甲醇和氨对腺嘌呤水解脱氨机理的影响
刘海英 孟凡翠 李 萍 丁世良
(1济南大学理学院,济南 250022; 2天津药物研究院,天津 300193; 3山东大学物理学院,济南 250100)
Adenine is an integral part of DNA and RNA,which is involved in base pairing with thymine in DNA and with uracil in RNA.In addition,adenine is also an important part of adenosine triphosphate(ATP)and adenosine diphosphate(ADP)in energy transfer process and cyclic adenosine monophosphate(cAMP) in signal transduction process.Several bases including adenine, guanine,and cytosine undergo slow but measurable loss of their amino groups,i.e.,deamination under physiological conditions in vitro.The deamination of adenine to produce hypoxanthine can cause codon changes in mRNA,which further induces the synthesis of variant protein structures.The base deamination has been investigated extensively[1-9].Almatarneh and coworkers[2]have studied the deamination reaction of cytosine with H2O and OH-by theoretical method.Two pathways for deamination with H2O were found but either pathway was unlikely due to the high barriers.They also have made further investigations on the deaminationofcytosinewithH2O/OH-and2H2O/OH-[3]andfound that all pathways underwent a tetrahedral intermediate and the obtained energy barrier agreed well with the experimental value. Labet et al.[4]have performed a theoretical study on cytosine deamination and they hold that the tautomerization of cytosine or assistant water was necessary for the deamination.They also investigated the deamination mechanism of 5-methylcytosine with H2O in protic medium[5]and that of the radical cation of 2′-deoxycytidine[6].Zhang et al.[7]have studied the hydrolytic deamination of adenine with one to four water molecules at the B3LYP/6-31G(d,p)level.
In our previous papers,both non-assisted[8]and water-assisted[9]hydrolyses of adenine have been studied and the results indicated that the water assistance could give rise to large decrease of the activation barrier of adenine deamination.As is well known,the surrounding environment plays an important role in many reactions of nucleic acid bases,such as tautomerism[10-11]and deamination[12].The interaction between nucleic acid bases with water[13], alcohol[14],metal cations[15],and other species[16]have been studied extensively.Then we wonder what effects could be caused by the surrounding environment on the reaction of adenine deamination?To our knowledge,no computational studies of the deamination of adenine under ammonia and methanol conditions have been reported.For this reason,we present a theoretical study of the methanol and ammonia assisted hydrolyses of adenine to simulate the hydroxyl group and amino group occurring in amino acid residues.Methanol and ammonia could act as both proton donor and proton acceptor in the reaction processes. The bond rotation steps are omitted since they are not important in this type of reaction[9].
1 Calculation methods
All the stationary points including reactants,intermediates, transition states,and products were obtained using B3LYP/6-311G(d,p)method[17-19]and verified by a vibrational frequency analysis at the same level.Zero point vibrational energies(ZPVE) were also determined through frequency analysis.Intrinsic reaction coordinate(IRC)calculations were performed on the transition states to ensure that the transition states were connected with the corresponding two minima.Single-point energy calculations were performed on the previously optimized geometries obtained by the IRC procedure of nTS01(the transition state of amine-imine tautomerization of adenine)to get the total atomic charge variation along the reaction coordinates.
To test the accuracy of B3LYP/6-311G(d,p)method,we also calculated all the stationary points using G3MP2//B3LYP/6-311G(d,p)methods.G3MP2//B3LYP[20-21]method is a composite scheme based on the three single point energies,i.e.,QCISD(T)/ 6-31G(d),MP2/6-31G(d),and MP2/G3MP2large.The ZPVE corrections obtained by B3LYP/6-311G(d,p)method were used without scaling due to the fact that the ZPVE corrected results agreed well with the experimental values[22].Single point calculations by using polarized continuum model in the integral equation formalism(IEFPCM)method[23-25]were performed on the obtained stationary points to consider the solvent effects,with the relative dielectric constant 78.39 to simulate the aqueous environment. The cavity was built using the all atom model applied on the atomic radii of the UFF force field[26-27].All calculations were performed using Gaussian 03 program[28].
