Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid*
ZHAO Xuhui (赵旭辉), YANG Lingfang (杨灵芳), ZUO Yu (左禹) and XIONG Jinping (熊金平)
Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid*
ZHAO Xuhui (赵旭辉), YANG Lingfang (杨灵芳), ZUO Yu (左禹)**and XIONG Jinping (熊金平)
School of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China
Hydroxyapatite coatings were directly prepared on anodized titanium by electro-deposition method in a modified simulated body fluid. The configuration, structure and bioactivity of the coating were investigated with scanning electron microscopy (SEM), X-ray diffraction analyzer (XRD) and Fourier transform infrared spectroscopy (FTIR) techniques. The results demonstrated that pure and homogeneous hydroxyapatite coating can be obtained without any post-treatment. The prepared coating showed good bioactivity in simulated body fluid (SBF). The time required for a fully covered dense hydroxyapatite coatings was 4 days immersion in SBF.
hydroxyapatite coating, electro-deposition, modified simulated body fluid, titanium, anodization
1 INTRODUCTION
Hydroxyapatite (HA) is a well established bioactive ceramic material capable of forming strong chemical bonds with natural bones. Its ability to bond chemically to bones is distinct from biocompatible surgical alloys such as titanium [1]. However, the mechanical strength of HA is too low to be used in any load-bearing applications. Titanium on the other hand has excellent corrosion resistance and mechanical properties. When hydroxyapatite is deposited on titanium, the composite material shows both high mechanical strength and good bioactivity. In the past decade, several methods have been reported on deposition of HA on implant surfaces, including plasma spray [2, 3], sputtering [4], pulsed laser-deposition [5], sol-gel [6, 7], electrophoresis method [8-11] and electrochemical deposition [12]. Plasma spray is one of the commercial methods for coatings on titanium implant surfaces, but the extremely high temperature used in plasma spray process may lead to decomposition of HA. Another issue of great concern is the poor adhesion of the plasma-sprayed HA coating to titanium substrate, resulting in the delamination of HA coating from the metal surface. Furthermore, the microstructure of the coating is difficult to control.
HA coating deposition by electrochemical method is an attractive process because it is quick and can be operated at relatively low temperature. Additionally, the thickness and chemical composition of coatings can be well controlled through the regulation of the processing parameters. Electro-deposition is potentially an attractive route for applying crystalline phosphate coatings on the metallic substrates and may be widely used in industry. However, the depositing solution frequently used was composed of Ca (NO3)2and NH4H2PO4with pH at 4-5 adjusted by HNO3and NaOH, which was far different from the condition of body fluid. Ban and Maruno [13] have prepared calcium phosphate coatings with electro-deposition method in a modified simulated body fluid, but the coating obtained needs to be further treated till it converts to HA coating. In this study, anodization was first applied to form a film of TiO2nanotubes on titanium surface, and HA coating was then deposited by electrochemical deposition in a modified simulated body fluid. By this method the HA coating can be directly prepared and better bioactivity are expected.
2 EXPERIMENTAL
2.1 Titanium anodization
Commercial pure titanium Ta0 [99.7% (by mass) Ti] sheet (Beijing Puri New Materials Science and Technology Co., Ltd) was cut into specimens with the size of 20 mm×10 mm×0.9 mm. The specimens were polished with 300# and 600# abrasive papers sequentially, and cleaned in an ultrasonic device (DSA-400, Fuzhou Desen Precision Instruments Co., Ltd) with acetone and ethanol, respectively. Then the specimens were polished chemically in a mixture of HF+HNO3(1︰3 in volume ratio) for about 30 s, rinsed with distilled water and dried in ambient air.
A 0.5 mol·L-1H3PO4and 5.79 g·L-1NaF mixed solution was used as the electrolyte for anodization. The titanium sheet was worked as the anode and a lead sheet was used as the cathode. The spacing between the two electrodes was 5.0 cm. Anodization was carried out at 20 V voltage for 60 min. During this period, the electrolyte was continuously stirred to maintain homogeneous temperature and solution composition. The titanium sheet was rinsed with distilled water and dried in ambient air after anodization.
