Measurements of Hydrate Equilibrium Conditions for CH4, CO2, and CH4 + C2H6 + C3H8 in Various Systems by Step-heating Method*
CHEN Litao (陈立涛), SUN Changyu (孙长宇), CHEN Guangjin (陈光进), NIE Yunqiang (聂运强), SUN Zhansong (孙占松) and LIU Yantao (刘延涛)
Measurements of Hydrate Equilibrium Conditions for CH4, CO2, and CH4+ C2H6+ C3H8in Various Systems by Step-heating Method*
CHEN Litao (陈立涛), SUN Changyu (孙长宇), CHEN Guangjin (陈光进)**, NIE Yunqiang (聂运强), SUN Zhansong (孙占松) and LIU Yantao (刘延涛)
State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing 102249, China
Phase equilibrium conditions of gas hydrate in several systems were measured by the step-heating method using the cylindrical transparent sapphire cell device. The experimental data for pure CH4or CO2+ deionized water systems showed good agreement with those in the literatures. This kind of method was then applied to CH4/CO2+ sodium dodecyl sulfate (SDS) aqueous solution, CH4/CO2+ SDS aqueous solution + silica sand, and (CH4+ C2H6+ C3H8) gas mixture + SDS aqueous solution systems, where SDS was added to increase the hydrate formation rate without evident influence on the equilibrium conditions. The feasibility and reliability of the step-heating method, especially for porous media systems and gas mixtures systems were determined. The experimental data for CO2+ silica sand data shows that the equilibrium pressure will change significantly when the particle size of silica sand is less than 96 μm. The formation equilibrium pressure was also measured by the reformation of hydrate.
equilibrium condition, hydrate, step-heating, sodium dodecyl sulfate, silica sand
1 INTRODUCTION
Gas hydrate is a kind of nonstoichiometric clathrate crystals formed from low molecular weight gases and water under the conditions of low temperature and high pressure. Such gases include light hydrocarbons (CH4, C2H6, C3H8, and C2H4,.), CO2, hydrogen sulfide, and nitrogen,. Natural gas hydrate exists both onshore in permafrost zone and offshore under the ocean bottom widely. It has been considered to be future substitute energy due to its estimated giant amounts in nature [1]. The equilibrium conditions of natural gas hydrate are necessary for locating hydrate occurring regions and estimating the total amount of gas hydrate in nature. Equilibrium conditions of gas hydrate in inhibitor or electrolyte containing systems are also necessary for safe production of gas/oil industries [2, 3].
In hydrate containing system, the existing phases may include gas (V), ice (I), liquid water (LW), liquid hydrocarbon (LH) and three structures hydrate (sI, sII, sH). According to the Gibbs phase rule, for hydrate formed by pure gas, the number of degrees of freedom is 1 if three phases exist in the system. That is to say the equilibrium pressure is unique at a determined temperature when three phase equilibrium is attained. As to gas mixtures, the equilibrium conditions are related to gases compositions. Three kinds of methods, isothermal pressure searching method, isobaric temperature searching method and isochoric curve method [4-8], are in general used to measure the equilibrium conditions of gas hydrate above ice point. One way of the isochoric curve method, called continuous-heating method, has also been used to measure hydrate equilibrium conditions in porous media systems [9-12].
In comparison, the other way of the isochoric curve method, called step-heating method, is significantly more reliable and repeatable than conventional continuous-heating method for porous media systems [13-16]. After the formation of hydrate, temperature is set to an interest value and the system pressure is adjusted to be slightly below the estimated dissociation pressure of the hydrate. Part of hydrate dissociates to establish the H-I-V or the H-LW-V equilibrium at a certain temperature. Afterward, the temperature is raised to another interest value and new pressure-temperature equilibrium is determined.
In this work, step-heating method was applied to bulk water, silica sand, and gas mixture hydrate systems. A kind of anionic surfactant was added to shorten the hydrate formation time. Hydrate equilibrium conditions were also measured by the reformation of hydrates. For gas mixture system, compositions of gas at every equilibrium stage were analyzed. This kind of measurement method exactly fits the meaning of “phase equilibrium” and it is expected to become a common method for hydrate equilibrium conditions measurement.