2 Results and discussion
In this paper,the prefix“Al”refers to those of methanolassisted system and“n”refers to those of ammonia-assisted system.The atom labels of the studied system are shown in Fig. 1.The optimized geometries of all the stationary points of methanol-assisted and ammonia-assisted mechanisms are shown in Fig.2 and Fig.3,respectively.The relative energy evolution of both methanol and ammonia assisted deaminations of adenine are shown in Fig.4(a)and 4(b),respectively.From Fig.4(a)and 4(b) we can see that the energy differences between B3LYP/6-311G (d,p)and IEFPCM//B3LYP/6-311G(d,p)are less than 13 kJ· mol-1for most of the stationary points.In addition,the difference between the relative energy barriers of these two methods is also small(less than 14 kJ·mol-1).These results reveal that solventeffects play a minor role for the studied systems and thus in the followingsectionwe use G3MP2//B3LYP/6-311G(d,p)to calculate energy values.
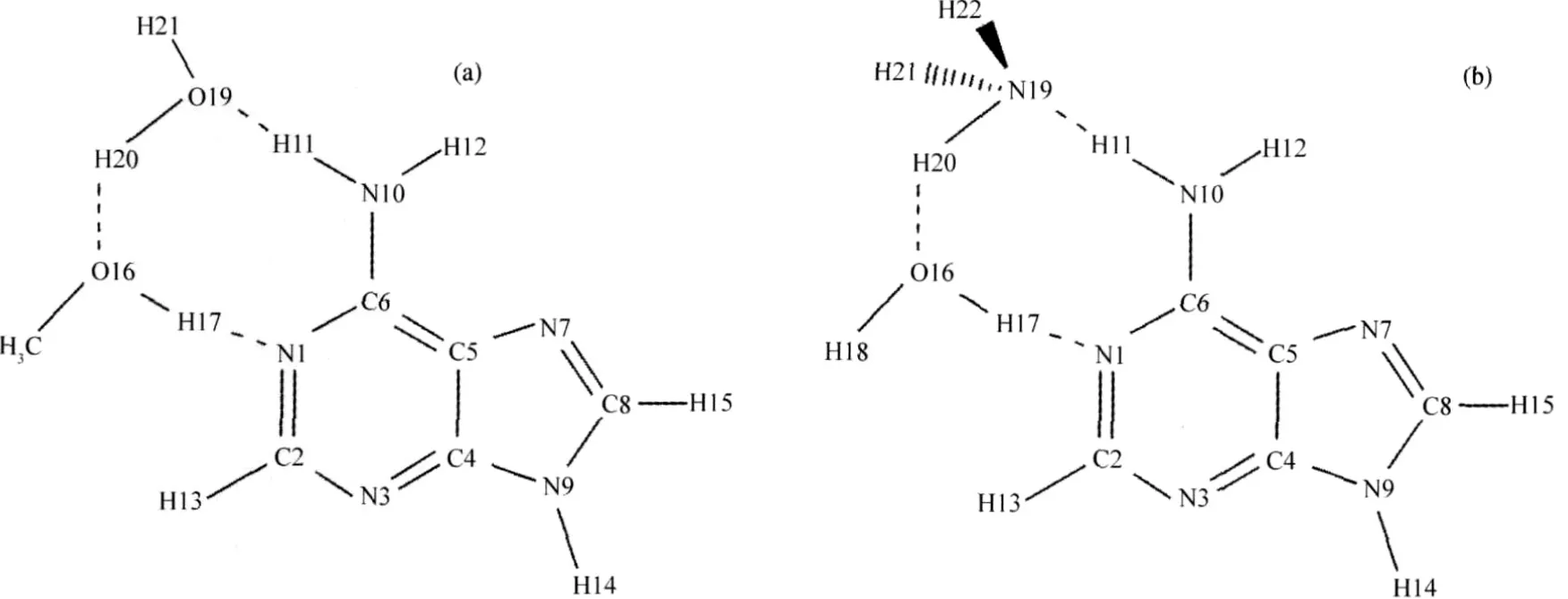
Fig.1 Molecular structures and atom labels of the studied methanol-assisted(a)and ammonia-assisted(b)systems
2.1 Methanol assisted hydrolytic deamination
2.1.1 Formation of the tetrahedral intermediate
In this step a tetrahedral intermediate has been created through the nucleophilic attacking of water molecule.Adenine forms a cyclic complex(AlInt1)with water and methanol molecules,in which three hydrogen bonds exist.AlInt1 is 71.04 kJ·mol-1more stable than the reactants.AlTS12 is the transition state connecting AlInt1 with the tetrahedral intermediate AlInt2.A six-member ring structure forms through the elongation of O16—H17 and O19—H20 bonds and the shortening of N1…H17,C6…O19 and O16…H20 bonds.The relative energy of AlTS12 is 101.58 kJ·mol-1higher than that of reactants.AlInt2 is a tetrahedral structure with one water molecule.There are two hydrogen bonds in AlInt2,one is N1—H17…O16 and the other is O16—H20…O19.The relative energy of AlInt2 is 32.38 kJ·mol-1.