2.2 Electrochemical deposition of HA coating
The electrolyte used in this section was composedof 137.8 mmol·L-1NaCl, 1.7 mmol·L-1K2HPO4·3H2O, 2.5 mmol·L-1of CaCl2and distilled water, a modified simulated body fluid without MgCl2·6H2O and Na2SO4. The solution was adjusted to a pH value of 7.2 with tris-hydroxymethylaminomethane [(CH2OH)3CNH2] and hydrochloric acid. All the chemicals used were reagent grade (Beijing Chemical Reagents Company). The anodized titanium was used as the cathodic electrode and a graphite electrode as the anode. Electrochemical deposition was carried out at 65°C for 60 min with a constant current of 1 mA·cm-2. The electrolyte was agitated by a magnetic stirrer during the electrochemical deposition. After deposition, the titanium electrodes were rinsed with distilled water and dried at room temperature in air for at least 24 hours.
2.3 Characterization of the coatings
The HA coating obtained was characterized with field emission scanning electron microscopy (FE-SEM, Type LEO-1450), X-ray diffraction analyzer (XRD) and Fourier transform infrared spectroscopy (FTIR).
3 RESULTS AND DISCUSSION
Figure 1 shows the surface morphology of titanium after anodization. The polished surface of titanium was very smooth before anodization. After anodization, a dense anodic film with nanotube structure was formed on titanium surface. Similar structures have been reported by other authors [14-16]. The nanotube structure is helpful in forming mechanical interlocks between titanium substrate and the HA coating. The pores are approximately 100-150 nm in diameter, in which the nucleation of calcium phosphate crystals may occur at the beginning of the deposition reaction, leading to the HA crystals embedded in the substrate. Hence anodization can improve the deposition efficiency and the tear strength of HA coating, as reported previously [17].

Figure 1 SEM observation of titanium after anodization
Figure 2 shows the morphology of the HA coating deposited on the anodized titanium at the current density of 1 mA·cm-2and 65°C for 60 min. It can be seen from this photo that the needle like crystals developed perpendicularly to the substrate, and the adjacent curled flakes joint together to construct the micro- porous structure. The microstructure of the HA crystals formed on anodized titanium is similar to that of bones, which is beneficial for healing up of the bones during a short period.
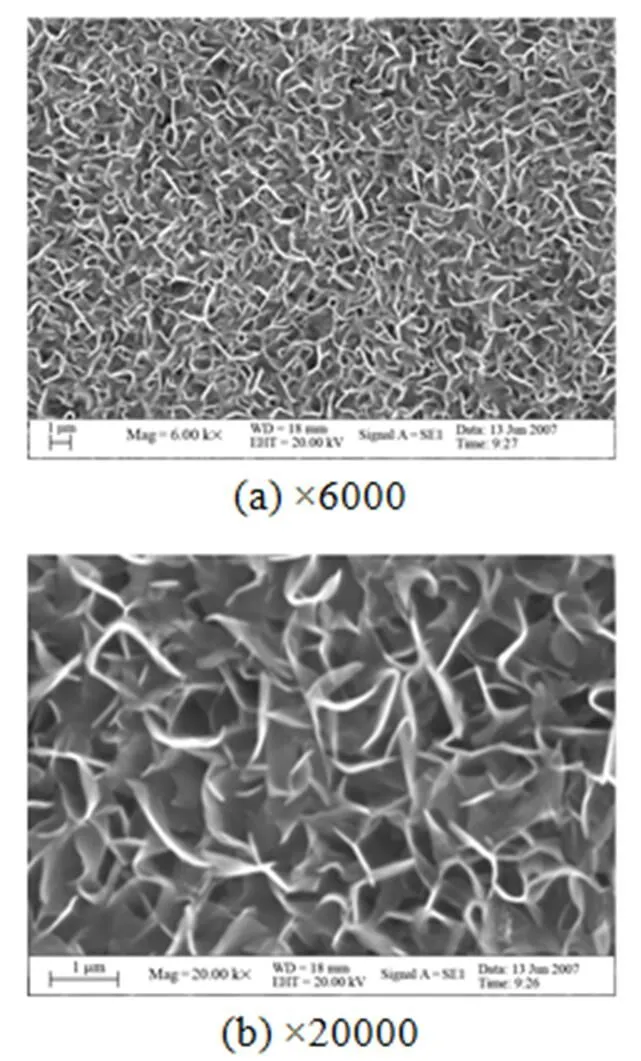
Figure 2 SEM observations of HA coating
Figure 3 shows XRD patterns of the HA coatings deposited on anodized titanium. The diffraction peaks can be indexed readily as standard HA patterns on anodized titanium. The diffraction peaks of titanium substrate were observed but no TiO2peaks were distinguished. The reason may be that the thickness of the TiO2film was too thin. The peaks at 25.84° and 32° belong to (002) and (211) crystal planes of HA coating, and the peaks at 28.09° and 49.32° also came from the coating. No diffraction peak of other matters was seen in this figure, which shows that a pure HA coating was prepared on anodized titanium by electro-deposition in the modified simulated body fluid. The result means that by this method HA coating can be directly formed without any other treatment.