2 EXPERIMENTAL
2.1 Apparatus
The experimental apparatus used in this work is similar to that used in our previous papers [8, 17-19], and the schematic diagram is shown in Fig. 1. The volume of the cylindrical transparent sapphire cell, which is the critical part of the apparatus, is about 50 cm3(the inner diameter is 25.4 mm and the length is 100 mm). As shown in Fig. 1, the sapphire cell is held by two flanges with gas inlet, liquid inlet, and thermal resistance ports. A movable piston driven by a hand volume pump is used to change the volume of the cell or compact the sample. The usually used hydraulic transmission fluid is aqueous solution of glycol. A magnetic stirrer for accelerating the equilibrium process could be chosen for aqueous liquid systems. The pressure is measured by MIDA-OEM pressure transducer manufactured by AdAstrA Company (Russia) with a precision of 0.1%. Temperature in the sapphire cell is measured by PT-100 platinum resistance thermometer with the precision of 0.1 K. The air bath temperature could be stable within ±0.1 K.
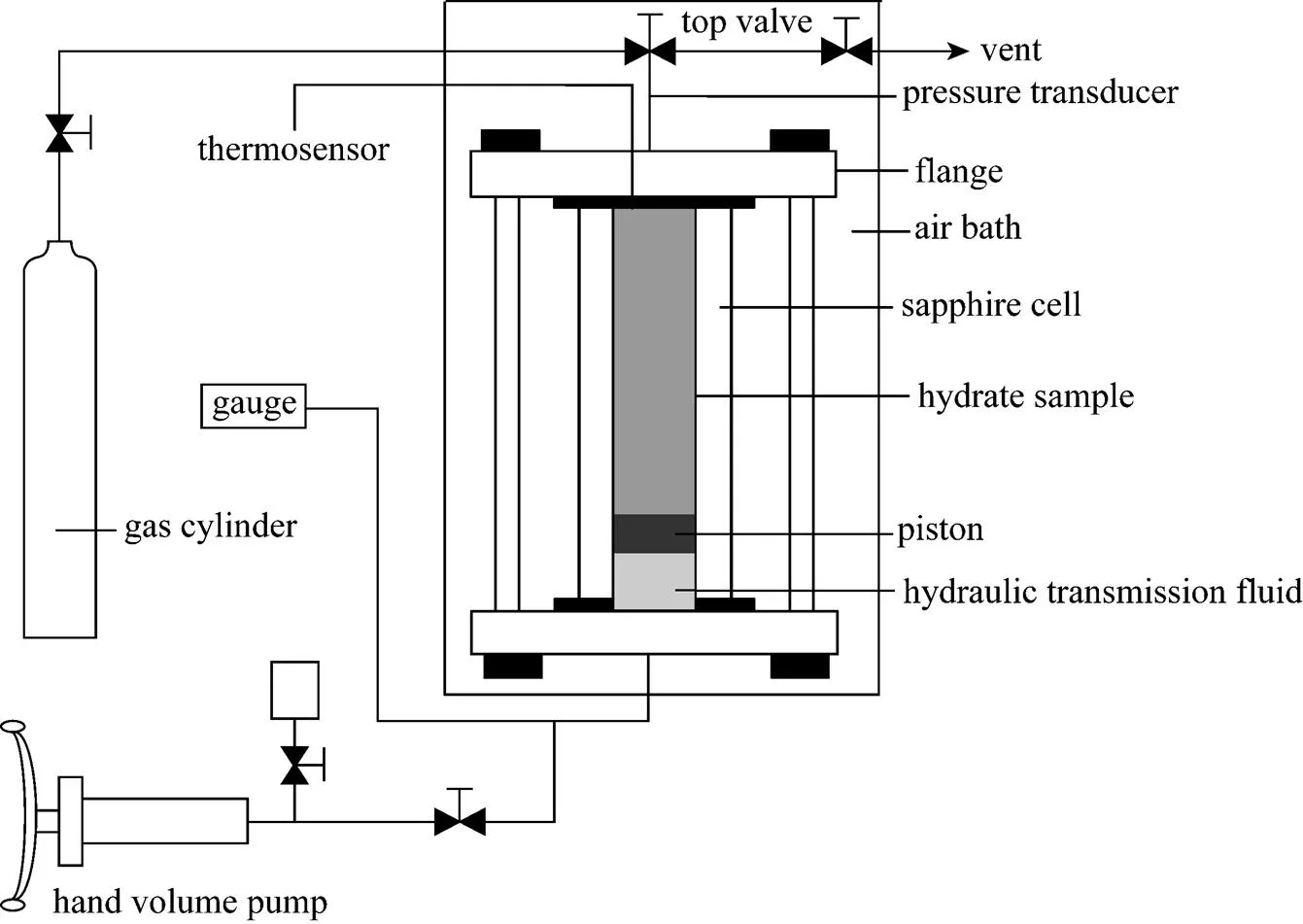
Figure 1 Schematic of experimental apparatus
2.2 Materials and preparation of samples
Analytical grade CH4(99.99%), C2H6(99.95%), C3H8(99.95%), and CO2(99.95%) supplied by Beifen Gas Industry Corporation were used in preparing the gas phase component. For gas mixtures system, a Hewlett-Packard gas chromatograph (HP 6890) was used to analyze the composition. The sodium dodecyl sulfate (SDS, analytical reagent) was supplied by Beijing Reagents Corporation. An electronic balance with a precision of ±0.1 mg was used in measuring SDS weight. Deionized distilled water was used in preparing the aqueous solution. The silica sand of various meshes came from natural river sand.
