臭氧与二乙胺和三乙胺气相反应的速率常数
盖艳波 葛茂发 王炜罡
(中国科学院化学研究所,分子动态与稳态结构国家重点实验室,北京分子科学国家实验室,北京 100190)
Low molecular weight alkylamines are emitted by a variety of widespread anthropogenic and biogenic sources,representing an important class of environmental pollutants due to their toxic and odorous properties.Animal husbandry is probably the most important anthropogenic emission source of amines into the troposphere,as several studies have reported high concentrations of gas-phaseaminesinareasofintenseanimalhusbandry[1-4].Though emission estimates vary widely,a number of short chain alkylamines have also been detected in industrial emissions and car exhaust[5-7],biomass burning[8],waste incinerators,and sewage treatment plants[9],as well as in marine,rural,and urban atmospheres.
Like most organic compounds,when released into the atmosphere,these amines become transformed mainly through reactions with a number of reactive species such as hydroxyl radicals and ozone.Ultimately,they may significantly contribute to the formation of ozone and other secondary photooxidants in polluted areas.Besides the environmental effects,amines are also the cause of many health problems for exposed workers[10-11],such as skin and eye irritation,dermatitis,pulmonary sensitization and asthma,and even the cause of carcinogenic effect.Moreover, there are 28 amino compounds,including triethylamine and diethanolamine,in the Federal Clean Air Act Amendments List of 189 Hazardous Air Pollutants[12].These various health effects are prompting different countries to regulate the maximum concentration of amines allowed in air[13-14].Therefore,in order to assess the impact of these chemical species on air quality and human health,a detailed understanding of the kinetics and mechanisms of their atmospheric degradation is required.
To date,rate constants for the reaction of mono-,di-,and trimethylamine(MMA,DMA and TMA)have been reported[15], which suggests that these reactions are relatively fast and give amines a lifetime on the order of hours in ambient air.Despite the relatively fast removal rate,several studies have detected amines in the particle phase[16-17]as well as within aqueous fog and rain drops[18-19].And intriguingly,most of the studies found that the concentrations of dialkylammonium were extremely higher than those of other alkylic ammonium salts in aerosol samples[20-22].In light of these findings,both homogeneous and heterogeneous reactions of amines in the atmosphere merit further investigation.
As part of series studies of amines,in this paper,we investigated the homogeneous kinetic reactions of diethylamine(DEA) and triethylamine(TEA)with ozone.Rate constants of these reactions were reported,and the lifetimes of DEA and TEA with respect to ozone were also evaluated.From this we hope to better understand the atmospheric process of amines.
1 Experimental
1.1 Reagents and equipment
Diethylamine(DEA,≥99%)and triethylamine(TEA,≥99%) were obtained from Alfa Aesar.C6H12(cyclohexane)in a purity of 99.5%was from Beijng Beihua Fine Chemical Company.N2(≥99.999%)and O2(≥99.999%)were supplied by Beijing Tailong Electronics Company.Ozone was produced from O2via electrical discharge using a commercial ozonizer(BGF-YQ,Beijing Ozone,China).
The experimental apparatus used here is similar to that we reported in previous publications[23-25]and just a brief description is given here.All the experiments were carried out in a 100 L FEP Teflon film chamber.With a self-made temperature controller, we now can control the temperature in the chamber accurately from room temperature to 350 K.At the two ends of the reactors, an inlet and an outlet made of Teflon are used for the introduction of reactants and sampling.The reactor and the analytical instruments are linked via Teflon tubes.Ozone analyzer(Model 49C,Thermo Electron Corporation,USA)was used for analyzing the ozone concentration in the reactor.Its flow rate and precision were 0.7 L·min-1and 1×10-9,respectively.Cyclohexane was added into the reactor to eliminate the OH radicals that may be generated during the reaction.With high purity of N2as the bath gas,the concentrations of DEA,TEA,and cyclohexane in the entire chamber were calculated from the amount of organics introduced and the total volume of the reactor.
1.2 Principle
Absolute rate constants for these ozone reactions were determined by monitoring the O3decay rates in the presence of known concentrations of the reactant organic.The temporal profile of O3is governed by the following processes:

With the initial organic concentration[organic]0being in large excess over the initial ozone concentration,the reactant organic concentration essentially remains a constant throughout the reaction.Similar to our previous works[25-26],the following equation could be obtained under pseudo-first-order conditions:

where k1and k2are the rate constants of reactions(1)and(2).
