Gaussia Luciferase Reporter Assay for Assessment of Gene Delivery Systems in Vivo△
Feng Chen, Zhen Xu, Jing Lu, Xiang Lü, Wen-li Mu, Ya-jun Wang, De-pei Liu,and Chih-chuan Liang
National Laboratory of Medical Molecular Biology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100005, China
GENE therapy has evolved into a promising therapeutic modality for treating a variety of diseases. One of the main merits of gene therapy is to treat diseases caused by loss- of-function mutations via delivering therapeutic genes and associated regulatory elements into the nucleus, by which the physiological expression of deficient gene is restored. The delivery of therapeutic genes into cells can be achieved by using either viral or non-viral vectors, whose effect and efficiency are critical in gene therapy. To date, there are several detection markers for analyzing and validating the effect and efficiency of these gene delivery systems in cell lines. For instance, enhanced green fluorescent protein, which has an intrinsic fluorescence that does not require any additional proteins, substrates, or cofactors and can be easily detected by fluorescence microscopy, flow cytometry, or macroscopic imaging,1-3is now routinely used as a reporter molecule to monitor gene expression in vitro, especially in studies on gene delivery systems.4,5However, there is currently a great demand for a detection marker which can be conveniently and sensitively detected to assess the in vivo function of gene delivery systems. The discovery of such a marker will improve our understanding of the in vivo regulation of transgene expression, the fate of implanted transduced cells, and the fine-tuning of the dose of gene therapy vectors.
A marker meeting the need should be easy to monitor in animals, such as by drawing a few microliters of blood or urine for assay of its activity. Reporters secreted into blood provide important tools for the quantitation and noninvasive monitoring of diverse biological processes in different experimental models.6-14The secreted alkaline phospha- tase (SEAP) reporter assay is a commonly used serum marker for monitoring different in vivo events, including tumor growth and drug efficacy,7promoter and transcription factors of activation and inhibition.6,9,14Besides SEAP, the marine copepod Gaussia princeps, named Gaussia luciferase (Gluc), can be naturally secreted from cultured mammalian cells in an active form, and it's activity in the conditioned medium is in linear relationship with cell number, growth, and proliferation.13,15Also, the Gluc reporter assay has been used to localize tumor, to monitor tumor growth and proliferation with bioluminescence imaging.15In addition, Gluc assay is much more sensitive than the routinely used firefly luciferase assay, Renilla luciferase assay,15and SEAP assay in cultured mammalian cells,13which makes Gluc more sensitive than SEAP when monitoring biological events in vivo.16In this study, we seek to investigate the feasibility of Gluc reporter assay for convenient and sensitive monitoring of in vivo functions of gene delivery systems.
MATERIALS AND METHODS
Materials
The Gluc reporter cDNA was amplified by polymerase chain reaction (PCR) from plasmid vector pCMV-GLuc (given by Professor Xiao-bing Wu of Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention). The primers used were synthesized by Shanghai Sangon Biological Engineering Technology & Services Corporation (Shanghai, China). Retroviral vector pMSCV-neo was kindly provided by Dr. Wei Cao of Peking Union Medical College, manufactured by Clontech (Mountain View, CA, USA). Restriction enzymes were produced by New England Biolabs (Ipswich, MA, USA). Lipopolysaccharide (LPS) and polybrene were produced by Sigma-Aldrich (St. Louis, MO, USA). Balb/c mice were purchased from Experimental Animal Center (PLA Academy of Military Medical Sciences, Beijing, China). All protocols regarding the care and use of animals were approved by the Animal Care and Use Committee. Gluc Assay Kit was purchased from New England Biolabs. Luminescence was measured with a luminometer (Turner BioSystems Luminometer Model TD-20, Promega Corporation, Madison, WI, USA).
Construction of retroviral vector pMGluc
After PCR amplification from the plasmid vector pCMV-Gluc, the Gluc reporter cDNA was subcloned into the EcoR I and Hind III sites of retroviral vector pMSCV-neo to substitute PGK promoter and neomycin resistance cassette. The resulting vector, named pMGluc, therefore contained a Gluc cDNA controlled by the long terminal repeat (5' LTR) promoter (Fig. 1).