2.3 Experimental procedure
Firstly, a desired quantity of the experimental samples, such as deionized distilled water, surfactant (SDS) aqueous solution, and/or SDS aqueous solution saturated silica sand with different size, was filled in the sapphire cell according to different experimental purpose. Afterward, the sapphire cell was sealed with flanges and equipped in the air bath. The gas space of the cell was purged with the prepared feed gas and evacuated to ensure the absence of air. Then the air-bath temperature was adjusted to the desired value. Once the cell temperature was kept constant, the gas sample was charged into the cell until the desired pressure was achieved. The gas sample might be pure CH4, pure CO2, or mixtures of CH4(89.3%, by mol) + C2H6(7.8%, by mol) + C3H8(2.9%, by mol) in different runs. The feed pressure was much higher than the estimated equilibrium pressure value at the specified temperature to ensure the hydrate formation. The estimated equilibrium pressure is calculated by CSMGem [1]. If it was bulk deionized water, the magnetic stirrer could be turned on to induce and accelerate the hydrate formation.
After the pressure drop was less than 0.01 MPa·h-1, at which hydrate growth was thought at a very low rate, the system pressure was decreased slowly by discharging the remaining gas in the cell. In the first dissociation stage, the system pressure was decreased to about 0.5 MPa lower than the estimated equilibrium pressure at the first experimental temperature. The top valve of the cell was then closed to make it be an enclosed system. Part of the hydrate would then dissociate into gas and water. The changes of system pressure and temperature of the samples with elapsed time were recorded on-line by computer. If the system pressure increased less than 0.01 MPa in three hours, the enclosed system was then considered as approaching equilibrium. And the current temperature and pressure was then deemed as a set of equilibrium condition data.
Afterward, the temperature of air bath was raised to the next experimental point. The residual hydrate would continue to dissociate because of the increasing temperature, and another group of-data would be obtained when equilibrium was attained. The experimental procedure was terminated until hydrate thoroughly dissociated. During the whole measurement process, gas was discharged only before the first dissociation stage. Therefore, the system was kept enclosed during the whole measurement process.

For some groups of experiments, after hydrate thoroughly dissociated, the air bath temperature was decreased to reform hydrate step by step. Similar to dissociation process, formation equilibrium was attained when pressure decreased less than 0.01 MPa in three hours. The-equilibrium data was then determined by the hydrate reformation. The reformation experiment was terminated when reaching the initial dissociation temperature.
For gas mixture systems, the compositions of gas mixtures changed with hydrate formation/dissociation because of the distillation of hydrate [20]. The composition of gas phase was no longer the same as the initial feed gas. In this work, gas compositions analyzed by gas chromatogram at the end of each equilibrium stage was assumed as the equilibrium composition at the equilibrium pressure and temperature.
3 RESULTS AND DISCUSSION
3.1 Pure gas + deionized water system
The equilibrium conditions of pure CH4and CO2hydrate in deionized water were firstly measured to verify the reliability of the experimental method. The initial formation conditions were 273.4 K, 8.00 MPa for CH4system and 273.4 K, 3.30 MPa for CO2system, respectively. With the higher driving force, most of the water transformed to hydrate in 1-2 days. The coexistence of hydrate, liquid water and gas in the transparent sapphire cell was observed by naked eyes. The equilibrium conditions were obtained according to the above experimental procedure described in Section 2.3. The corresponding variations of pressure and temperature with time for CH4hydrate dissociation are shown in Fig. 2.