2.1.2 Detachment of ammonia
In pathway a(Fig.2),AlInt3a is obtained through the conformational change of AlInt2,which is 9.50 kJ·mol-1more stable than AlInt2.The hydroxyl group of methanol forms two hydrogen bonds,one is O16—H20…N10 and the other is O19—H21…O16.This facilitates the creation of cyclic transition state. AlTS34a is the transition state obtained from AlInt3a.It is a sixmember ring structure through the decreasing of O16…H21 and N10…H20 bond distances and increasing of O19—H21 and O16—H20 bond distances.The relative energy of AlTS34a is 111.88 kJ·mol-1.AlInt4a is the product obtained from AlTS34a, which is the complex of hypoxanthine with ammonia and methanol.The bond length of C6—O19 changes from 0.140 nm in AlInt3a to 0.122 nm in AlInt4a,implying the formation of C=O bond in AlInt4a.AlInt4a has three hydrogen bonds with a lower energy of-59.45 kJ·mol-1compared with reactants.
In pathway b(Fig.2),AlInt3b is another intermediate obtained through conformation change of AlInt2,in which the methanol also forms two hydrogen bonds but one of them is formed with cyclic N1.AlInt3b is 7.57 kJ·mol-1more stable than AlInt2.In AlTS34b,the hydrogen atom of hydroxyl group O19—H21 transfers to amino group to release ammonia.The methanol moleculedoesnotparticipateinthe formation of the four-member ring transition structure and only forms hydrogen bond with N1—H17,which results in the higher relative energy of AlTS34b(166.14 kJ·mol-1).AlInt4b is the intermediate obtained from AlTS34b with a relative energy of-38.11 kJ·mol-1.AlInt4b is not as stable as AlInt4a,due to the different locations of ammonia and methanol molecules.
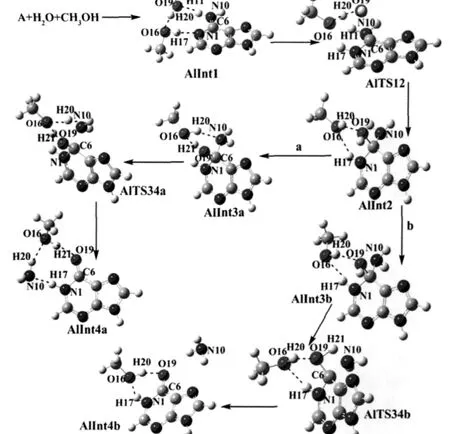
Fig.2 Optimized geometries in methanol-assisted adenine deaminationThe dashed line indicates hydrogen bond.
Compared the methanol-assisted hydrolyticdeamination mechanism with the water-assisted one[9],we can find that the methanol assistance does not change the reaction mechanism and the preliminary bond distances and bond angles have little difference.Moreover,the activation energies of the two mechanisms are similar(as shown in Table 1).
2.2 Ammonia assisted hydrolytic deamination
2.2.1 Tautomerization of adenine
As for the ammonia-assisted deamination of adenine,the adenine tautomerized from amine type to imine type structure.In this step,firstly,the canonical adenine forms a complex with ammonia and water molecules,in which three hydrogen bonds exist.nInt0 is 54.14 kJ·mol-1more stable than the reactants. Secondly,with the elongation of bond distances of O16—H17, N19—H20,N10—H11 and the shortening of N1…H17,O16…H20,N19…H11,an eight-member ring transition state nTS01 is formed.In nTS01,the ammonia and water molecules act as both proton donor and proton acceptor,which facilitate the proton transfer from exocyclic amine nitrogen to cyclic N1.The activation energy of the transition state nTS01 is as low as 30.37 kJ· mol-1,which indicates that the amine-imine tautomerization of adenine occurs easily.At the end of this step,nInt1 comes into being.It is a complex of imine form adenine with one water and one ammonia molecules.The relative energy of nInt1 is-18.74 kJ·mol-1.