Thus,from the ozone decay rates,k,measured at various organic concentrations and with a knowledge of the background O3decay rate(k1),the rate constant(k2)can be obtained.
1.3 Experimental method
Thoroughly cleaning the chamber was performed for at least 24 h in presence of ozone prior to each set of experiments to remove any residue from last experiment.Attenuation experiments of the reactants(DEA,TEA,and O3)in pure N2were performed separately to study the wall effect.In all experiments,the reactant organic and cyclohexane were introduced in the chamber by injecting certain volumes of the liquid into a 3-way glass tube and by flushing the contents of the glass tube into the chamber using pure N2as the carrier gas.Sufficient time was allowed for the concentration inside the chamber to reach steady state.Then with ozone introduced,the chamber was connected to the ozone analyzer and ozone concentration measurements integrated over 10 s time intervals were collected up to about a total of 30 min. All experiments were conducted at(298±1)K and 1.01×105Pa.
2 Results and discussion
2.1 Wall effect
In the attenuation experiments,stable concentrations of the investigated amines were confirmed by at least seven measurements made over the course of 3 h and giving decreases of the integrated peak areas below 3%of the initial values,which was monitored using gas chromatograph-coupled with flame-ionization detection(GC/FID,GC6820,Agilent Technologies,USA). And the loss of ozone caused by the wall was of negligible importance after continuously measured by the ozone analyzer. When measured once an hour,the ozone decay rate constant of 7.44×10-6s-1was finally obtained after 8 h.This value is consistent with our previously reported value(6.95×10-6s-1)[26],and is about 2 orders of magnitude lower than the values of the pseudofirst-order reaction rate constants listed in Table 1.Thus the background ozone decay accounts for only a small part of all the loss of ozone in the reactor in our experiments.Obviously,the loss of the reactants caused by background decay in this work is negligible.
2.2 Effect of cyclohexane
Previous work has shown that OH radicals are often formed from the gas-phase reactions of O3with organic compounds under atmospheric conditions[27-30].Considering that OH reacts with these compounds several orders faster than O3does,so it would result in certain error to the rate constants for the reactions of ozone.In order to avoid the impacts of OH radicals,high concentrations of cyclohexane were added into the reaction system as OH scavenger.The rate constant for the reaction of OH with cyclohexane is high enough(6.38×10-12cm3·molecule-1·s-1)to scavenge a significant fraction of the OH formed in the ozonolysis reaction[31].At the same time,the reaction of cyclohexane with ozone is negligibly slow and would not interfere with the determination of the rate constants of interest[32].
Comparative experiments were carried out for TEA and ozone in the presence and absence of 2.51×1015molecule·m-3of cyclohexane,the results of which were listed in Table 1.With the addition of cyclohexane,the rate constants in all of the comparative experiments reduced by 3%-5%,further demonstrating that there are certain effects of OH radicals on the rate constant.
From the results of No.6,7,16,and 17 listed in Table 1,we can see that,when cyclohexane concentration increased,the k value was almost unaltered,and the small difference can be considered as an experimental error.So the amounts of cyclohexane used in this work were enough for scavenging OH radicals generated in the present experimental system.
2.3 Determination of rate constants
As described above,the rate constants are determined under pseudo-first-order conditions.The initial O3concentration was in the range of 1.77×1012-8.59×1012molecule·cm-3while the initial concentrations of DEA and TEA were in the range of 5.84× 1013-17.5×1013molecule·cm-3and 2.08×1013-4.16×1013molecule· cm-3,respectively.In all experiments,decays of O3concentration were obtained as a function of time,and the logarithms of the ratios of the concentrations([O3]0/[O3])in the presence of reactants were plotted for different reaction time(Figs.1,2).As shown in Figs.1 and 2,straight lines were obtained for all these pseudo-first order plots.All the lines have excellent correlation coefficients(>0.998),which demonstrates that Eq.(I)is suitable for kinetic study in this work.The slope of such plots yields the pseudo-first order rate constant,k.The results were also listed in Table 1.Then,the values of k vs[organic]0data(Fig.3),according to Eq.(I),were also subjected to linear least-squares analysis to obtain k2.
It can be known from Table 1 and Fig.3 that k values increase linearly with increasing the initial concentrations of DEA and TEA.The slopes of the lines in Fig.3,which are just the absolute values of the second-order rate constants for DEA and TEA,are determined to be(1.33±0.15)×10-17and(8.20±1.01)× 10-17cm3·molecule-1·s-1,respectively.The quoted errors for the determinedrateconstants include 2σ(σ:standard deviation)from the least-squares analysis and an estimated systematic error of10%.Both least-squares linear regressions yielded near-zero intercepts.