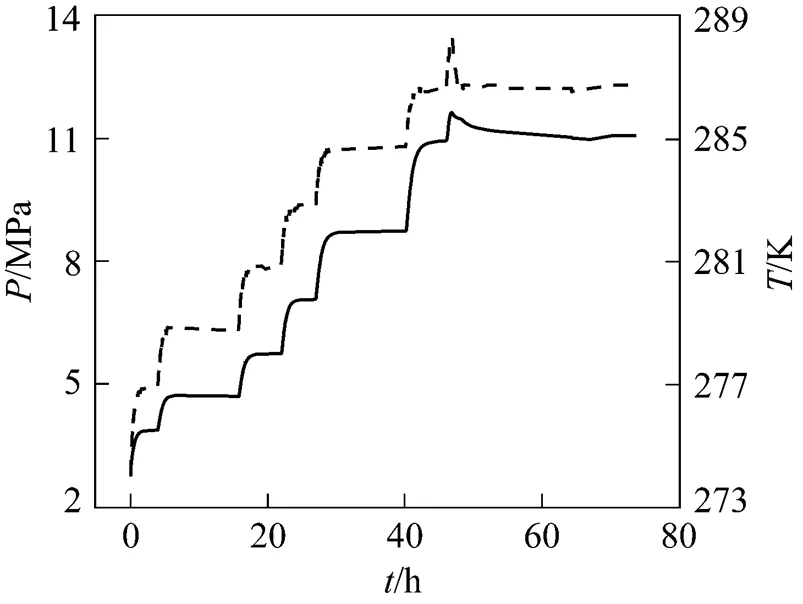

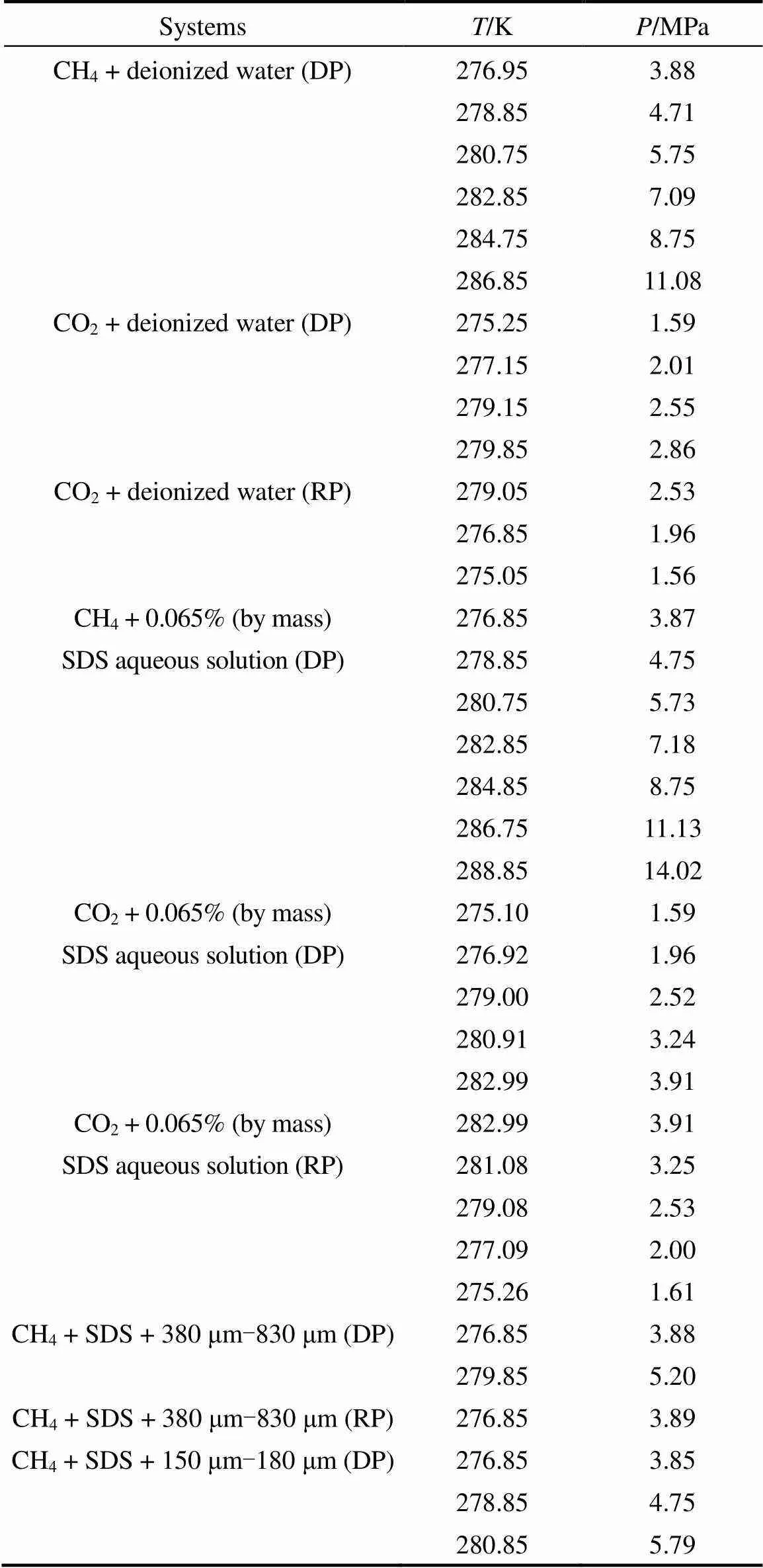
Table 1 Equilibrium conditions measured in this work

Table 1 (Continued)
Note: DP, phase equilibrium data determined by dissociation process; RP, phase equilibrium data determined by reformation process.