Table 1 The ratio of n(Ca)/n (P) and thickness of HA after immersion in SBF

Figure 3 XRD patterns of the HA coatings■ HA;○ Ti

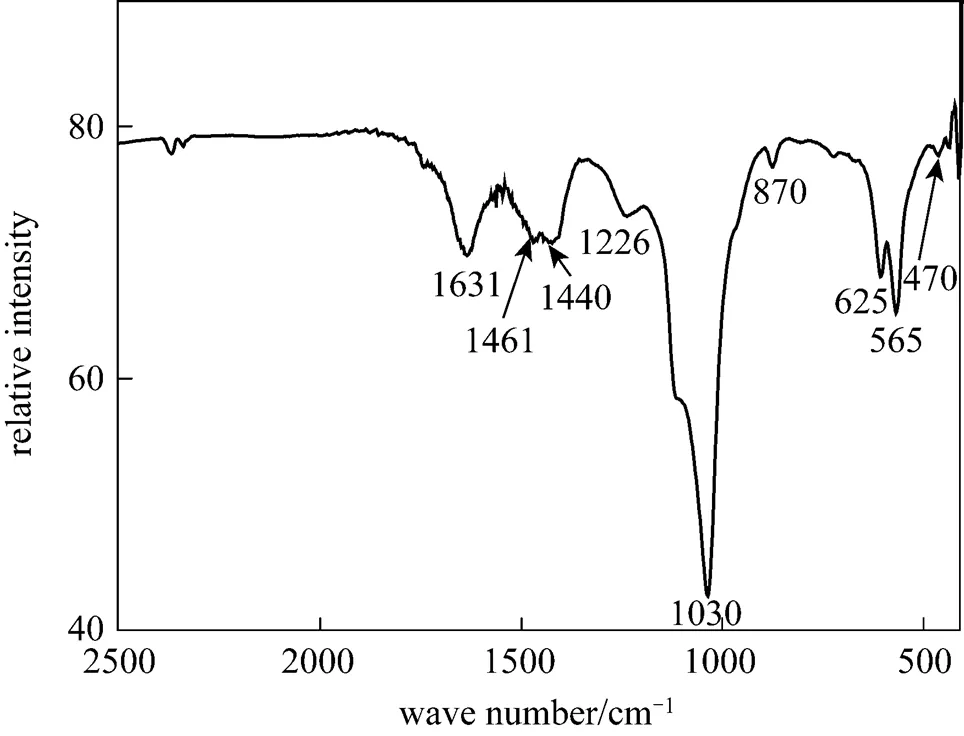
Figure 4 FTIR spectra of the HA coatings

Figure 5 shows the surface and cross section SEM images of the coatings immersed in the SBF for 1 day and 4 days, respectively. It is seen that the needle like crystallites got widen after one day. Four days later, the samples were fully covered by newly grown HA coatings. After the immersion, the coating became much thicker as shown in Fig. 5 and Table 1. Energy dispersive spectrum (EDS) analysis showed that after only one day of immersion, titanium could not be detected on the surface. The mole mass ratio of Ca and P in HA coating,(Ca)/(P), increased with immersion time and was very close to the value of perfect HA, as shown in Table 1.
