Application of Ionic Liquid in Upgrading 6# Solvent Oil*
SUN Xuewen (孙学文) and ZHAO Suoqi (赵锁奇)
Application of Ionic Liquid in Upgrading 6#Solvent Oil*
SUN Xuewen (孙学文)**and ZHAO Suoqi (赵锁奇)
State Key Laboratory of Heavy Oil Processing, China Petroleum University, Beijing 102249, China
A new method of upgrading 6#solvent oils using different ionic liquids as catalysts in a continuous apparatus is studied in this paper. The results show that aromatics, olefins and small quantity of sulfurs can be removed simultaneously. Using complex ionic liquid modified with CuCl as catalyst, olefins are removed completely, the mass concentrations of aromatics and sulfurs in solvent oil are 0.36% and 0.0058%, respectively, and the bromic index is zero. The sulfur removal rate decreases gradually with increasing of running time. The refined 6#solvent oil is corresponded to the quality standards of GB16629-1996, which request that the mass concentrations of aromatics, sulfurs and bromic index are 1%, 0.012% and 1000, respectively. The loss of solvent oil is less than 3%.
upgrading, alkylation, ionic liquid, 6#solvent oil
1 INTRODUCTION
In recent years, the development of hydrocarbon solvent oil is very fast. It is applied widely in food oil, printing oil, leather, pesticide, rubber, cosmetic, spicery, polymerization, and the cleanout of integrated circuit (IC) electric parts [1]. For different solvent oils, the requirements on the content of aromatics, olefins, and sulfur are quite different. If the content of aromatics in 6#solvent oil exceeds a certain level, it can threat the health of people. Thus, the strict standards for solvent oil are proposed in the world,.., the mass concentration of benzene and aromatic in solvent-petroleum ether and aliphatic hydrocarbon are 0.05% and 0.20% in England, respectively [2]. The mass concentration of aromatics and bromic index in 6#solvent oil in China are restricted to 1% and 1000, respectively. Therefore, the methods of reducing the content of aromatics have been studied in many companies [3]. At present, the key products in China are 6#, 120#, and 200#solvent oils. As the extension of feedstock, some undesired components such as sulfurs and olefins are taken out from feed and entered solvent oils with processing. Thus, all solvent oils must be refined to improve their color, stability, corrosion and toxicity such as alkaline washing, clay refining, hydrotreating, or to produce general solvent oil through molecular sieve refining. After treated by the conventional processes, the contents of sulfur, nitrogen, aromatics and olefins in products are still high and the taste, toxicity and color are not satisfied. With a new food oil standard carried into execution in May 1, 2004, the standards of solvent oil are strictness gradually and the food oil market used for immersion will be increased. The method of removing aromatics from 6#solvent oil is completed by Daqing Petroleum Institute, which can be operated at mild conditions (130-150°C, no more than 0.3 MPa ) [4-6]. The conventional methods of removing sulfur, olefins and aromatics are catalytic hydrogenation or adsorption. However, the hydrogenation needs severe conditions, such as a high ratio of hydrogen to oil and high hydrogen consumption, which is not possible to practice for a small scale refinery, while the adsorbent is deactivated easily and regenerated frequently in the adsorption process. Thus, it is necessary to develop a new process which can be operated under mild conditions and the aromatics, olefins and small amount of sulfur can be removed simultaneously.

2 EXPERIMENTAL
2.1 Materials
1-methyl imdazole (99% above) was purchased from Aldrich, 98.5% aluminium chloride and 99% 1-chlorobutane used for preparation of the ionic liquid were from Beijing Chemical Reagents Company, and 6#solvent oil was provided by Daqing Linyuan Refinery, which contains aromatics, olefins and sulfurs of 3.13% (by mass), 0.47% (by mass) and 0.0282% (by mass), respectively.
2.2 Synthesis of [bmim]Cl
A certain amount of 1-methyl imdazole was put into a stainless-steel autoclave, and then a slight molar excess of 1-chlorobutane was added. The autoclave was sealed, pressurized to 0.51 MPa with nitrogen and then heated to 90°C for 18 h. After cooling to room temperature, the materials inside were transferred to a rotary evaporator where the unreacted chlorobutane and 1-methyl imdazole were removed at 95°C for several hours with nitrogen stripping, and then extracted with acetonitrile [8]. The 1-butyl-3-methyl imdazolium was analyzed by liquid chromatogram and the result shows that the purity of [bmim]Cl prepared is over 98%.
2.3 Ionic liquid preparation
The Al-ionic liquid employed in this work was prepared by slowly adding the desired amount of AlCl3into the 1-butyl-3-methyl imdazolium salt under a dry N2atmosphere in a glove box. The reaction was carried out with stirring overnight at room temperature in order to attain a perfect homogenization of the resulting Al-ionic liquid. The synthesis method of Fe-ionic liquid was the same as Al-ionic liquid. Ionic liquids containing H+was prepared by stirring ionic liquids under HCl atmosphere with HCl partial pressure of 0.05 MPa for 30 min in a reactor before alkylation. The whole process was kept under an inert atmosphere to avoid the hydrolysis of AlCl3. The complex ionic liquid used in this study was synthesized by mixing 1-butyl-3-methylimidazolium chloride (BMIC) and purified anhydrous CuCl in a BMIC︰CuCl molar ratio of 1︰2 at temperature of 80°C in heptane. The all products above were stored in an inert atmosphere before further experiments.
2.4 Experimental and analytic methods
Experiments were carried out at room temperature with mass velocity 3.0 h-1and reaction pressure of 0.1 MPa. The simplified flow scheme is shown in Fig. 1.
The ionic liquid and 6#solvent oil in mass ratio 1︰10 were pumped respectively through the static mixer (reactor) together, and then separated in a separator. The upper layer containing solvent oil was separated from the ionic liquids phase by decantation and then transported to products pot. The ionic liquid was recycled. The products were taken out periodically to analyze paraffins, olefins, naphthene, aromatics (PONA) and sulfur content. With increasing of running time, the ratio of ionic liquid to 6#solvent oil may be adjusted according to the analytical results.