Figure 3 Comparison of measured equilibrium conditions in deionized water with literature data [1]■ CH4measured;○ CH4literature;▲ CO2measured;▽ CO2literature

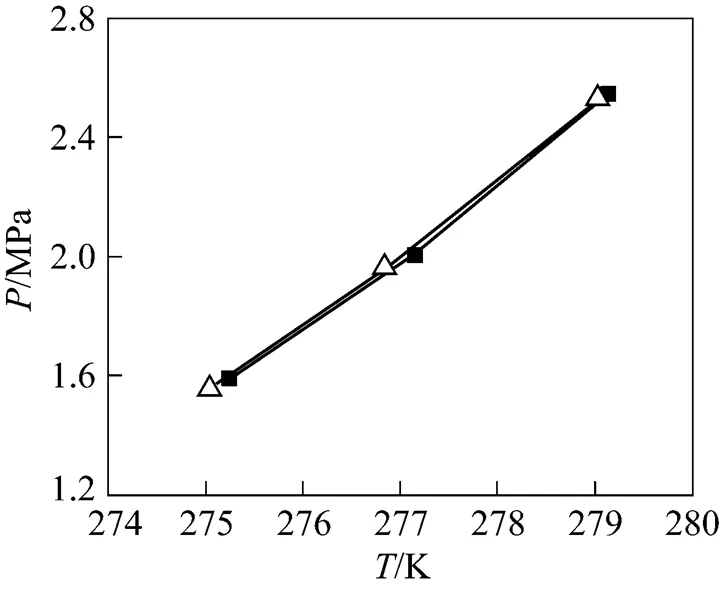
Figure 5 Comparison of equilibrium conditions for CO2+ deionized water system■ dissociation;△ reformation
The obtained-equilibrium data for pure CH4and CO2in deionized water system are listed in Table 1 and shown in Fig. 3. The literature data [1] for the two systems are also depicted in Fig. 3 for comparison. The measured data and those in literatures are in good agreement. It is known that almost all the available literature data are measured either by isothermal pressure searching method or by isobaric temperature searching method [4-8]. These two methods have the common procedure of “searching” by changing either the pressure or temperature. It will take a long time on approaching the final pressure or temperature equilibrium. It may need 1-2 days to obtain a group of-data. In addition, the equilibrium conditions obtained from these two methods are determined by observing the existence of trace hydrate formation or dissociation. It is actually the formation conditions but not the equilibrium conditions although the difference among them is small and could always be neglected. The step-heating method, however, exactly determines the equilibrium conditions.
For CO2+ deionized water hydrate system, hydrate was reformed to check the measured dissociation equilibrium-data. The variation of pressure and temperature with time, and the equilibrium data obtained are shown in Fig. 4 and Fig. 5, respectively. It could be found that the formation equilibrium conditions are coincided with dissociation equilibrium conditions, except a longer time is needed for one set of-data during the reformation process.
3.2 Pure gas + SDS aqueous solution system
SDS is a kind of hydrate formation promoter used to shorten the induction time and enhance the formation rate of hydrate [21-24]. For the step-heating method, adequate amount of hydrate should be prepared for the subsequent procedure. For bulk water system, the formation rate is slow and a great deal of hydrate is hard to be prepared quickly even stirring is used. For porous media system, stirring is impossible. SDS could be added in water to form hydrate quickly. The influence of SDS on the hydrate equilibrium condition was firstly checked.