Virus-producing cell lines
293T and RetroPack TM PT67 cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM)-high glucose supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. The 293T cells were kept in 100 mm tissue culture plates at 80% confluence before transfected with a mixture of 12.5 μg pMGluc, 9.375 μg pMD, and 3.125 μg pV for virus producing. Twenty-four hours after transfection, the cells were washed and fresh DMEM medium was added. Viral vector-containing supernatant was collected at 12-hour intervals and used for transduction of RetroPack TM PT67 cells. After repetitive transduction for four times, supernatant from RetroPack TM PT67 cells culture was harvested to transduce primary spleen lymphocytes, which were activated in advance by LPS stimulation. For the transduction of lymphocytes, retrovirus packaged in RetroPack TM PT67 cells rather than in 293T cells was used because the efficiency of the dualtropic 10A1 serotype retrovirus packed by RetroPack TM PT67 is much higher than VSV-G serotype retrovirus packed by 293T for transducing primary mouse lymphocytes,17,18and because using retrovirus packed by 293T to transduce RetroPack TM PT67 cells will produce higher titre of retrovirus vector than that yielded after using plasmid for transfection.18

Figure 1. Map of retroviral vector pMGluc. The Gluc reporter cDNA was subcloned into the EcoR I and Hind III sites of retroviral vector pMSCV-neo. The expression of Gluc is controlled by the long terminal repeat (5' LTR) promoter.
Preparation of spleen lymphocytes and gene transfer
Spleen lymphocytes obtained from 8-week-old mice were kept in RPMI 1640 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, then stimulated with LPS (50 μg/mL) for 16 hours before retroviral vector infection. Gene transfer was performed by co-culturing primary spleen lymphocytes with virus-pro- ducing RetroPack TM PT67 cells. In brief, 1×107LPS- stimulated spleen cells were plated in 10 mL of RPMI 1640 medium onto a confluent lawn of mitomycin pretreated virus-producing RetroPack TM PT67 cells in 100 mm tissue culture plates. Meanwhile, polybrene (6 μg/mL) and LPS (50 μg/mL) were added to the medium. Spleen lymphocytes were harvested 24 hours later, and then adoptively transferred into 8-week-old Balb/c mice for in vivo research (1.5×107-3×107cells per mouse were intraveneously injected into the tail vein). Age and sex matched mice which were not injected with cells were chosen as control.
Gluc assay
After cell transplantation, blood was taken from tail vein of the mice on days 0, 1, 3, 6, and 9 for the detection of Gluc concentration. The blood samples (50-100 μL) were directly injected into tubes containing 5 μL of 100 mmol/L ethylenediaminetetraacetic acid (EDTA). Gluc activity of whole blood and plasma was detected by using a commercial Gluc assay kit. Briefly, 10 μL whole blood or plasma was added into 50 μL GLuc assay solution and the luminescence was promptly measured with a luminometer.
RESULTS
Retroviral vector encoded Gluc expressed and biologically active in vivo
We chose retroviral vector as a representative of gene delivery systems in our study for its ability to integrate stably into genome and mediate long-term expression of transgene. Mice injected with Gluc-expressing cells showed an equal initial Gluc value on day 0, which gradually rose and peaked on day 1 or day 3 after implantation with different numbers of cells (Figs. 2, 3). This result indicated that the Gluc encoded in retrovirus was successfully expressed in vivo, and that a substantial number of Gluc-expressing spleen cells survived the injection procedure. It was also demonstrated that lymphocyte-derived Gluc was biologically active in vivo. However, the Gluc activity was found dramatically decrease after the peak and finally became undetectable on day 9.
In the implantation process, different numbers of cells were injected (1.5×107, 2×107, and 3×107) in order to assess whether Gluc activity is in good correlation with the number of genetically modified spleen lymphocytes implanted to the mice. Such a correlation was observed as presumed (Fig. 2). An identical relationship was also observed in the detection of whole blood (Fig. 3). These findings demonstrated that the Gluc activity was cell number-dependent.