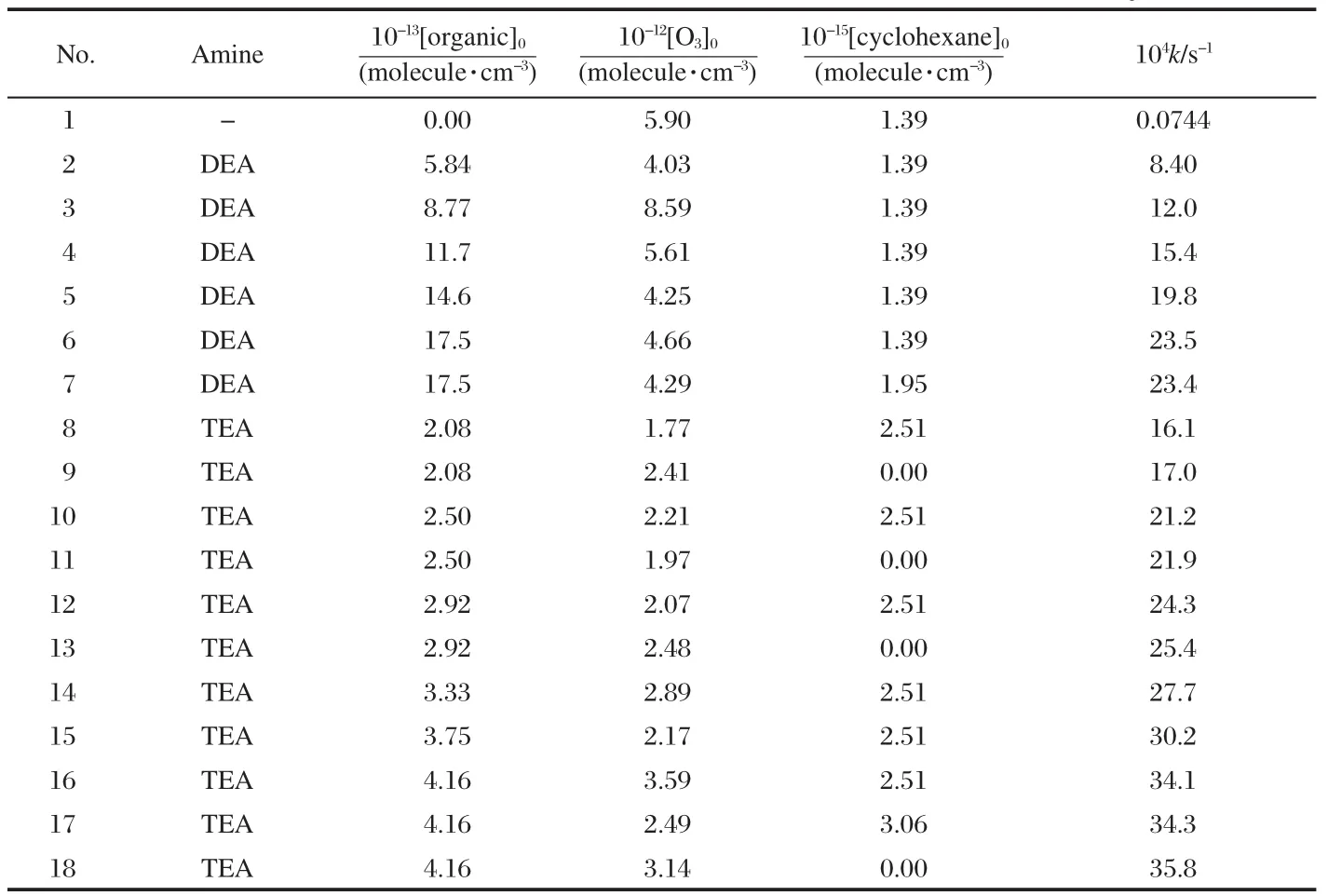
Table 1 Results under different initial concentrations of amine,ozone,and cyclohexane

Fig.1 Plots of ln([O3]0/[O3])versus reaction time for different initial DEA concentrations10-13[DEA]0/(molecule·cm-3):(a)5.84,(b)8.77,(c)11.7,(d)14.6,(e)17.5
The results presented here represent the first experimental measurement of the reaction rate constants of ozone with DEA and TEA.We can compare our results with data for the reactions of analogous amines with ozone,which are summarized in Table 2.With one ethyl group replacing—H in DEA,reaction of TEA with ozone is about 6 times faster than that of DEA with ozone.And similarly,the substitution of methyl group in DMA to give TMA also increases the reactivity by a factor of 4.7. Comparing DMA with DEA,and TMA with TEA,we can find that the substitution of ethyl group has a more significant effect on the reactivity than that of methyl group.As methyl and ethyl groups are all electron-donating groups,so it is probable that the reaction of ozone with amines may involve electrophilic reaction mechanism.The introduction of methyl and ethyl groups increases the electronic density at N atom and thus the reactivity of amines.And the more substitutions of methyl or ethyl groups in amines,the faster the reactions of amines with ozone.In order to fully understand the mechanism of these reactions,further investigacions on products are required.