The equilibrium condition data of CH4+ 0.065% (by mass) SDS aqueous solution and CO2+ 0.065% (by mass) SDS aqueous solution are listed in Table 1 and shown in Fig. 6. In comparison, the experimental results of CH4and CO2in deionized water systems are also illustrated. It could be found that, in the experimental temperature and pressure range, equilibrium pressures hardly change after adding SDS at the same temperatures. This has been affirmed in CH4+ 0.02% (by mass) SDS aqueous solution system by other researchers [25]. Therefore, 0.065% (by mass) SDS aqueous solution was used in the subsequent measurementassuming it having no influence on the phase equilibrium. During the measurements, it was found that the nucleation and growth of hydrate occurred readily.
Hydrate was reformed in CO2+ 0.065% (by mass) SDS aqueous solution system. The profiles of pressure and temperature during the whole dissociation and reformation processes are shown in Fig. 7, and the equilibrium data obtained from dissociation and reformation are shown in Fig. 8. It could be seen that good agreements are obtained for the two measurement process as shown in deionized water system.
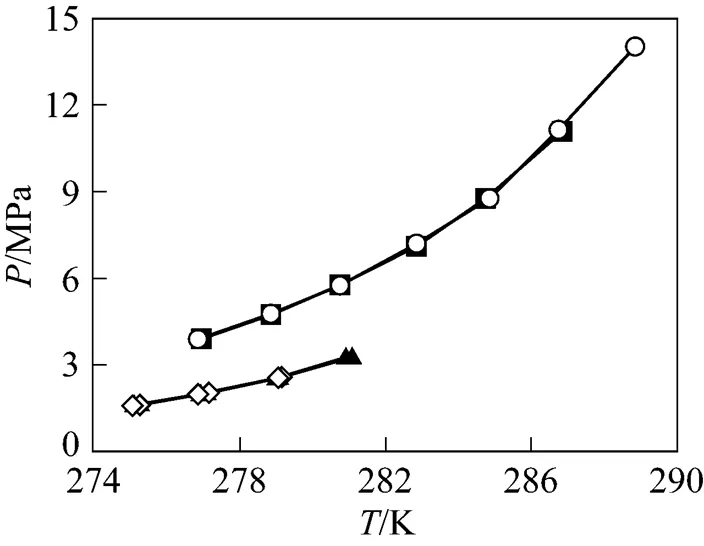
Figure 6 Comparison of CH4/CO2hydrate equilibrium conditions between SDS aqueous solutions and deionized water systems■ CH4+ deionized water;○ CH4+ 0.065% (by mass) SDS aqueous solution;▲ CO2+ deionized water;◇ CO2+ 0.065% (by mass) SDS aqueous solution


Figure 8 Hydrate equilibrium conditions of CO2+ 0.065% (by mass) SDS aqueous solution system■ dissociation;○ reformation equilibrium
3.3 Pure gas + SDS + silica sand system
It is well known that the existing searching methods mainly focus on the bulk water system, of which the hydrate formation and dissociation could be observed and confirmed by naked eye. For porous media system, the formation of hydrate can not be observed directly, so step-heating/continuous-heating method is then used. Equilibrium conditions of CH4/CO2+ silica sand systems were determined accordingly in this work. The sand used were 380-830 μm, 150-180 μm for CH4system and 96-109 μm, 80-96 μm for CO2system, respectively. SDS was used to enhance the hydrate formation rate with a concentration of 0.065% (by mass). Hydrate was reformed after dissociation for CH4system. The equilibrium conditions of CH4/CO2+ 0.065% (by mass) SDS aqueous solution + silica sand system determined by dissociation and reformation process are listed in Table 1 and shown in Figs. 9 and 10. Excellent coincidence could be found.

Figure 9 Hydrate equilibrium conditions of CH4+ 0.065% (by mass) SDS aqueous solution + silica sand system ■ 150-180 μm, dissociation;○ 150-180 μm, reformation; ▼ 380-830 μm, dissociation;▲ 380-830 μm, reformation;△ bulk water
Figure 10 Hydrate equilibrium conditions of CO2+ 0.065% (by mass) SDS aqueous solution + (80-96) μm/(96-109) μm silica sand system ■ 96-109 μm;○ 80-96 μm;△ no silica sand


3.4 Gas mixtures + 0.065% (by mass) SDS aqueous solution system
The measured equilibrium conditions of CH4+ C2H6+ C3H8+ 0.065% (by mass) SDS aqueous solutions at 275.2 K are listed in Table 2. The initial composition of the mixture is CH4(89.3%, by mol) + C2H6(7.8%, by mol) + C3H8(2.9%, by mol). Equilibrium gas compositions at the equilibrium pressure and temperature are also listed in Table 2. It could be found that the C3H8mole fraction increases as hydrate dissociates. The lowest mole fraction of C3H8is 0.45% at the first equilibrium stage. Table 2 also lists the equilibrium pressures at every stage calculated by CSMGem [1]. The largest difference between measured and calculated pressure is 0.17 MPa at the first equilibrium. The relative larger difference at the first stage might be due to the coexistence of sI and sII hydrate for the mixture with higher CH4and lower C3H8contents [26].
