Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
ZOU Weihua (邹卫华), ZHAO Lei (赵蕾) and HAN Runping (韩润平)
Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
ZOU Weihua (邹卫华)1, ZHAO Lei (赵蕾)1and HAN Runping (韩润平)2,3,**
1School of Chemical and Energy Engineering, Zhengzhou Univerity, Zhengzhou 450001, China2Department of Chemistry, Zhengzhou University, Zhengzhou 450001, China3Luoyang Company of China Petrolum & Chemical Corporation, Luoyang 471012, China
The adsorption of uranium (VI) on the manganese oxide coated zeolite (MOCZ) from aqueous solution was investigated in a fixed-bed column. The experiments were conducted to investigate the effects of bed height, flow rate, particle size, initial concentration of uranium (VI), initial pH, presence of salt and competitive ions. The U-uptake by MOCZ increased with initial uranium (VI) concentration and bed height, but decreased as the flow rate and particle size increased. In the presence of salt and competitive ions, the breakthrough time was shorter. The adsorption capacity reached a maximum at pH of 6.3. The Thomas model was applied to the experimental data to determine the characteristic parameters of the column for process design using linear regression. The breakthrough curves calculated from the model were in good agreement with the experimental data. The BDST model was used to study the influence of bed height on the adsorption of uranium (VI). Desorption of uranium (VI) in the MOCZ column was investigated. The column could be used for at least four adsorption-desorption cycles using 0.1 mol·L-1NaHCO3solution as the elution. After desorption and regeneration with deionized water, MOCZ could be reused to adsorb uranium (VI) at a comparable capacity. Compared to raw zeolite, MOCZ showed better capacity for uranium (VI) removal.
adsorption, uranium (VI), manganese oxide coated zeolite, regeneration
1 INTRODUCTION
Uranium is one of the most serious contamination concerns because of its radioactivity and heavy-metal toxicity. Uranium and its compounds are highly toxic, which is a threat to human health and ecological balance. Dissolved uranium usually occurs in most natural waters at very low concentrations, but uranium mining, milling, processing, enriching, and disposal all contribute to contaminate surface water and groundwater, where the concentrations of uranium may increase to above 1 μg·L-1. Hence the research on separation of uranium from wastewater is important. Adsorption by lowcost adsorbents provides an environmentally and economically favorable method for removing uranium from wastewaters. Although their adsorption capacities are usually less than those of synthetic or modified adsorbents, the minerals may be inexpensive substitutes for the treatment of heavy metal-laden wastewaters. To enhance the adsorption capacity of natural adsorbents for heavy metals, many attempts have been made,.., chemical modification for their surfaces using metal oxides[1-3].
Natural zeolites have received considerable attention for heavy metal removal due to their valuable properties, such as ion exchange ability. The physical structure is highly porous, enclosing interconnected cavities, in which the metal ions and water molecules are captured [4]. In order to improve the performance, such as adsorption capacity, mechanical strength, and resistance to chemical environments, several physical and chemical methods have been used to modify zeolite.

The objective of this study is to test the properties of manganese oxide coated zeolite (MOCZ) as an adsorbent for removing uranium (VI) from synthetic solutions using a fixed-bed column. The effects of main variables, such as bed height, flow rate, particle size, initial uranium (VI) concentration, initial pH, presence of salt and competitive ions, on the adsorption of uranium (VI) are investigated.
2 MATERIALS AND METHODS
2.1 Preparation of MOCZ

The raw zeolite sample was crushed and sieved through the mesh screens, and a fraction of particles with an average size were soaked in tap water for 24 h to decrease its alkalescence, rinsed with distilled water and dried at 373 K in the oven for preparation of surface coating. Manganese oxide coated zeolite (MOCZ) was prepared in a reductive procedure to precipitate colloids of manganese oxide on the particle surface. A boiling solution containing potassium permanganate was poured over dried zeolite particles in a beaker, and then hydrochloric acid (mass fraction 37.5%) was added dropwise into the solution. After stirring for 1 h, the material was filtered (60-80 μm, 80-100 μm, 160-200 μm), washed to reach pH 7.0 using distilled water, dried at room temperature and stored in a polypropylene bottle until use.
2.2 Reagents and solution
All chemicals and reagents used for experiments and analyses were of analytical grade. The solution of uranium (VI) was prepared by dissolving appropriate amount of UO2(NO3)2·6H2O (A.R.) in deionized water. Arsenazo III solution with 0.5 g·L-1was prepared by dissolving 0.5 g of the reagent in 1000 ml of deionized water. Samples were analyzed by the Arsenazo III colorimetric method for uranium (VI) concentration.
2.3 Determination of uranium contents in solution
A simple and sensitive spectrophotometric method based on colored complexes with Arsenazo III in an aqueous medium was used for determination of uranium (VI) [13]. The concentration in the solution was determined with a Shimadzu Brand UV-3000 spectrophotometer by measuring absorbance atmaxof 588 nm for uranium complex.
2.4 Methods for adsorption studies

2.5 Analysis of experimental data
The fraction of uranium (VI) uptake from a solution in a fixed-bed is usually expressed by/0as a function of time or volumetric flow rate for a given bed height, resulting in a breakthrough curve [14]. The maximum column capacity,total(mg), for a given feed concentration and the flow rate, is equal to the area under the curve of the adsorbed uranium (VI) concentrationad(ad0)time (min) and is calculated by Eq. (1).