2.2.2 Formation of the tetrahedral intermediate
In this step,nTS12 is the transition state connecting nInt1 and nInt2.nTS12 is also a six-member ring structure like AlTS12. But nTS12 is formed through exocyclic nitrogen atom instead of cyclic ring nitrogen,which is different from AlTS12.The relative energy of nTS12 is 169.15 kJ·mol-1,which is higher than that of AlTS12.nInt2 is a tetrahedral structure with an ammonia molecule.
2.2.3 Detachment of ammonia

Fig.3 Optimized geometries in ammonia-assisted adenine deaminationThe dashed line indicates hydrogen bond.
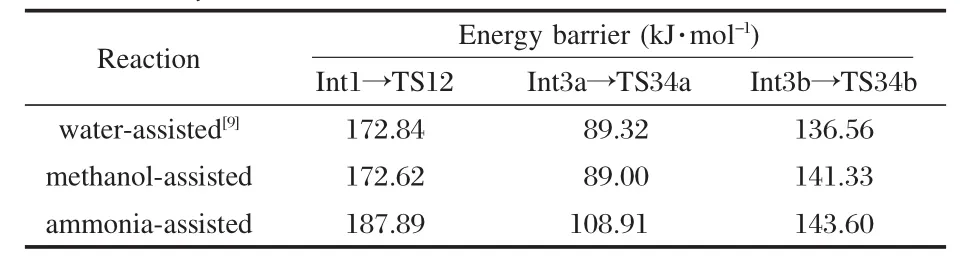
Table 1 Energy barriers of the water-assisted,methanolassisted,and ammonia-assisted reaction mechanisms
nInt2 transforms to nInt3a and nInt3b through different conformational changes and relocations of water and ammonia molecules.Similar to the methanol-assisted mechanism,in pathway a(Fig.3)ammonia attends in the formation of transition state nTS34a.nTS34a is obtained through the elongation of C6—N10, N19—H22,and O16—H18.The energy difference between nTS34a and the reactants is 149.74 kJ·mol-1,which is about 37 kJ·mol-1larger than that of AlTS34a.nInt4a is the product of pathway a.It is a complex of hypoxanthine with two ammonia molecules.
nInt3b is another intermediate obtained from nInt2,in which the ammonia forms two hydrogen bonds:N1—H17…N19 and N19—H22…N10.Inthepathwayb(Fig.3)theammoniamolecule is not involved in the creation of transition state.In nTS34b,the hydrogen atom transfers directly from hydroxyl group to ammine nitrogen and the ammonia molecule only acts as a medium.Due to the lack of the ammonia,the hydrogen transfer is difficult to happen.nTS34b has a higher activation energy(143.60 kJ·mol-1) just as expected.nInt4b is also a complex of hypoxanthine with two ammonia molecules.Owning to the different location of the two ammonia molecules,the relative energy of nInt4b is 32.55 kJ·mol-1higher than that of nInt4a.