4 CONCLUSIONS
Step-heating method was applied to several systems using the cylindrical transparent sapphire cell device. The systems include: CH4/CO2+ deionized water, CH4/CO2+ SDS aqueous solution, CH4/CO2+ SDS aqueous solution + silica sand, (CH4+ C2H6+ C3H8) + SDS aqueous solution. Comparisons of experimental data with the literature value show the feasibility and reliability of the step-heating method, especially for porous media systems and gas mixtures systems. Besides, it could be applied to almost all kinds of systems, this kind of measurement method is also simpler and less time consumption compared with the other searching methods.
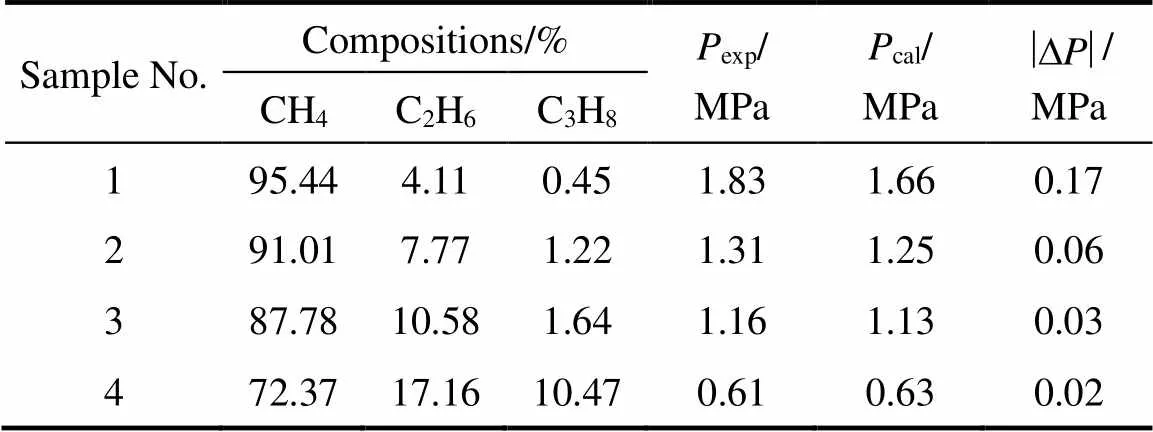
Table 2 Hydrate equilibrium conditions of CH4 + C2H6 +C3H8 + 0.065% (by mass) SDS aqueous solutions at 275.2 K
1 Sloan, E.D., Koh, C.A., Clathrate Hydrates of Natural Gases, 3rd edition, CRC Press, New York (2008).
2 Makogon, Y.F., Hydrates of Hydrocarbons, Pennwell Publishing Company, Tulsa Oklahoma (1997).
3 Qiu, J.H., Guo, T.M., “Kinetics of methane hydrate formation in pure water and inhibitor containing systems”,...., 10 (3), 316-322 (2002).
4 Nixdorf, J., Oellrich, L.R., “Experimental determination of hydrate equilibrium conditions for pure gases, binary and ternary mixtures and natural gases”,, 139, 325-333 (1997).
5 Kang, S.P., Chun, M.K., Lee, H., “Phase equilibria of methane and carbon dioxide hydrates in the aqueous MgCl2solutions”,, 147, 229-238 (1998).
6 Fan, S.S, Liang, D.Q., Guo, K.H., “Hydrate equilibrium conditions for cyclopentane and a quaternary cyclopentane-rich mixture”,..., 46 (4), 930-932 (2001).
7 Majumdar, A., Mahmoodaghdam, E., Bishnoi, P.R., “Equilibrium hydrate formation conditions for hydrogen sulfide, carbon dioxide, and ethane in aqueous solutions of ethylene glycol and sodium chloride”,..., 45, 20-22 (2000).
8 Sun, C.Y., Chen, G.J., Lin, W., Guo, T.M., “Hydrate formation conditions of sour natural gases”,..., 48, 600-602 (2003).
9 Cha, S.B., Ouar, H., Wildeman, T.R., Sloan, E.D., “A third-surface effect on hydrate formation”,..., 92, 6492-6494 (1988).