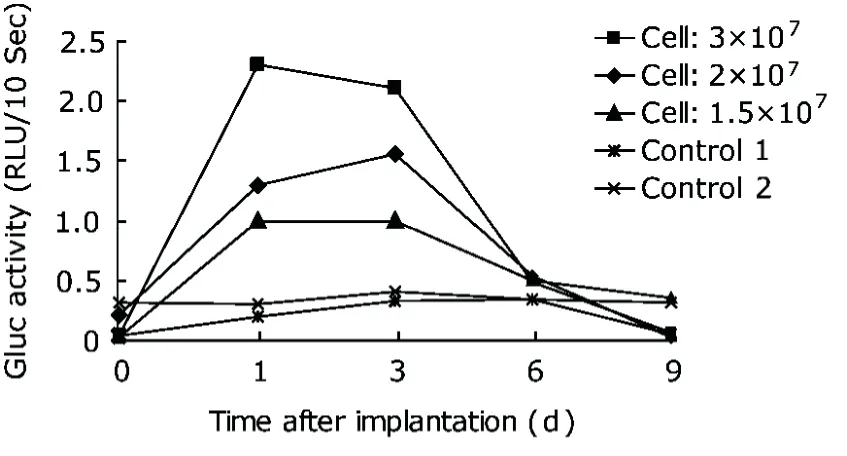
Figure 2. Time-course and dosage-dependence of plasma Gluc activity in mice implanted with retrovirally modified spleen lymphocytes. Different numbers of spleen lymphocytes (1.5×107, 2×107, and 3×107) were injected into Balb/c mice at tail vein. Two age and sex matched mice without injection were set as controls. Whole blood was collected through that vein at different time points, and the plasma was separated for the detection of Gluc activity with a luminometer. RLU: relative light units. Each curve represents the results from one mouse.
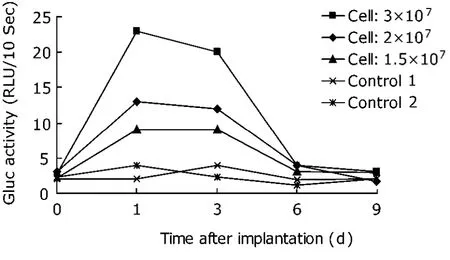
Figure 3. Time-course and dosage-dependence of whole blood Gluc activity in mice implanted with retrovirally modified spleen lymphocytes. Gluc activity in whole blood of the same batch of samples in Figure 2. The results obtained using whole blood were comparable with those using plasma samples.
Identical Gluc activity in whole blood and plasma
Whole blood samples were evaluated in parallel for Gluc activity in the mice implanted with retrovirally modified spleen lymphocytes. No luminescence signal was initially detected in any whole blood samples. Considering that hemoglobin may affect the emission of photons, we increased the sensitivity of the luminometer from 50% to 90%. Consequently, relatively strong luminescence signals were detected in all samples of mice implanted with Gluc-expressing cells after increasing the sensitivity of the luminometer. Gluc activity in whole blood was found identical to that in plasma. In particular, the Gluc activity in plasma and whole blood both reached the highest level on the same day after injection, and both declined to undetectable level after day 6 (Figs. 2, 3) .
DISCUSSION
In this study, we used Gluc as a reporter to detect the in vivo events of gene delivery system. We constructed a retroviral vector harboring a Gluc expression cassette for in vitro general modification of primary spleen lymphocytes. In mice implanted with these modified cells, high level expression of Gluc was detected in a few microliters of whole blood or plasma drawn from the tail vein, allowing convenient monitoring of in vivo function of gene delivery system. We found that the Gluc level was in good correlation with the number of genetically modified spleen lymphocytes implanted to the mice, and that Gluc activity in whole blood was almost identical to that in plasma, although the sensitivity of luminometer was tuned higher for the detection of Gluc expression in whole blood. This may result from the large overlapping between Gluc emission spectrum (450-600 nm) and haemoglobin absorption spectrum (474-616 nm).15,20
By day 6 after cell implantation, the expression of Gluc could not be detected, which might be caused by the presence of neutralizing antibodies in immunocompetent mice. Based on this consideration, Gluc might be more applicable in immunodeficient animal models.16
Our study found that merely 10 μL of whole blood or plasma is sufficient for detecting the expression of Gluc. Real-time monitoring of in vivo function of gene delivery systems in animal models is therefore theoretically feasible by repetitive blood collection at short intervals. Gluc assay can be completed in a few seconds, with equal efficiency in whole blood and plasma, thereby the assay could be made more convenient by omitting the plasma separating step in sample preparation. Additionally, the half-life of Gluc in vivo is about 20 minutes,16which means that Gluc accumulation over time contributes little to the total Gluc signal detected in blood samples, allowing dynamic in vivo events of gene delivery system to be monitored.
We described the application of Gluc, a secretable reporter, as a tool to quantitatively assess the in vivo process of genetically modified spleen cells implanted into mouse. Gluc was shown in this research to be a useful reporter for gene therapy researches, and Gluc blood assay provides an alternative method for assessment of gene delivery systems in vivo.
ACKNOWLEDGEMENT
We thank Prof. Xiao-bing Wu (Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention) for providing pCMV-GLuc vector; Dr. Wei Cao (Peking Union Medical College) for providing pMSCV- neo vector.