2.3 Comparisons of methanol and ammonia effects
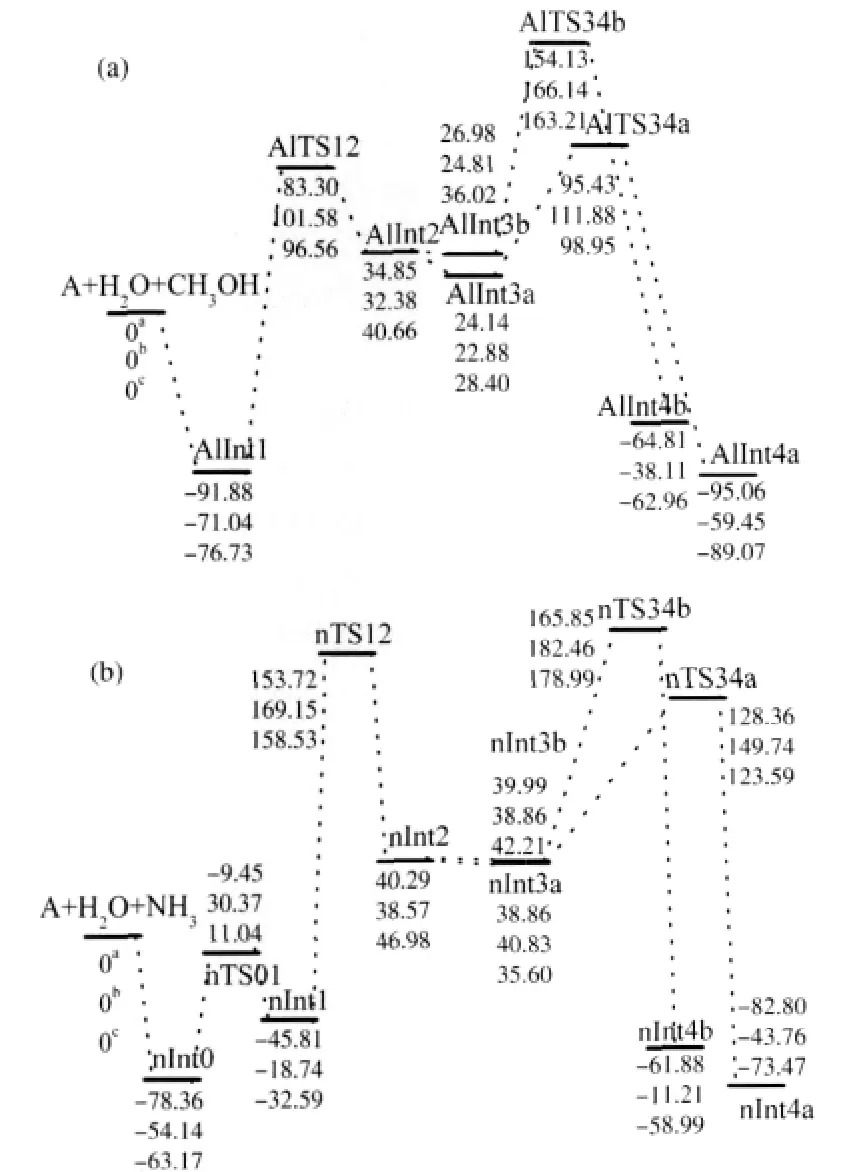
Fig.4 Energy profiles of methanol-assisted(a)and ammonia-assisted(b)deamination of adnineaB3LYP/6-311G(d,p),bG3MP2//B3LYP/6-311G(d,p), cIEFPCM//B3LYP/6-311G(d,p);the relative energy in kJ·mol-1
The reaction mechanism of methanol-assisted hydrolytic deamination of adenine is the same as that of water-assisted hydrolyticdeamination[9].However,this is not the case for ammoniaassisted hydrolytic deamination,in which tautomerization of adenine is necessary.The structure of the transition state in the following(nTS12)is also different from that of water-assisted mechanism.The six-member ring transition state has been formed through exocyclic amino group and carbon atom under ammonia condition,while it has been formed by cyclic nitrogen and carbon atoms under methanol condition.The more favorable pathway is still pathway a under both conditions.Methanol-assisted hydrolytic deamination of adenine has similar reaction barriers with those of water-assisted reaction,while the energy barriers of the ammonia-assisted reaction are higher than those of waterassisted reaction(as shown in Table 1),which may give useful information on the deamination mechanism of adenine involving amino acid residues.
2.4 Total atomic charge evolution along adenine tautomerization
Fig.5(a)is the evolution of total atomic charges of C6 along nTS01.Fig.5(a)indicates that the tautomerization of adenine makes the atomic charge of C6 less positive,thus results in the nucleophilic reaction more difficult to happen.Fig.5(b)presents the evolution of the Mulliken overlap populations between C6 and N1 and between C6 and N10,which can be correlated with the electronic populations between C6 and N1 and between C6 and N10,respectively.Fig.5(b)shows that the overlap populations between C6 and N1 and between C6 and N10 decrease and increase along the reaction coordinate,respectively.The variation of the overlap population is consistent with the change of bond type.In this step,the C6=N1 double bond is transformed into a single one,while the C6—N10 single bond is transformed into a double one.The crossing point of the two bond population can be considered as the starting point of the whole reaction.After the tautomerization,the overlap population between C6 and N10 is larger than that between C6 and N1,thus results in the water attack for C6=N10 instead of C6—N1 in the following step.