Figure 1 Simplified flow scheme of continuous apparatus

Table 1 The effect of AlCl3 and FeCl3 anions in the refining
The 6#solvent oil was refined by distillation to take out high boiling point products, and then the PONA of 6#solvent oil was analyzed by a multidimensional gas chromatograph system, which is composed of a flame ionization detector (FID), adsorption trap of olefins and aromatics, 5A molecular sieve adsorption column, Pt hydrotreating reactor, an OV-275 packed column(3 m long and 3 mm in o.d.) and an HP-1 capillary column (15 m long and 0.53 mm in o.d.). The temperature of column and detector were 130°C and 180°C, respectively. The concentrations of olefins and aromatics are given by normalization according to the area of each chromatograph peak. ANTEK 7000NS was used to analyze the total sulfurs in oil phase and to calculate the sulfur removal.
3 RESULTS AND DISCUSSION
3.1 The effect of AlCl3 and FeCl3 anions in the refining
The effect of AlCl3and FeCl3anions in the refining are listed in Table 1.
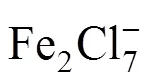
3.2 The effect of ionic liquid modified with HCl on refining of 6# solvent oil
The effects of ionic liquid modified with HCl on refining of 6#solvent oil are listed in Table 2.

Table 2 The effects of ionic liquid modified with HCl on refining of 6# solvent oil
The results in Table 2 show that the removal of aromatics, olefins and sulfurs is improved apparently after the ionic liquid is modified with HCl. For modifiedAlCl3ionic liquid, the removal of olefins, aromatics and sulfurs is increased from 98.30%, 90.41% and 71.28% to 100%, 93.02% and 72.46%, respectively, while for FeCl3ionic liquids it is improved from 91.06%, 83.06% and 65.25% to 93.42%, 84.54% and 66.55%, respectively. Thus, the refining efficiency of modified AlCl3ionic liquid is better than that of FeCl3ionic liquid. This may be attributed to the formation of super acidity after modification with HCl [12] due to the presence of the free H+in the system to improve the alkylation efficiency. At the same time, as the sulfurs and aromatics are very similar in physical and chemical properties, the alkylations of sulfurs and olefins are also improved using modified ionic liquid as catalyst.
3.3 The effect of complex ionic liquid on the removal of sulfurs
Better effects are attained when removing olefins and aromatics using ionic liquids. In order to remove the sulfurs from 6#solvent oil, the effect of complex ionic liquid modified with CuCl on the sulfur removal was studied. The results are shown in Fig. 2.
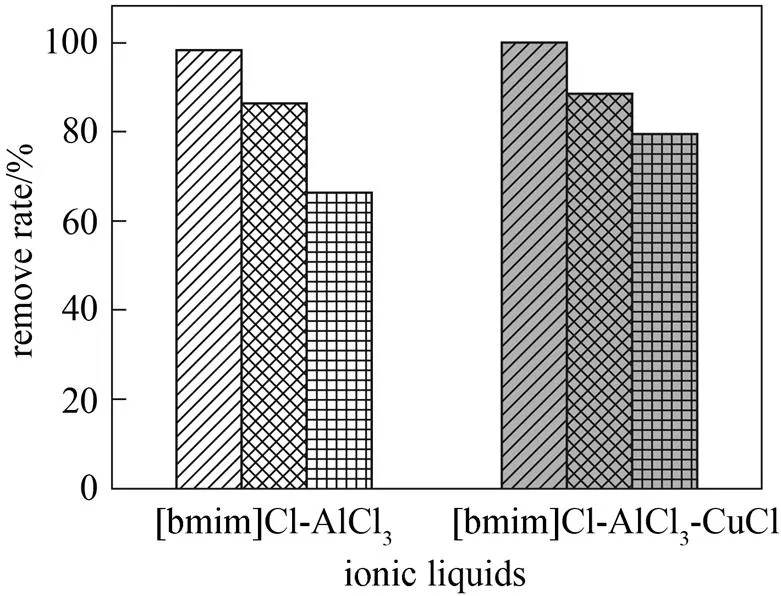