1. Chalfie M, Tu Y, Euskirchen G, et al. Green fluorescent protein as a marker for gene expression. Science 1994; 263:802-5.
2. Heim R, Cubitt AB, Tsien RY. Improved green fluorescence. Nature 1995; 373:663-4.
3. Zolotukhin S, Potter M, Hauswirth WW, et al. A "humanized" green fluorescent protein cDNA adapted for high- level expression in mammalian cells. J Virol 1996; 70: 4646-54.
4. Larochelle A, Choi U, Shou Y, et al. In vivo selection of hematopoietic progenitor cells and temozolomide dose intensification in rhesus macaques through lentiviral transduction with a drug resistance gene. J Clin Invest 2009; 119:1952-63.
5. Chin JY, Kuan JY, Lonkar PS, et al. Correction of a splice-site mutation in the beta-globin gene stimulated by triplex-forming peptide nucleic acids. Proc Natl Acad Sci U S A 2008; 105:13514-9.
6. Bao R, Connolly DC, Murphy M, et al. Activation of cancer-specific gene expression by the survivin promoter. J Natl Cancer Inst 2002; 94:522-8.
7. Bao R, Selvakumaran M, Hamilton TC. Use of a surrogate marker (human secreted alkaline phosphatase) to monitor in vivo tumor growth and anticancer drug efficacy in ovarian cancer xenografts. Gynecol Oncol 2000; 78: 373-9.
8. Hiramatsu N, Kasai A, Hayakawa K, et al. Secreted protein-based reporter systems for monitoring inflammatory events: critical interference by endoplasmic reticulum stress. J Immunol Methods 2006; 315:202-7.
9. Meng Y, Kasai A, Hiramatsu N, et al. Real-time monitoring of mesangial cell-macrophage cross-talk using SEAP in vitro and ex vivo. Kidney Int 2005; 68:886-93.
10. Murata T, Yasukawa T, Shiku H, et al. Electrochemical single-cell gene-expression assay combining dielectrophoretic manipulation with secreted alkaline phosphatase reporter system. Biosens Bioelectron 2009; 25:913-9.
11. Nishijo K, Hosoyama T, Bjornson CR, et al. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J 2009; 23:2681-90.
12. Hiramatsu N, Kasai A, Meng Y, et al. Alkaline phosphatase vs luciferase as secreted reporter molecules in vivo. Anal Biochem 2005; 339:249-56.
13. Badr CE, Hewett JW, Breakefield XO, et al. A highly sensitive assay for monitoring the secretory pathway and ER stress. PLoS One 2007; 2:e571.
14. Shiraiwa T, Kaneto H, Miyatsuka T, et al. Establishment of a non-invasive mouse reporter model for monitoring in vivo pdx-1 promoter activity. Biochem Biophys Res Com- mun 2007; 361:739-44.
15. Tannous BA, Kim D, Fernandez JL, et al. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther 2005; 11:435-43.
16. Wurdinger T, Badr C, Pike L, et al. A secreted luciferase for ex vivo monitoring of in vivo processes. Nat Methods 2008; 5:171-3.
17. Hagani AB, Rivière I, Tan C, et al. Activation conditions determine susceptibility of murine primary T-lymphocytes to retroviral infection. J Gene Med 1999; 1:341-51.
18. Annenkov AE, Daly GM, Chernajovsky Y. Highly efficient gene transfer into antigen-specific primary mouse lymphocytes with replication-deficient retrovirus expressing the 10A1 envelope protein. J Gene Med 2002; 4:133-40.
19. Colin M, Moritz S, Schneider H, et al. Haemoglobin interferes with the ex vivo luciferase luminescence assay: consequence for detection of luciferase reporter gene expression in vivo. Gene Ther 2000; 7:1333-6.
 Chinese Medical Sciences Journal2010年2期
Chinese Medical Sciences Journal2010年2期
- Chinese Medical Sciences Journal的其它文章
- Lipids-induced Apoptosis Is Aggravated by Acyl-coenzyme A: Cholesterol Acyltransferase Inhibitor△
- A Case of Thoracic Spinal Stenosis Secondary to Paget's Disease
- DTNBP1 Gene Is Associated with Some Symptom Factors of Schizophrenia in Chinese Han Nationality△
- Association between the Epidermal Growth Factor Gene and Intelligence in Major Depression Patients△
- Role of Acetylated p53 in Regulating the Expression of map2 in Retinoic Acid-induced P19 Cells△