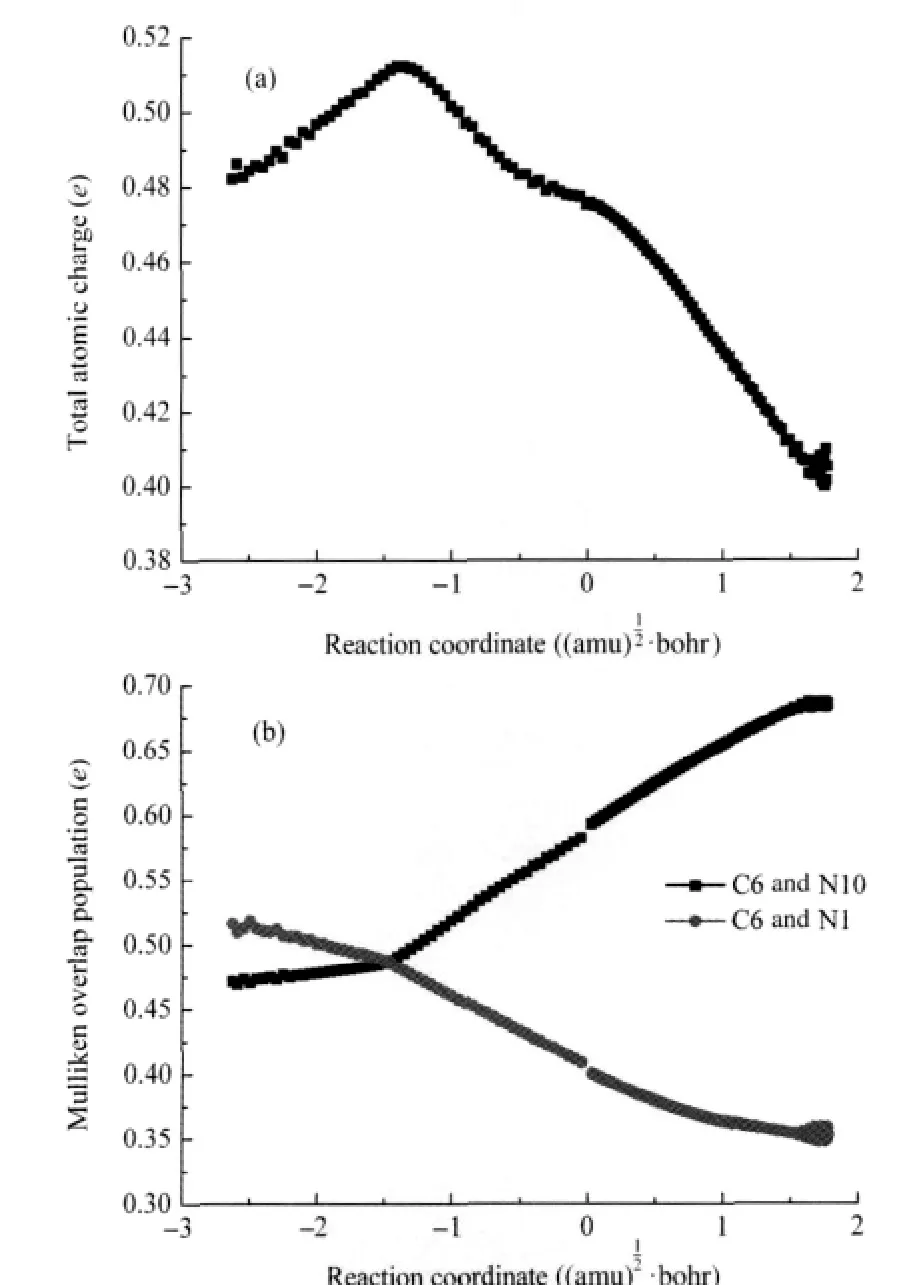
Fig.5 Evolution of the total atomic charge on C6(a)and the overlap populations between C6 and N1 and between C6 and N10(b)along the reaction path of nInt0-nTS01-nInt1 step
3 Conclusions
In this paper both the methanol and ammonia assisted hydrolytic deamination mechanisms of adenine were investigated. The geometries of all the stationary points were optimized at the B3LYP/6-311G(d,p)level and single point energies were obtained by both IEFPCM and G3MP2//B3LYP methods.The results show that both the mechanism and the activation energies of methanol-assisted deamination are similar to those of waterassisted deamination.A tetrahedral intermediate forms through nucleophilic attack,then two intermediates are obtained by different conformational changes.The methanol molecule involves in the formation of transition state in pathway a and only acts as a medium in pathway b.In the ammonia-assisted hydrolytic deamination,amine-imine tautomerization of adenine occurs firstly.Then the nucleophilic attack of water takes place and a tetrahedral intermediate forms.There are also two pathways in the following step.In pathway a the ammonia participates in the six-member ring transition state while in pathway b it only acts as a medium.The ammonia-assisted reaction is more difficult to occur compared with methanol-assisted reaction due to higher energy barriers.Pathway a has lower energy barrier than pathway b under both conditions.