The equilibrium uptake (eq), the amount of uranium (VI) adsorbed per unit mass of dry adsorbent (mg·g-1) in the column, is calculated as follows:
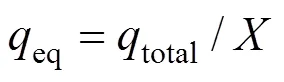
whereis the total mass of dry adsorbent in the column.
The total amount of uranium (VI) added to the column (total) is

The total removal percent of uranium (VI) is the ratio of the maximum capacity of the column,total, to the total amount of uranium (VI) added to column,total.

The effluent volume,eff, can be calculated by Eq. (5)
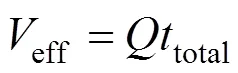
2.6 Mathematical model
The Thomas model [15] was used to predict the adsorption performance. The non-linearized and linearized form of the model are given as Eqs. (6) and (7), respectively.

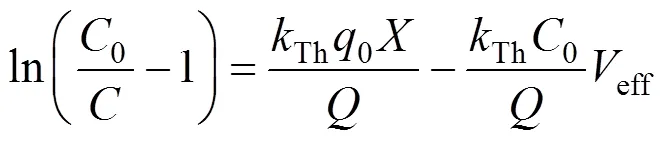

3 RESULTS AND DISCUSSION
3.1 Effects of main variables on the adsorption of uranium (VI) by MOCZ
3.1.1
The bed height of column for uranium (VI) adsorption was about 5, 10, 15, and 20 cm, corresponding to 5, 10, 15, and 20 g of MOCZ, respectively. The breakthrough curves at flow rate of 5.45 ml·min-1and initial uranium (VI) concentration of 50 mg·L-1are illustrated in Fig. 1 (a). The total adsorbed amount, the maximum uranium (VI) uptake and removal percent with respect to the bed height are presented in Table 1.

Table 1 Comparison of adsorption capacities on MOCZ and raw zeolite for removal of uranium (VI)
Figure 1 (a) shows thatbandeincrease with the bed height. The slopes of the curves from breakthrough time to exhaustion time decrease as the bed height increases from 5 to 20 cm, and the shape and gradient of the breakthrough curves are slightly different. The uranium (VI) uptake capacity,total, increases from 65.4 to 286 mg with the increase of bed height in the column. The increase in adsorption with higher bed height is due to the increase in adsorbent mass, providing more adsorption sites for uranium (VI). The removal efficiencies and equilibrium capacities (eq) also increase, as shown in Table 1.

Figure 1 Effect of bed height on breakthrough curve of uranium (VI) (a) and pH value of effluent (b)
The effluent pH is changed dramatically, as shown in Fig. 1 (b). The pH value drops rapidly at the beginning, and then increases to about 4.8 at the exhausting point. The pH profile can be explained by the surface ion exchange between uranium (VI) and H+. At the beginning of adsorption, more uranium (VI) ions are bounded on MOCZ, and more H+are released into the solution by ion exchange, so the value of pH decreases rapidly. As the bed height increases from 5 to 20 cm, the minimum pH value decreases from 4.3 to 4.1, since more adsorbent mass releases more H+by exchanging with uranium (VI) ions. Although the H+diffusivity is higher, the concentration fronts of H+and uranium (VI) move at the same velocity for maintaining the electro-neutrality of solution. Therefore, the breakthrough curve of pH may be used as an indicator of metal breakthrough, which is a simple method for ending a column cycle since monitoring pH values is easier than monitoring metal concentrations.
Bed depth service time (BDST) is a simple model, in which the bed heightand service timeof a column bears a linear relationship. The equation can be expressed as follows [16, 17]:
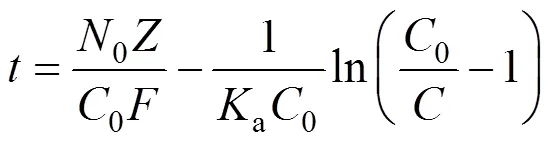