It is also seen from Table 1 that after one day of immersion, the thickness of the HA coating kept unchanged, which indicates that the dissolution rate and growth rate of the HA coating during this period were almost the same. With the immersion time prolonged, growth rate of the HA coating was finally faster than its dissolution rate, and the coating thickness increased obviously during the third and fourth days of immersion. HA coating dissolves and grows in following form:
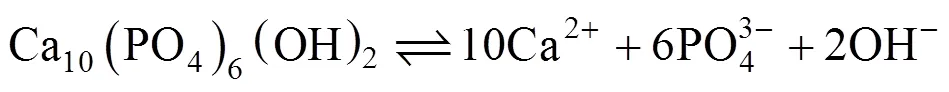
Figure 5 SEM of HA coating after soaking in SBF solution
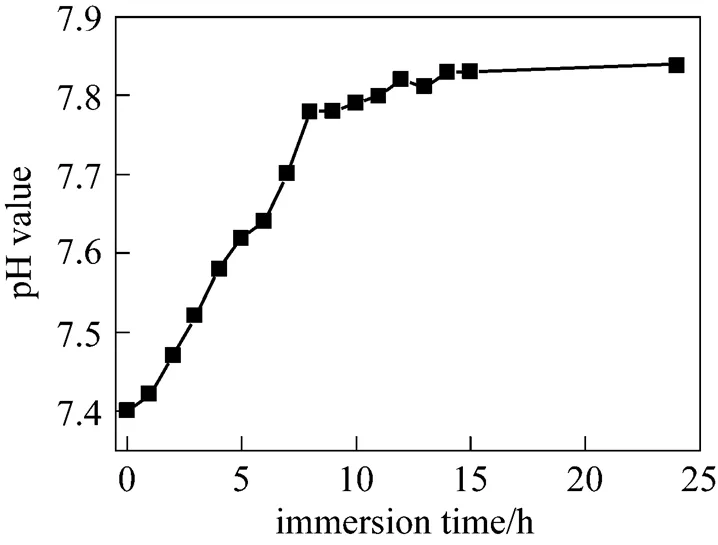
Figure 6 Changes in pH value of the SBF around the coating after soaking in SBF
Figure 7 shows XRD patterns of the coatings after immersion in SBF for 1-4 days. With increasing time, the diffraction peaks of Ti became smaller while the diffraction peaks of HA coating such as peaks at 25.84° and 32° became stronger. It indicates that the HA coatings on sample surface developed with the immersion time.
The above phenomena show that the HA coating deposited on anodized titanium surface has good bioactivity. The deposited HA coating may induce further growth of bone-like HA coating in SBF. It is suggested that the Ti-OH groups in the anodic films may provide preferred nucleus positions when immersed in SBF. Also, the coating morphology is similar to that of the structural cells of bones, and the composition of the modified simulated body fluid is similar to that of the SBF, both of which are helpful for the growth of HA.

Figure 7 XRD patterns of the HA coatings after soaking in SBF■ HA;○ Ti
4 CONCLUSIONS
(1) Pure and homogeneous hydroxyapatite coatings were directly prepared on anodized titanium by electro-deposition method in a modified simulated body fluid without any post-treatment.
(2) The deposited coating showed good bioactivity in simulated body fluid. Further growth of HA coating was induced in SBF by the deposited coating and a dense HA coating with a thickness up to 76 μm was obtained after 4 days of immersion.
1 Suchank, W., Yoshimura, M., “Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants”,..., 13 (1), 94-117 (1998).
2 Porter, A.E., Hobbs, L.W., Benzra, R.V., “The ultrastructure of the plasma-sprayed hydroxyapatite—bone interface predisposing to bone bonding”,, 23 (3), 725-733 (2002).
3 Lu, Y.P., Li, M.S., Wang, Z.G., “Plasma-sprayed hydroxyapatite+titania composite bond coat for hydroxyapatite coating on titanium substrate”,, 25 (18), 4393-4403 (2004).
4 Ding, S.J., Ju, C.P., Chern, L.J.H., “Characterization of hydroxyapatite and titanium coatings sputtered on Ti-6Al-4V substrate”,..., 44 (3), 266-279 (1999).
5 Wang, C.K., Chern, L.J.H., Ju, C.P., Ong, H.C., Chang, R.P.H., “Structural characterization of pulsed laser-deposited hydroxyapatite film on titanium substrate”,, 18 (20), 1331-1338 (1997).