Figure 3 Anions SIMS spectrum of complex ionic liquid
It can be seen that complex ionic liquid shows remarkable ability for sulfur removal. The removal of olefins is increased slightly and that of aromatics is not changed, while that of sulfurs is increased from 66.31% to 79.42%. This may be attributed to the alkylation of aromatic sulfurs with olefins catalyzed by the new anions formed in the modified ionic liquid. The analysis of anions in CuCl modified ionic liquid is performed through SIMS(the second ion mass spectrum). The analytical results are shown in Fig. 3.
It can be seen from Fig. 3 that some new complex anions appeared in the ionic liquid after modified with CuCl. As the π-complexation of thiophene and Cu(I) is also one of the important desulfurization mechanisms for CuCl-based ionic liquid, the sulfur removal ability for complex ionic liquid is improved remarkably. This is coincidence with above results.
At the same time, the reusability of ionic liquids was tested. The results are shown in Table 3.

Table 3 The results of refining oil with the reusing ionic liquid
It can be found that the content of sulfur increased gradually with increasing running time. This may be attributed to that the ionic liquid is saturated and loses gradually its adsorption capacity to sulfurs, leading to the decrease of the sulfur removal, while the removal of aromatics and olefins is not changed obviously. This indicates that the complex ionic liquids may be reused for a long time. The yield of 6#solvent oil is over 97% after treated with complex ionic liquid.
The olefins, aromatics and partial sulfurs in 6#solvent oil can be removed simultaneously when processed with acidic ionic liquids. The removal rate for olefins and aromatics after processed with AlCl3ionic liquid modified with HCl is 100% and 93.02%, respectively. 79.42% sulfur can be removed using AlCl3ionic liquid modified with CuCl. The contents of aromatics, olefins and sulfurs are 0.36%, 0.00% and 0.0058% in the upgrading solvent oil with zero bromic index, respectively. The qualities of 6#solvent oil are coincidence with GB16629-1996. The yield of refined solvent oil is over 97%.
1 Ou, F., Application Technology of Petroleum Products, Petroleum Industry Press, Beijing, 72 (1983). (in Chinese)
2 Hobsom, G.D., Modern Petroleum Technology, 5th edition, The Institute of Petroleum, London, 956 (1984).
3 Editor group, Handbook of Synthetic Material Additives, Chemical Industry Press, Beijing, 10 (1985). (in Chinese)
4 Wang, G.W., Li, F.R., Tang, J.X., “Study on catalytic hydrodearomatization of 6#extraction solvent oil with high aromatics content”,, 21 (3), 32-34 (1997). (in Chinese)
5 Wang, G.W., Wang, Z.Y., Ma, C.X., “Study on catalytic hydrodearomatization of Jianghan oil field petroleum refining solvent oil blended”,, 24 (2), 28-30 (2000). (in Chinese)
6 Tang, N.H., Yu, Z.M., Hao, A.X., “Refining process for removing arene-including impurities from solvent oil”,(), 25 (4), 27-30 (2004). (in Chinese)
7 Welton, T., “Room-temperature ionic liquids: Solvents for synthesis and catalysis”,.., 99, 2071-2083 (1999).
8 Kabalka, G.W., Malladi, R.R., “Reduction of aldehydes using trialkylboranes in ionic liquids”,.., (22), 2191-2192 (2000).
9 Earle, M.J., McCormac, P.B., Seddon, K.R., “Regioselective alkylation in ionic liquids”,.., (20), 2245-2246 (1998).
10 Sun, X.W., Zhao, S.Q., “Effect of CuCl modification on catalytic performances of FeCl3-(butyl-methyl-imidazolium) ionic liquid in alkylation”,, 34 (5), 433-436 (2005). (in Chinese)
11 Sun, X.W., “The alkylation of benzene with ethylene in ionic liquid and research of catalysis mechanism”, Ph. D. Thesis, China Petroleum University (Beijing), Beijing (2003). (in Chinese)
12 Qiao, K., Deng, Y.Q., “Alkylation of benzene in room temperature ionic liquids modified with HCl”,...:., 171, 81-84 (2001).
2008-08-11,
2009-05-12.
the National Natural Science Foundation of China (20676150), the Natural Science Foundation of Beijing (2052010), and the Science and Technology Venture Foundation of the PetroChina Company Limited (2005 [68]).
** To whom correspondence should be addressed. E-mail: sunxwb2000@cup.edu.cn
 Chinese Journal of Chemical Engineering2009年4期
Chinese Journal of Chemical Engineering2009年4期
- Chinese Journal of Chemical Engineering的其它文章
- Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
- Phase Equilibrium of Isobutanol in Supercritical CO2
- Conversion of Methane by Steam Reforming Using Dielectric-barrier Discharge*
- Permeability and Selectivity of Sulfur Dioxide and Carbon Dioxide in Supported Ionic Liquid Membranes*
- Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid*
- Model Study on a Submerged Catalysis/Membrane Filtration System for Phenol Hydroxylation Catalyzed by TS-1*