wherebis the breakthrough uranium (VI) concentration (mg·L-1). The value of0was found to be 6.0 cm, below which the column of MOCZ would be not efficient enough under the condition. The parameters in the BDST model will be helpful to scale up the process.
3.1.2
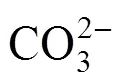
The breakthrough curves at different pH are shown in Fig. 2. The influent concentration of uranium (VI) was 50 mg·L-1, the mass of MOCZ in the column was 15 g, and the flow rate was 7.69 ml·min-1. The precipitate of uranium (VI) and colloid formation were not observed in the pH range of 3.2 to 8.1 under the experimental condition. As shown in Fig. 2, the pH of aqueous phase is a controlling factor in uranium (VI) adsorption. As pH increases from 3.2 to 6.3, the breakthrough time reaching saturation increases significantly, and the uptake capacityeqalso increases from 8.2 to 15.4 mg·g-1. The highest maximum bed capacity and the longest breakthrough time are obtained at pH 6.3. At lower pH, H+competes with uranium (VI) ions for the surface of adsorbent, so that the uranium (VI) uptake is less. It was also observed that the uptake capacity of uranium (VI) decreases from 15.4 to 11.5 mg g–1as pH increases from 6.3 to 8.1. Such a pH-dependence of uranium (VI) sorption on MOCZ is similar to that for uranium (VI) adsorption on ferrihydrite, hematite, goethite, and amorphous iron hydroxide with solutions exposing to the atmosphere [18-20]. This behavior may be explained by that the carbonate concentration increases with pH at a constant carbon dioxide partial pressure, resulting in an increase in the concentration of soluble uranium (VI) carbonate complexes. They compete with uranium (VI) for adsorption sites, so that the adsorption of uranium (VI) ions decreases as the concentration of dissolved carbonate and bicarbonate anions increase [21].


Predicted breakthrough curves with respect to solution pH are also shown in Fig. 2, which are in good agreement with experimental data. The correlation coefficients () at pH from 3.2 to 8.1 are between 0.966 and 0.985. The calculated0values are similar to experimentaleqvalues.
3.1.3
The effect of initial concentration of uranium (VI) on adsorption by MOCZ was investigated for flow rate 5.45 ml·min-1and bed height 15 cm, shown in Fig. 3. The initial concentration of uranium (VI) varied from 50 to 120 mg·L-1.
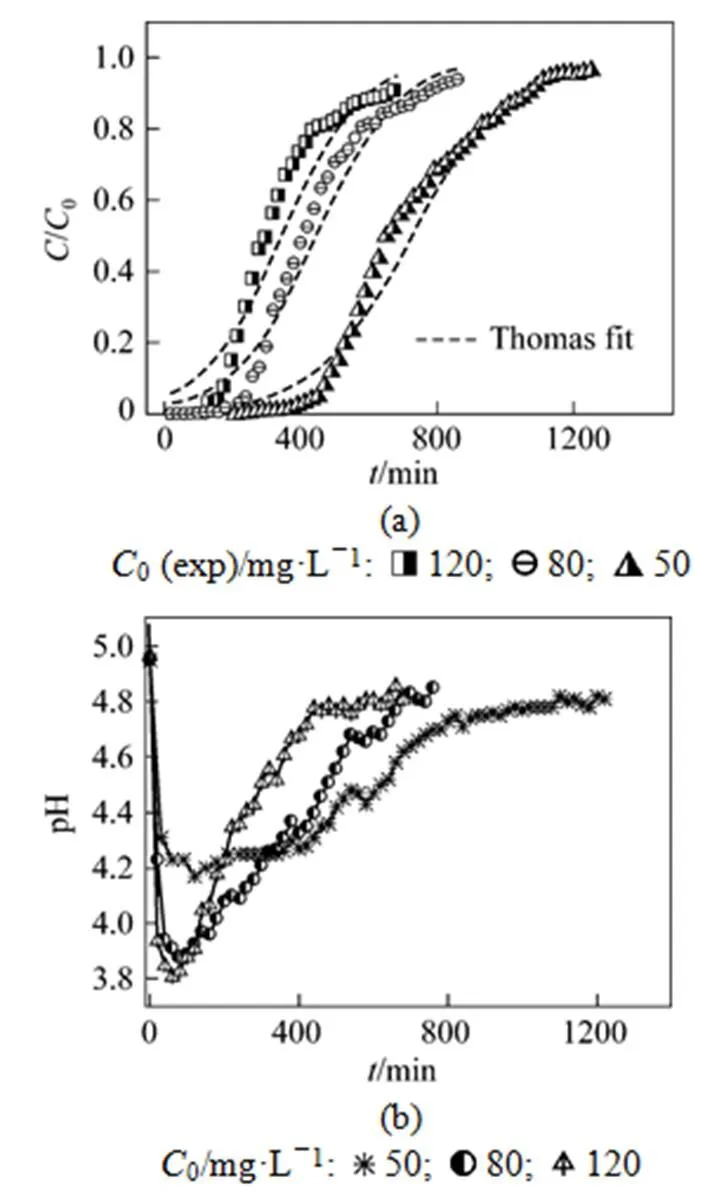
Figure 3 Effect of initial uranium (VI) concentration on breakthrough curve (a) and pH value of effluent (b)