6 Hsieh, M.F., Perng, L.H., Chin, T.S., “Hydroxyapatite coating on Ti6Al4V alloy using a sol-gel derived precursor”,...,74 (3), 245-250 (2002).
7 Stoch, A., Jastrzebski, W., Dlugon, E., Lejda, W., “Sol-gel derived hydroxyapatite coatings on titanium and its alloy Ti6Al4V”,..., 744-747, 633-640 (2005).
8 Manso, M., Jiménez, C., Morant, C., Herrero, P., Martínez-Duart, J.M., “Electrodeposition of hydroxyapatite coatings in basic conditions”,, 21 (17), 1755-1761 (2000).
9 Park, J.H., Lee, D.Y., Oh, K.T., “Bioactivity of calcium phosphate coatings prepared by electrodeposition in a modified simulated body fluid”,.., 60 (11/12), 2573-2577 (2006).
10 Zhang, Y.Y., Tao, J., Pang, Y.C., Wang, L., Wang, W., “Electrochemicallydeposited coatings of hydroxyapatite on titanium”,.., 35 (3), 459-462 (2006). (in Chinese)
11 Wang, Y.Q., Tao, J., “Hydroxyapatite coatings deposited electrochemically on titanium substrate”,.., 19 (6), 47-50 (2006). (in Chinese)
12 Stoch, A., Brozek, A., Kmita, G., Stoch, J., Jastrzebski, W., “Electrophoretic coating of hydroxyapatite on titanium implants”,..., 596 (1-3), 191-200 (2001).
13 Ban, S., Maruno, S., “Morphology and microstructure of electrochemically deposited calcium phosphates in a modified simulated body fluid”,, 19 (14), 1245-1253 (1998).
14 Tsuchiya, H., Macak, J.M., Gicov, A., “Characterization of electronic properties of TiO2nanotube films”,.., 49 (1), 203-210 (2007).
15 Zhao, J.L., Wang, X.H., Sun, T., Li, L.T., “Crystal phase transition and properties of titanium oxide nanotube arrays prepared by anodization”,..., 434-435, 792-795 (2007).
16 Kaneco, S., Chen, Y., Westerhoff, P., Crittenden, J., “Fabrication of uniform size titanium oxide nanotubes: Impact of current density and solution conditions”,, 56 (5), 373-376 (2007).
17 Yang, L.F., Zuo, Y., Xiong, J.P., Zhao, X.H., “Properties of hydroxyapatite coatings prepared by electrodeposition in a modified simulated body fluid”,..., 22 (4), 444-448 (2008). (in Chinese)
18 Fowler, B.O., Moreno, E.C., Brown, W.E., “Infra-red spectra of hydroxyapatite, octacalcium phosphate and pyrolysed octacalcium phosphate”,, 11 (5), 477-492 (1966).
19 Ban, S., Maruno, S., “Deposition of calcium phosphate on titanium by electrochemical process in simulated body fluid”,...., 32 (10B), L1577-L1580 (1993).
20 Sauer, G.R., Wuthier, R.E., “Fourier transform infrared characterization of mineral phases formed during induction of mineralization by collagenase-released matrix vesicles”,..., 263 (27), 13718-13724 (1988).
21 Kokubo, T., Kawashita, H.M., “Novel bioactive materials with different mechanical properties”,, 24 (13), 2161-2175 (2003).
2008-12-08,
2009-05-24.
the Young Scholars Fund of Beijing University of Chemical Technology (QN0713).
** To whom correspondence should be addressed. E-mail: zuoy@mail.buct.edu.cn
 Chinese Journal of Chemical Engineering2009年4期
Chinese Journal of Chemical Engineering2009年4期
- Chinese Journal of Chemical Engineering的其它文章
- Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
- Phase Equilibrium of Isobutanol in Supercritical CO2
- Conversion of Methane by Steam Reforming Using Dielectric-barrier Discharge*
- Permeability and Selectivity of Sulfur Dioxide and Carbon Dioxide in Supported Ionic Liquid Membranes*
- Model Study on a Submerged Catalysis/Membrane Filtration System for Phenol Hydroxylation Catalyzed by TS-1*
- Molecular Dynamics Simulation of Effect of Salt on the Compromise of Hydrophilic and Hydrophobic Interactions in Sodium Dodecyl Sulfate Micelle Solutions*