Fig.2 Plots of ln([O3]0/[O3])versus reaction time for different initial TEA concentrations10-13[TEA]0/(molecule·cm-3):(a)2.08,(b)2.50,(c)2.92,(d)3.33, (e)3.75,(f)4.16

Fig.3 Plots of k against[TEA]0and[DEA]0
2.4 Atmospheric implications
The atmospheric lifetimes τ of these amines with respect to removal by ozone can be estimated based on the corresponding rate constants summarized in Table 2 and the estimated ambient tropospheric concentration of ozone,according to Eq.(II):

where[O3]is the estimated ambient tropospheric concentration of ozone.In this work,a typical ozone concentration of 7×1011molecule·cm-3was used[33].
As illustrated in Table 2,atmospheric lifetimes against removal by ozone are 29.8 and 4.8 h for DEA and TEA,respectively.In polluted areas,where the concentration of ozone could be high up to 2.5×1012molecule·cm-3[34],the lifetimes of DEA and TEA would be even shorter,about 8.4 and 1.4 h,respectively.Under these conditions,the ozone reactions would serve as an important loss pathway for these amines.In addition,as also can be seen in Table 2,the reactions of trialkylamines with ozone are all extremely faster than those of dialkylamines with ozone.This would more or less help to explain why higher concentrations of dialkylammonium were detected in the aerosol samples[20-22].That is,the homogenous reactions of trialkylamines are relatively fast and they can be rapidly removed when released into the atmosphere;however,dialkylamines have a relatively longer lifetime and may be easily participated into particle phase through other reactions.Furthermore,in order to fully understand the atmospheric process of amines,further studies including OH reactions and heterogeneous reactions of amines are needed in the future.

Table 2 Summary of rate constants(k2)and estimated atmospheric chemical lifetimes(τ)for the reactions of amines with ozone at room temperature
3 Conclusions
The kinetics of the reactions of DEA and TEA with ozone were investigated at 298 K and 1.01×105Pa in our smog chamber.With cyclohexane as the OH scavenger,the absolute rate constants we obtained were(1.33±0.15)×10-17cm3·molecule-1· s-1for DEA and(8.20±1.01)×10-17cm3·molecule-1·s-1for TEA. Comparing our results with the data for the reactions of analogous amines with ozone,we can see that the reactions of trialkylamines with ozone are all extremely faster than those of dialkylamines with ozone.That is,the introduction of methyl or ethyl group increases the reactivity of amines in the homogenous reactions.And the more substitutions of methyl or ethyl group in amines,the faster the reactions with ozone.This may help to explain the intriguing finding in field studies that higher concentrations of dialkylammonium were detected in the aerosol samples.The atmospheric lifetimes of DEA and TEA with respect to removal by ozone have also been estimated based on the measured rate constants and ambient tropospheric concentration of ozone,which indicates that reaction with ozone is an important loss pathway for these amines in the atmosphere,especially in polluted areas.