Table 2 Effect of initial pH on the adsorption of uranium (VI)
Figure 3 (a) shows that the Thomas model give a good fit of the experimental data in the concentration range studied. The value of0increases from 13.0 to 15.1 mg·g-1, while the value ofThobtained from Thomas model decreases from 0.142 to 0.0727 ml·mg-1·min-1as the initial concentration of uranium (VI) changes from 50 to 120 mg·L-1(Table 3).
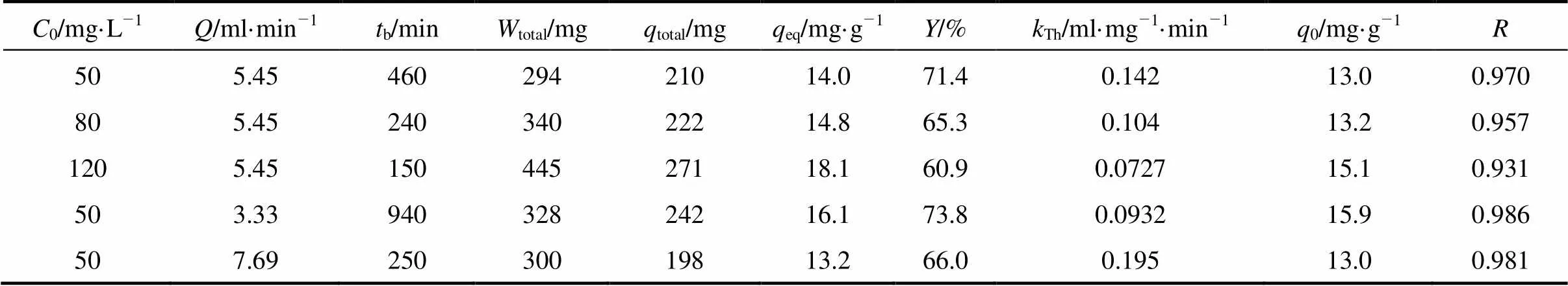
Table 3 Effect of initial concentration and flow rate on the adsorption of uranium (VI)

Figure 4 Effect of flow rate on breakthrough curve (a) and pH value of effluent (b)

3.1.4
Flow rate is one of the important parameters in column design [22]. The breakthrough curves at different flow rate are shown in Fig. 4 (a). The initial uranium (VI) concentration is 50 mg·L-1and the bed height is 15 cm. Fig. 4 (a) shows thateincreases significantly as the flow rate decreases from 7.69 to 3.33 ml·min-1. The breakthrough curves are much steeper at higher flow rates. As the flow rate increases from 3.33 to 7.69 ml·min-1, the uranium (VI) uptake capacity,eq, decreases from 16.1 to 13.2 mg·g-1(Table 3), and the minimum value of pH increases from 4.0 to 4.3 [Fig. 4 (b)]. This is because lower liquid velocity in the column results in longer contact time between phases. With the Thomas model, the calculated breakthrough curves are close to the experimental curves, as shown in Fig. 4 (a), and the correlation coefficients are between 0.957 and 0.986. The calculated0values are similar to experimentaleqvalues.
3.1.5
Figure 5 shows the experimental results for different particle sizes ranged from 60-200 μm at a flow rate of 5.45 ml·min-1and initial uranium (VI) concentration of 80 mg·L-1. Larger adsorbent particle size gives an earlier breakthrough, and the slope of the breakthrough curve increases as particle size increases. The equilibrium adsorption capacity,eq, increases from 14.2 to 15.1 mg·g-1, and the total removal percent of uranium (VI) increases from 54.6% to 78.8% as particle size decreases, as shown in Table 4. This may be due to the fact that the adsorption is a surface phenomenon and the extent of adsorption is expected to be proportional to the specific surface. The effective surface area increases as particle size decreases and as a consequence, the saturation adsorption per unit mass of adsorbent increases [9, 23].
In Fig. 5 the Thomas model give a good fit of the experimental data for all tested particle sizes with high correlation coefficient greater than 0.929. As the particle size of MOCZ increases, the value ofThdecreases (Table 4). The calculated0values from Thomas model are similar to experimentaleqvalues.
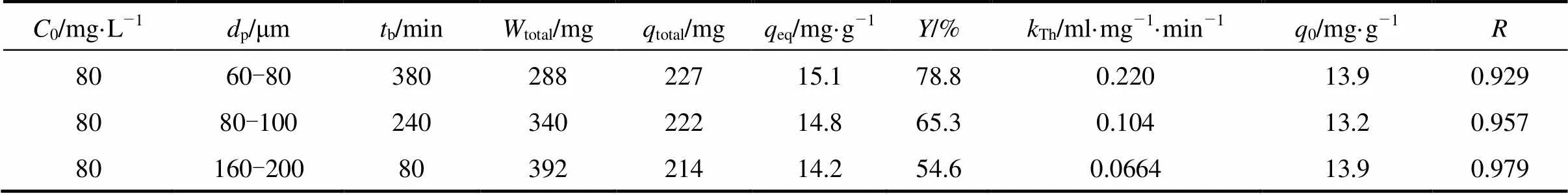
Table 4 Effect of particle size of MOCZ on the adsorption of uranium (VI)
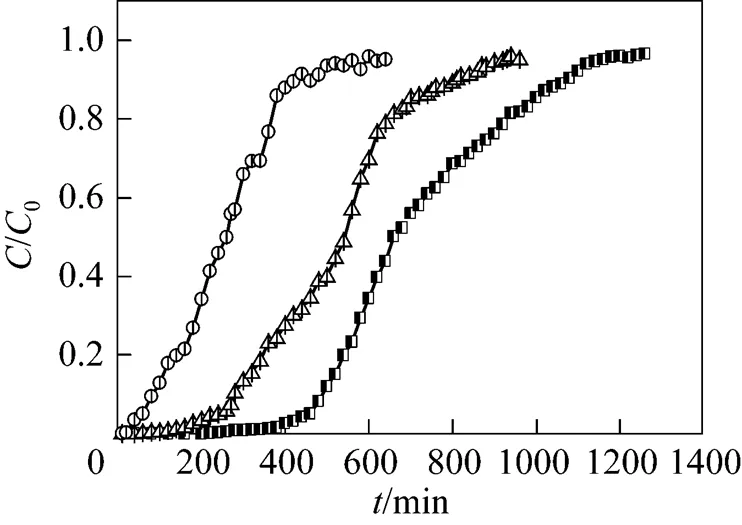