10 Uchida, T., Ebinuma, T., Ishizaki, T., “Dissociation condition measurements of methane hydrate in confined small pores of porous glass”,..., 103, 3659-3662 (1999).
11 Seo, Y.T., Lee, H., “Multiple-phase hydrate equilibria of the ternary carbon dioxide, methane, and water mixtures”,..., 105, 10084-10090 (2001).
12 Dicharry, C., Gayet, P., Marion, G., Graciaa, A., Nesterov, A.N., “Modeling heating curve for gas hydrate dissociation in porous media”,..., 109, 17205-17211 (2005).
13 Anderson, R., Llamedo, M., Tohidi, B., Burgass, R.W., “Experimental measurement of methane and carbon dioxide clathrate hydrate equilibria in mesoporous silica”,..., 107, 3507-3514 (2003).
14 Handa, Y.P., Stupin, D., “Thermodynamic properties and dissociation characteristics of methane and propane hydrates in 70Å radius silica gel pores”,..., 96, 8599-8603 (1992).
15 Zhang, W., Wilder, J.W., Smith, D.H., “Methane hydrate-ice equilibria in porous media”,..., 107, 13084-13089 (2003).
16 Seshadri, K., Wilder, J.W., Smith, D.H., “Measurements of equilibrium pressures and temperatures for propane Hydrate in silica gels with different pore-size distributions”,..., 105, 2627-2631 (2001).
17 Ma, C.F., Chen, G.J., Wang, F., Sun, C.Y., Guo, T.M., “Hydrate formation of (CH4+ C2H4) and (CH4+ C3H6) gas mixtures”,, 191, 41-47 (2001).
18 Zhang, L.W., Huang, Q., Sun, C.Y., Ma, Q.L., Chen, G.J., “Hydrate formation conditions of methane + ethylene + tetrahydrofuran + water systems”,..., 51, 419-422 (2006).
19 Huang, Q., Sun, C.Y., Chen, G.J., Yang, L.Y., “Hydrate formation conditions of (CH4+ CO2+ H2S) ternary sour natural gases”,.... (), 56 (7), 1159-1163 (2005). (in Chinese)
20 Luo, Y.T., Zhu, J.H., Chen, G.J., “Numerical simulation of separating gas mixtures via hydrate formation in bubble column”,...., 15 (3), 345-352 (2007).
21 Zhong, Y., Rogers, R.E., “Surfactant effects on gas hydrate formation”,..., 55, 4175-4187 (2000).
22 Karaaslan, U., Uluneye, E., Parlaktuna, M., “Effect of an anionic surfactant on different type of hydrate structures”,...., 35, 49-57 (2002).
23 Lin, W., Chen, G.J., Sun, C.Y., Guo, X.Q., Wu, Z.K., Liang, M.Y., Chen, L.T., Yang, L.Y., “Effect of surfactant on the formation and dissociation kinetic behavior of methane hydrate”,..., 59, 4449-4455 (2004).
24 Sun, C.Y., Chen, G.J., Ma, C.F., Huang, Q., Luo, H., Li, Q.P., “The growth kinetics of hydrate film on the surface of gas bubble suspended in water or aqueous surfactant solution”,.., 306, 491-499 (2007).
25 Gayet, P., Dicharry, C., Marion, G., Graciaa, A., Lachaise, J., Nesterov, A., “Experimental determination of methane hydrate dissociation curve up to 55 MPa by using a small amount of surfactant as hydrate promoter”,..., 60, 5751-5758 (2005).
26 Schicks, J.M., Naumann, R., Erzinger, J., Hester, K.C., Koh, C.A., Sloan, E.D., “Phase transitions in mixed gas hydrates: Experimental observations versus calculated data”,..., 110, 11468-11474 (2006).
2008-10-06,
2009-04-17.
the National Natural Science Foundation of China (20676145, U0633003), the National Basic Research Program of China (2009CB219504) and the Program for New Century Excellent Talents in University of the State Ministry of Education.
** To whom correspondence should be addressed. E-mail: gjchen@cup.edu.cn
 Chinese Journal of Chemical Engineering2009年4期
Chinese Journal of Chemical Engineering2009年4期
- Chinese Journal of Chemical Engineering的其它文章
- Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
- Phase Equilibrium of Isobutanol in Supercritical CO2
- Conversion of Methane by Steam Reforming Using Dielectric-barrier Discharge*
- Permeability and Selectivity of Sulfur Dioxide and Carbon Dioxide in Supported Ionic Liquid Membranes*
- Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid*
- Model Study on a Submerged Catalysis/Membrane Filtration System for Phenol Hydroxylation Catalyzed by TS-1*