1 Mosier,A.R.;Andre,C.E.;Viets Jr.,F.G.Environ.Sci.Technol., 1973,7:642
2 Schade,G.W.;Crutzen,P.J.J.Atmos.Chem.,1995,22:319
3 Rabaud,N.E.;Ebeler,S.E.;Ashbaugh,L.L.;Flocchini,R.G. Atmos.Environ.,2003,37:933
4 Filipy,J.;Rumburg,B.;Mount,G.;Westberg,H.;Lamb,B.Atmos. Environ.,2006,40:1480
5 Finlayson-Pitts,B.J.;Pitts Jr.,J.N.Atmospheric chemistry.New York:Wiley-Interscience,1986:1098
6 Cadle,S.H.;Mulawa,P.A.Environ.Sci.Technol.,1980,14:718
7 Westerholm,R.;Li,H.;Almen,J.Chemosphere,1993,27:1381
8 Mace,K.A.;Artaxo,P.;Duce,R.A.J.Geophys.Res.,2003,108: 4512
9 Manahan,S.E.Environmental chemistry.4th ed.Chelsea:Lewis, 1990:612
10 Lauwerys,R.R.Toxicologie industrielle et intoxications professionnelles.4th ed.Paris:Masson,1999:961
11 Greim,H.;Bury,D.;Klimisch,H.J.;Oeben-Negele,M.;Ziegler-Skylakakis,K.Chemosphere,1998,36:271
12 The clean air act amendments,1990,section 112:hazardous air pollutants(b)
13 OSHA,Occupational safety and health standards,1910.1000,Table Z-1:limits of air contaminants,www.osha.gov,2006
14 HSE,workplace exposure limits,EH40/2005,Table 1:list of approved workplace exposure limits,www.hse.gov.uk,2006
15 Tuazon,E.C.;Atkinson,R.;Aschmann,S.M.;Arey,J.Res.Chem. Intermed.,1994,20:303
16 Angelino,S.;Suess,D.T.;Prather,K.A.Environ.Sci.Technol., 2001,35:3130
17 Murphy,D.M.;Thomson,D.S.J.Geophys.Res.,1997,102:6341 18 McGregor,K.G.;Anastasio,C.Atmos.Environ.,2001,35:1091
19 Zhang,Q.;Anastasio,C.Atmos.Environ.,2003,37:2247
20 Makela,J.M.;Yli-Koivisto,S.;Hiltunen,V.;Seidl,W.;Swietlicki, E.;Teinila,K.;Sillanpaa,M.;Koponen,I.K.;Paatero,J.;Rosman, K.;Hameri,K.Tellus B,2001,53:380
21 Sorooshian,A.;Murphy,S.M.;Hersey,S.;Gates,H.;Padro,L.T.; Nenes,A.;Brechtel,F.J.;Jonsson,H.;Flagan,R.C.;Seinfeld,J.H. Atmos.Chem.Phys.,2008,8:5489
22 Facchini,M.C.;Decesari,S.;Rinaldi,M.;Carbone,C.;Finessi,E.; Mircea,M.;Fuzzi,S.;Moretti,F.;Tagliavini,E.;Ceburnis,D.; O′Dowd,C.D.Environ.Sci.Technol.,2008,42:9116
23 Du,L.;Xu,Y.F.;Ge,M.F.;Jia,L.;Wang,G.C.;Wang,D.X. Acta Chim.Sin.,2006,64:2133 [杜 林,徐永福,葛茂发,贾 龙,王庚辰,王殿勋.化学学报,2006,64:2133]
24 Gai,Y.B.;Ge,M.F.;Wang,W.G.Chem.Phys.Lett.,2009,473: 57
25 Gai,Y.B.;Ge,M.F.;Wang,W.G.Atmos.Environ.,2009,43: 3467
26 Du,L.;Xu,Y.F.;Ge,M.F.;Jia,L.;Yao,L.;Wang,W.G.Chem. Phys.Lett.,2007,436:36
27 Atkinson,R.;Aschmann,S.M.;Arey,J.;Shorees,B.J.Geophys. Res.,1992,97:6065
28 Paulson,S.E.;Orlando,J.J.Geophys.Res.Lett.,1996,23:3727
29 Paulson,S.E.;Sen,A.D.;Liu,P.;Fenske,J.D.;Fox,M.J. Geophys.Res.Lett.,1997,24:3193
30 Donahue,N.M.;Kroll,J.H.;Anderson,J.G.;Demerjian,K.L. Geophys.Res.Lett.,1998,25:59
31 Wilson,E.W.;Hamilton,W.A.;Kennington,H.R.;Evans,B.; Scott,N.W.;DeMore,W.B.J.Phys.Chem.A,2006,110:3593
32 Atkinson,R.;William,P.L.C.Chem.Rev.,1984,84:437
33 Logan,J.A.J.Geophys.Res.,1985,90:463
34 Wang,C.X.;Chen,Z.M.Atmos.Environ.,2008,42:6614