3.1.6
In order to investigate the effect of coexisting ions on the adsorption of MOCZ, a solution containing Cu(II) or Zn(II) was used, with a concentration of 22 mg·L-1. Fig. 6 shows the experimental results at a flow rate of 5.45 ml·min-1and initial uranium (VI) concentration of 80 mg·L-1. In binary systems, a shorter time was required to attain the breakthrough and saturation in comparison with single metal solutions. This is primarily because the presence of competitive metal ions Cu2+or Zn2+reduces the adsorption capacity of uranium (VI) ions on MOCZ.
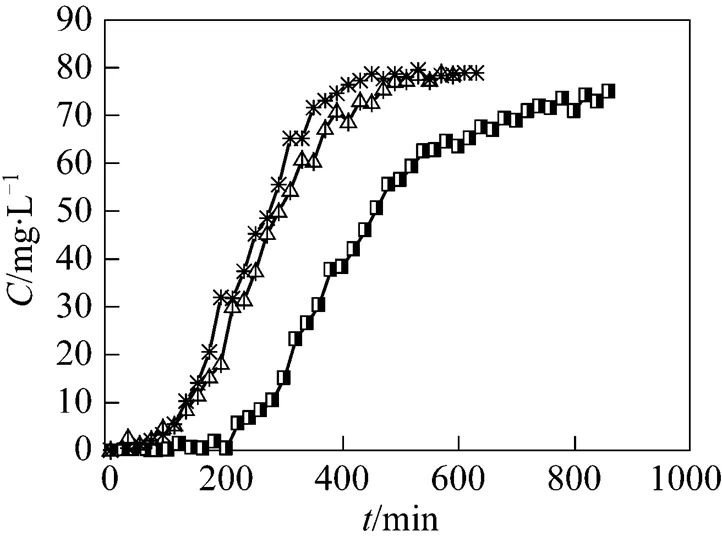

3.1.7
The effect of coexisting common salt was studied with uranium (VI) of 50 mg·L-1in NaCl or CaCl2of 0.02 mol·L-1as background electrolytes. The flow rate was 5.45 ml·min-1. As shown in Fig. 7, the existence of salt in the solution results in steeper slope and smaller breakthrough time. The effect of CaCl2is stronger than that of NaCl. The result is in good agreement with our previous study in a batch system [24]. The reason may be the competitive effect between uranium (VI) ions and metal cations from the salt for the adsorption sites available. Another reason is that as ionic strength increases, the activity of uranium (VI) and the active sites decrease, so the adsorptive capacity of uranium (VI) decreases. As Ca2+has more contribution to ionic strength and more positive charges than Na+, the effect of Ca2+on the adsorption is more serious than Na+at the same concentration [25].
3.2 Regeneration
Disposal of the adsorbent loaded with heavy metal ions creates another environmental problem, as it is a hazardous material to the environment. This problem may be solved to some extent by using one of the elimination methods,.., elution, incineration and pyrolysis. Regeneration of the adsorbent material is of crucial importance in the economic development [26]. The regeneration of MOCZ fixed bed column was studied to assess the possibility for the reuse of adsorbent and recovery of metal ions.
The column was packed with 15 g MOCZ. The flow rate was 5.45 ml·min-1and initial uranium (VI) concentration was 50 mg·L-1. Once the adsorbent MOCZ was saturated, the ions can be removed by desorbing agents and the MOCZ can be reused in further adsorption processes.
The elution curves are shown in Fig. 8. The elution curves obtained in all cases exhibit a similar trend. The concentration of the effluent uranium (VI) is very high at the beginning of the desorption process, and then drops quickly to a very low level. The maximum concentrations of uranium (VI) are 7875 mg·L-1for 0.5 mol·L-1HNO3, 5308 mg·L-1for 0.1 mol·L-1HNO3, 5283 mg·L-1for 0.5 mol·L-1NaHCO3, and 3850 mg·L-1for 0.1 mol·L-1NaHCO3. The solution of 0.5 mol·L-1HNO3was the most effective desorbing agent among the eluting agents, but it would destroy the adsorbent MOCZ. The solution of 0.1 mol·L-1NaHCO3did not cause damage to MOCZ and the efficiency of desorption was significant, so that it was selected as the desorbing agent. Following desorption, the bed was washed with distilled water until the pH of the effluent was corresponded to the inlet water. This treatment was carried out after each cycle.
The regenerated MOCZ was reused for four adsorption-desorption cycles, as shown in Fig. 9 and Table 5. The efficiency of uranium (VI) adsorption- desorption process changes little in the second cycle and remains almost the same in the following cycles. Therefore, MOCZ is an excellent adsorbent for uranium (VI), and has promising potential applications for removal of uranium (VI) from industrial effluents.



