS3;diethylenetriamine;metal complex;magnetic property
O614
A
1001-4861(2010)12-2237-06
2010-04-07。收修改稿日期:2010-09-11。
国家自然科学基金资助项目(No.20774071)。
*通讯联系人。E-mail:xgchen@whu.edu.cn
颜小兵,男,26岁,博士研究生;研究方向:光电功能材料。