1 Glaser,R.;Rayat,S.;Lewis,M.;Son,M.S.;Meyer,S.J.Am. Chem.Soc.,1999,121:6108
2 Almatarneh,M.H.;Flinn,C.G.;Poirier,R.A.;Sokalski,W.A. J.Phys.Chem.A,2006,110:8227
3 Almatarneh,M.H.;Flinn,C.G.;Poirier,R.A.J.Chem.Inf. Model.,2008,48:831
4 Labet,V.;Morell,C.;Grand,A.;Toro-Labbé,A.J.Phys.Chem.A, 2008,112:11487
5 Labet,V.;Morell,C.;Cadet,J.;Eriksson,L.A.;Grand,A.J.Phys. Chem.A,2009,113:2524
6 Labet,V.;Grand,A.;Cadet,J.;Eriksson,L.A.ChemPhysChem, 2008,9:1195
7 Zhang,A.;Yang,B.;Li,Z.J.Mol.Struct.-Theochem,2007,819: 95
8 Zhu,C.;Meng,F.Struct.Chem.,2009,20:685
9 Zheng,H.;Meng,F.Struct.Chem.,2009,20:943
10 Kim,H.S.;Ahn,D.S.;Chung,S.Y.;Kim,S.K.;Lee,S.J.Phys. Chem.A,2007,111:8007
11 Gu,J.;Leszczynski,J.J.Phys.Chem.A,1999,103:2744
12 Matsubara,T.;Ishikura,M.;Aida,M.J.Chem.Inf.Model.,2006, 46:1276
13 Danilov,V.I.;van Mourik,T.;Kurita,N.;Wakabayashi,H.; Tsukamoto,T.;Hovorun,D.M.J.Phys.Chem.A,2009,113: 2233
14 Harańczyk,M.;Rak,J.;Gutowski,M.;Radisic,D.;Stokes,S.T.; Bowen,K.H.J.Phys.Chem.B,2005,109:13383
15 Kabelác,M.;Hobza,P.J.Phys.Chem.B,2006,110:14515
16 Shukla,M.K.;Dubey,M.;Zakar,E.;Namburu,R.;Leszczynski,J. Chem.Phys.Lett.,2010,493:130
17 Becke,A.D.J.Chem.Phys.,1993,98:5648
18 Lee,C.;Yang,W.;Parr,R.G.Phys.Rev.B,1988,37:785
19 Miehlich,B.;Savin,A.;Stoll,H.;Preuss,H.Chem.Phys.Lett., 1989,157:200
20 Baboul,A.G.;Curtiss,L.A.;Redfern,P.C.J.Chem.Phys.,1999, 110:7650
21 Curtiss,L.A.;Raghavachari,K.J.Chem.Phys.,1998,109:7764
22 Tang,Y.Z.;Sun,J.Y.;Sun,H.;Pan,Y.R.;Wang,R.S.Theor. Chem.Acc.,2008,119:297
23 Cancès,M.T.;Mennucci,B.;Tomasi,J.J.Chem.Phys.,1997, 107:3032
24 Cossi,M.;Barone,V.;Mennucci,B.;Tomasi,J.Chem.Phys.Lett., 1998,286:253
25 Mennucci,B.;Tomasi,J.J.Chem.Phys.,1997,106:5151
26 Rappé,A.K.;Casewit,C.J.;Colwell,K.S.;Goddard III,W.A.; Skiff,W.M.J.Am.Chem.Soc.,1992,114:10024
27 Rappé,A.K.;Goddard III,W.A.J.Phys.Chem.,1991,95:3358
28 Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;et al.Gaussian 03. Revision D.01.Wallingford,CT:Gaussian Inc.,2004
———理学院