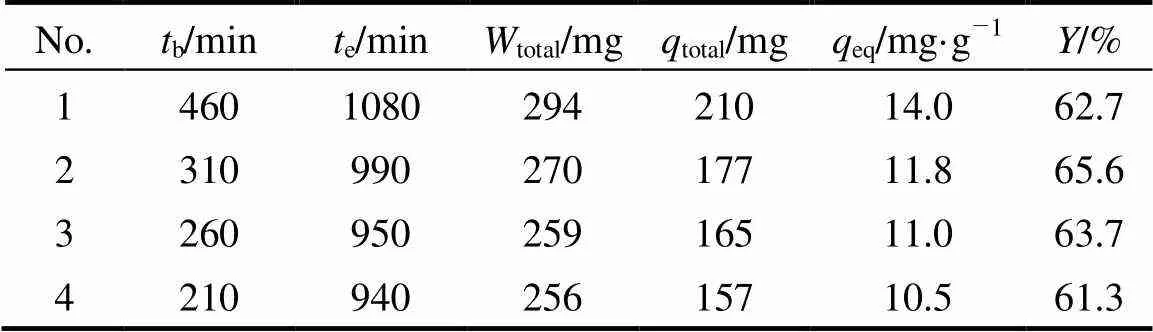
Table 5 Adsorption parameters for four adsorption-desorption cycles
3.3 Comparison with raw zeolite
For inlet concentration of uranium (VI) 50 mg·L-1, flow rate 5.45 ml·min-1with a bed height of 15 cm, the breakthrough curves of zeolite column and MOCZ column are given in Fig. 10, respectively. The values of equilibrium uptakeeqand removal percents are also presented in Table 1. The breakthrough time is 180 and 460 min for uranium (VI) to be adsorbed on zeolite and MOCZ, respectively. The MOCZ has higher adsorption capacity than raw zeolite, which is attributed to the highly negative surface charge on the modified surface [27]. Furthermore, the increment of surface area will play a significant role in the overall removal process.The surface area of MOCZ increased from 24.87 to 28.23 m2·g-1after coating manganese oxide on the surface of zeolite[12].
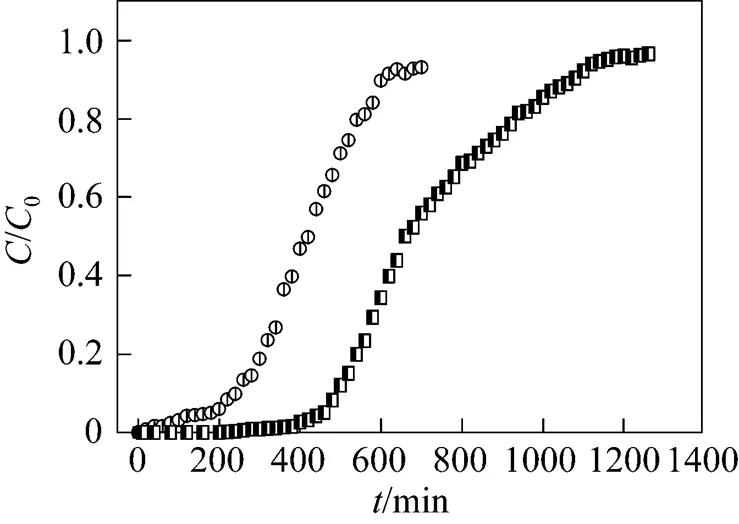

3.4 Comparison with other solid adsorbents
Various forms of natural and synthetic adsorbents were used in heavy metal removal from aqueous solutions and wastewaters. Uranium adsorption on various minerals was widely described in the literature. Table 6 provides a comparison on uranium (VI) uptake capacities of some natural and synthetic adsorbents, based on mg of uranium (VI) ion adsorbed per gram mass of adsorbent.

Table 6 Comparison of adsorption/retention capacities of MOCZ and various adsorbents for uranium (VI)
From Table 6, the adsorption capacity of chelate modified resin is higher than that of natural and modified minerals reported in literature so far. However, the flow rate for natural and modified minerals may be higher than that for resin due to their larger particle sizes in the column. On the other hand, the particle shapes of natural and modified minerals do not deform in water treatment. The size of MOCZ is larger than that of ion-exchange resin, so the process can be operated at higher flow rate in a fixed-bed column. The adsorption capacity of MOCZ is comparable to other adsorbents. The maximum adsorption capacityeqachieved with MOCZ is 18.1 mg·g-1, which is satisfactory for MOCZ to be an adsorbent, with the advantage of easy handling and cost effectiveness.
4 CONCLUSIONS
The adsorption of uranium (VI) from aqueous solutions on MOCZ was investigated in a continuous fixed bed column. The uranium (VI) adsorption capacity increased with decreasing initial uranium (VI) concentration, flow rate, and particle size, but increased with bed height. Presence of Cu2+or Zn2+resulted in a shorter breakthrough period, as compared to the single uranium (VI) system. The Thomas model was applied to the experimental data to predict the breakthrough curves and to determine the kinetic parameters for the column. MOCZ was regenerated and used for four cycles with almost the same efficiency. Compared to raw zeolite and other mineral adsorbents, MOCZ had better ability to adsorb uranium (VI) from the solution, so that MOCZ can be used to remove uranium (VI) from aqueous solutions.
NOMENCLATURE
area under the breakthrough curve
effluent uranium (VI) concentration, mg·L-1

bbreakthrough uranium (VI) concentration, mg·L-1
0influent or initial uranium (VI) concentration, mg·L-1
pparticle size of MOCZ, μm
influent linear velocity, cm·min-1
arate constant in BDST model, L·mg-1·min-1
Thrate constant in Thomas model, ml·min-1·mg-1
0adsorption capacity from BDST model, mg·L-1
volumetric flow rate, ml·min-1
eqequilibrium uranium (VI) uptake per gram of adsorbent from experiment, mg·g-1
totaltotal adsorbed quantity of uranium (VI) in the column, mg
0equilibrium uranium (VI) uptake per gram of adsorbent from Thomas model, mg×g-1
btime at breakthrough, min
etime at exhaustion, min
totaltotal flow time, min
effeffluent volume, ml
totaltotal amount of uranium (VI) added into the column, mg
amount of adsorbent in the column, g
total removal percent of uranium (VI), %
bed height, cm
0critical bed height, cm
1 Al-Degs, Y., Khraisheh, M.A.M., “The feasibility of using diatomite and Mn-diatomite for remediation of Pb2+, Cu2+, and Cd2+from water”,...,35, 2299-2310 (2000).
2 Edwards, M., Benjamin, M.M., “Adsorption filtration using coated sand: a new approach for treatment of metal-bearing wastes”,...,61, 1523-1533 (1989).
3 Kuan, W.H., Lo, S.L., Wang, M.K., “Removal of Se (IV) and Se (VI) from water by aluminum oxide coated sand”,., 32, 915-923 (1998).
4 Al-Haj, A.A., El-Bishtawi, R., “Removal of lead and nickel ions using zeolite tuff”,...., 69, 27-34 (1997).
5 Birsen, A., Timothy, A.D., “Application of MnO2coated scintillating and extractive scintillating resins to screening for radioactivity in groundwater”,.., 505, 458-461 (2003).
6 Catts, J.G., Langmuir, D., “Adsorption of Cu, Pb, and Zn byd-MnO2: Applicability of the side binding-surface complexation model”,.., 1, 255-264 (1986).
7 Fu, G., Allen, H.E., Cowan, C.E., “Adsorption of cadmium and copper by manganese oxide”,., 152, 72-81 (1991).
8 Zou, W.H., Liu, C.X., Jiang, L., Han, R.P., “Single and binary component adsorption of copper cation and lead cation from aqueous solutions using fresh δ-MnO2”,.. (..), 26, 15-19 (2005).
9 Al-Degs, Y., Khrasisheh, M.A.M., Tutunji, M.F., “Sorption of lead ions on diatomite and manganese oxides modified diatomite”,., 35, 3724-3728 (2001).
10 Han, R.P., Zou, W.H., Zhang, Z.P., Shi, J., Yang, J.J., “Removal of copper (II) and lead (II) from aqueous solution by manganese oxide coated sand (I) Characterization and kinetic study”,..., 137, 384-395 (2006).
11 Han, R.P., Zou, W.H., Li, H.K., Li, Y.H., Shi, J., “Copper (II) and lead (II) removal from aqueous solution in fixed-bed columns by manganese oxide coated zeolite”,..., 137, 934-942 (2006).
12 Zou, W.H., Han, R.P., Chen, Z.Z., Shi, J., Liu, H.M., “Characterization and properties of manganese oxide coated zeolite (MOCZ) as adsorbent for removal of copper (II) and lead (II) ions from solution”,..., 51, 534-541 (2006).
13 Misaelides, P., Godelitsas, A., Filippidis, A., Charistos, D., Anousi, I., “Thorium and uranium uptake by natural zeolitic materials”,..,173/174, 237-246 (1995).
14 Guibal, E., Lorenzelli, R., Vincent, T., Cloirec, L., “Application of silica gel to metal ion sorption: static and dynamic removal of uranyl ions”,.., 16, 101-114 (1995).
15 Thomas, H.C., “Heterogeneous ion exchange in a flowing system”,....,66, 1664-1666 (1944).
16 Goel, J., Kadirvelu, K., Rajagopal, C., Garg, V.K., “Removal of lead (II) by adsorption using treated granular activated carbon: batch and column studies”,..., 125, 211-220 (2005).
17 Othman, M.Z., Roddick, F.A., Snow, R., “Removal of dissolved organic compounds in fixed-bed columns: evaluation of low-rank coal adsorbents”,., 35, 2943-2949 (2001).
18 Lenhart, J.J., Honeyman, B.D., “Uranium VI sorption to hematite in the presence of humic acid”,..., 63, 2891-2901 (1999).
19 Waite, T.D., Davis, J.A., Payne, T.E., Waychunas, G.A., Xu, N., “Uranium(VI) adsorption to ferrihydrite: Application of a surface complexation model”,..., 58, 5465-5478 (1994).
20 Wazne, M., Korfiatis, G.P., Meng, X., “Carbonate effects on hexavalent uranium adsorption by iron oxyhydroxide”,..., 37, 3619-3624 (2003).
21 Barnett, M.O., Jardine, P.M., Brooks, S.C., Selim, H.M., “Adsorption and transport of uranium(VI) in subsurface media”,...., 64, 908-917 (2000).
22 Chu, K.H., “Improve fixed bed models for metal biosorption”,...,97, 233-239 (2004).
23 Banergee, K., Cheremisinoff, P.N., Cheng, L.S., “Adsorption kinetics of-xylene by fly ash”,., 31, 249-261 (1997).
24 Han, R.P., Zou, W.H., Wang, Y., Zhu, L., “Removal of uranium(VI) from aqueous solutions by manganese oxide coated zeolite: discussion of adsorption isotherms and pH effect”,..., 93, 127-143 (2007).
25 Han, R.P., Zhang, J.J., Han, P., Wang, Y.F., Zhao, Z.H., Tang, M.S., “Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite”,..., 145, 496-504 (2009).
26 Han, R.P., Zou, L.N., Zhao, X., Xu, Y.F., Xu, F., Li, Y.L., Wang, Y., “Characterization and properties of iron oxide-coated zeolite as adsorbent for removal of copper (II) from solution in fixed bed column”,..., 149, 123-131 (2009).
27 Kanungo, S.B., Paroda, K.M., “Interfacial behavior of some synthetic MnO2samples during their adsorption of Cu2+and Ba2+from aqueous solution at 300 K ”,.., 98, 252-260 (1984).
28 Tran, H.H., Roddick, F.A., O'Donnell, J.A., “Comparison of chromatography and desiccant silica gels for the adsorption of metal ions (I) Adsorption and kinetics”,., 33, 2992-3000 (1999).
29 Kilislioglu, A., “The effect of various cations and pH on the adsorption of U(VI) on Amberlite IR-118H resin”,.., 58, 713-717 (2003).
30 Gabriel, U., Gaudet, J.P., Spadini, L., Charlet, L., “Reactive transport of uranyl in a goethite column: an experimental and modelling study”,.., 151, 107-128 (1998).
31 Štamberg, K., Venkatesan, K.A., Vasudeva Rao, P.R., “Surface complexation modeling of uranyl ion sorption on mesoporous silica”,, 221, 149-162 (2003).

33 Sylwester, E.R., Hudson, E.A., Allen, P.G., “The structure of uranium(VI) sorption complexes on silica, alumina, and montmorillonite”,..., 64, 2431-2438 (2000).
34 Barton, C.S., Stewart, D.I., Morrisb, K., Bryant, D.E., “Performance of three resin-based materials for treating uranium-contaminated groundwater within a PRB”,..., 116, 191-204 (2004).
35 Kütahyal, C., Eral, M., “Selective adsorption of uranium from aqueous solutions using activated carbon prepared from charcoal by chemical activation”,...,40, 109-114 (2004).
2008-07-08,
2009-03-30.
the National Science Foundation for Postdoctoral Scientists of China (20070420811) and the Science and Technology Department of Henan Province in China (200510459016).
** To whom correspondence should be addressed. E-mail: rphan67@zzu.edu.cn
 Chinese Journal of Chemical Engineering2009年4期
Chinese Journal of Chemical Engineering2009年4期
- Chinese Journal of Chemical Engineering的其它文章
- Phase Equilibrium of Isobutanol in Supercritical CO2
- Conversion of Methane by Steam Reforming Using Dielectric-barrier Discharge*
- Permeability and Selectivity of Sulfur Dioxide and Carbon Dioxide in Supported Ionic Liquid Membranes*
- Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid*
- Model Study on a Submerged Catalysis/Membrane Filtration System for Phenol Hydroxylation Catalyzed by TS-1*
- Molecular Dynamics Simulation of Effect of Salt on the Compromise of Hydrophilic and Hydrophobic Interactions in Sodium Dodecyl Sulfate Micelle Solutions*
