Isolation of Cordyceps ophioglossoides L2 from Fruit Body and Optimization of Fermentation Conditions for Its Mycelial Growth*
XU Qinqin (许勤勤), LÜ Longxian (吕龙贤), CHEN Shaoyun (陈少云), ZHENG Jing (郑静), ZHENG Gaoli (郑高利)and LI Yongquan (李永泉),**
Isolation ofL2 from Fruit Body and Optimization of Fermentation Conditions for Its Mycelial Growth*
XU Qinqin (许勤勤)1, LÜ Longxian (吕龙贤)1, CHEN Shaoyun (陈少云)1, ZHENG Jing (郑静)1, ZHENG Gaoli (郑高利)2and LI Yongquan (李永泉)1,**
1 College of Life Sciences, Zhejiang University, Hangzhou 310058, China 2 Institute of Materia Medica, Zhejiang Academy of Medical Sciences, Hangzhou 310013, China
A strain isolated from the fruiting body of a fungus parasitized onwas identified asbased on the morphological characteristics and the analysis of ITS-5.8s rDNA sequence. The optimal medium composition (g·L-1), containing sucrose 66.0, yeast powder 10.0, silkworm chrysalises digest 30.0, MgSO4·7H2O 0.4, and KH2PO40.4, was found using fractional factorial design and a central composite design, and the optimization of cultural conditions obtained a result of seed age 6 days, inoculum size 6% (by volume), initial pH 5.6, temperature 24°C, shaking speed 160 r·min-1 by one-factor-at-a-time method. The maximum biomass reached about 20.2 g·L-1 after 90 hours culture under the optimal conditions. Elementary pharmacological activities showed that mycelia of.L2 from submerged culture promoted uterus growth in estrogen- depleted mice. In the 15-litre scale-up fermentation, the mycelial biomass was around 19.1 g·L-1, indicating a promising prospect for this biotechnology and the potency to develop its medical value.
, identification, fermentation, optimization, fractional factorial design, central composite design
1 INTRODUCTION
have been used in China for thousands of years as a tonic food. Recently, they have become more and more attractive as functional food and sources of drugs in several countries [1, 2].is a unique species of, which parasitized on certain types of[3, 4]. It was first described in New China Compendium of Materia Medica and the wild mycelia were usually used as medicined diet for women’s Uterine Bleeding, the dysfunctional condition, and the menstruation syndrome in folk [5, 6]. Recent studies have discovered its mycelia combined multiple pharmacological effects, including antitumor activity from a protein-bound polysaccharide extract from its mycelia, estrogenic activity and anti-ageing activity also from its mycelia extract [7-9].
However, the growth of wildis restricted by season, region, climate, and especially host. Meanwhile, their collection will cause catastrophic destroy on vegetative cover as described by Shih. in other species of[10]. In practice, it has been nearly impossible to get the crudefor medical or experimental material till now. Some efforts were made on the artificial cultivation, however, this process proved expensive and time consuming. Since the submerged culture was a simple, fast way to produce fungal biomass with nearly the similar pharmacological efficacy of wild growth [11-13], it may also be an efficient method for mycelial production of.. To the best of our knowledge, there is still no study on the fermentation technique of.. Therefore, it is timely to isolate the strain from crudeand to achieve a large-scale production by fermentation.
This study aims to isolate a fungus from fruit body of.. Then, the medium composition and culture conditions for submerged culture are studied using statistical method. After that, this biotechnology is scaled up in a 15-litre fermentor. To confirm whether the mycelia after fermentation has estrogen-like activities, elementary pharmacological activities experiment is studied.
2 MATERIALS AND METHODS
2.1 Isolation and identification of the fungus
The fruiting body was obtained from the forest of Xi Shuang Ban Na in Yunnan Province, Southern China. It was thoroughly washed by axenic water several times, cut to pieces, and placed on solid potato sucrose agar (PSA)containing (g·L-1) potato 200, sucrose 20, agar 20, and distilled water, cultured at 25°C for 8-12 days. Then, the strain was stored in a refrigerator at 4°C, and subcultured every 3 months. This isolated strain was phylogenetically identified by ITS-5.8S rDNA sequencing analysis. The nucleotide sequence obtained was compared with that of GenBank using the National Center for Biotechnology Information (NCBI) Blast program. Sequence homology was comparatively analyzed using the Clustal X program. Then, the phylogenetic analysis was performed using Neighbor-Joining (N-J).
2.2 Inoculums preparation and flask cultures
The mycelia were removed by punching them out from the slant with a sterilized cutter. They were transferred into a liquid medium containing (g·L-1) saccharine 30.0, yeast powder 5.0, peptone 5.0 MgSO4·7H2O 1.0, KH2PO4 0.5, and distilled water, as described by Xiao[14]. After cultivation for 3-5 days at 25°C on a rotary shaker (170 r·min-1.), the mycelia were used as the liquid seed to be inoculated within the submerged medium. The flask culture experiments were performed in a 500ml flask containing 100 ml of the medium after inoculating with 10% (by volume) of the seed culture.
2.3 Optimization of media and culture conditions
To determine the optimal culture requirements, the following factors were investigated using the one-factor-at-a-time method, namely, selection of optimal carbon and nitrogen resource, temperature, initial pH value, medium capacity, agitation rate, seed age, and inoculum size based on basal medium. According to the results of the single factor experiment, the fractional factorial design (FFD) and central composite design (CCD) were used to find the relationship between different variables of medium components and their concentrations for mycelial growth.
2.4 Experimental design
A 25-2 fractional factorial design (FFD) leading to 12 sets of experiments, performed in triplicate, was used to verify the most significant factors affecting the biomass. The variables were coded according to the following equation:

where,is the coded value of an independent variable,xis the real value of an independent variable,0 is the real value of an independent variable at the center point, and Δis the step change value. The range and the levels of the variables’ both coded values and natural values investigated in this study are given. The biomass is considered as the dependent variable or response (), and then, the focus is on the critical subset of media components; central composite design (CCD) with five-coded levels is performed to optimize the critical components and maximize the mycelial biomass. The quadratic model for predicting the optimal point is expressed according to the following equation:

where,is the response variable,0,b,,are the regression coefficient variables, for intercept, linear, quadratic, and interaction terms, respectively, andxandare independent variables. Data were analyzed using SAS 8.0 software. These designs can decrease the experiment times, screen the most essential factor quickly, and estimate the major effects and the part interaction terms [15, 16].
2.5 Batch fermentation in 15-litre fermentors
The batch fermentation was carried out in a 15-litre fermentor equipped with two 6-bladed disc impellers (Biostar, Shanghai GuoQiang, China), oxygen, and pH electrodes, under the following conditions: working medium volume 8 L, inoculation volume 6% (by volume), culture temperature 23°C, initial pH 5.8, aeration rate 4.8 L·min-1, and agitation speed 160 r·min-1.
2.6 Elementary pharmacological activities experiment
Dry mycelia prepared from fermentation were weighed and then mixed with deionized water. The suspension was used in screening of pharmacological activities. Cleaning degree ICR (Institute of Cancer Research) mice, provided by the experimental animal center of Zhejiang Province, China [No. SCXK (Zhe) 2003-0001], were used in this study. The animals were housed under normal laboratory conditions and maintained on standard rodent chow. For determination of elementary pharmacological activities experiment, the mice were pretreated by force feeding using a stomach tube with the suspension of wet mycelial powder.
Sixty immature mice (ICR 8-10 g) were randomly assigned into 5 groups: control, positive group, and varying doses of wet powder of.L2 2.86 g·kg-1, 1.43 g·kg-1, 0.715 g·kg-1, respectively, for immature mouse uterotropic bioassay. Mice were fed for 5 consecutive days and killed by cervical dislocation after the last feed for 24 hours; then, the uterine wet mass: body mass ratios were calculated and the ratios of thymus gland, spleen, and ovary mass to body mass were also studied simultaneously. Meanwhile, fifty female mice (ICR 22-26 g) were ovariectomized (OVX) for 7 days and randomly divided into 5 experimental groups and control groups while the other 10 were obtained as sham-operation (SHAM) group for ovariectomized mice uterotropic bioassay. Seven days later, the mice were killed and the body and uteri were weighed.
2.7 Analytical methods

3 RESULTS AND DISCUSSION
3.1 Isolation and identification of strain Cordyceps ophioglossoides L2
The colony of the isolated strain on PSA medium was white and floccose throughout on the surface after culture at 25°C for 15 days [Fig. 1 (a)]. The hyphae of mycelia were Hyaline, smooth-walled, and septate [Fig. 1 (b)]. They usually had irregular hyaline cylindricalconidia [Fig. 1 (c)] at the end of hyphae. Fig. 2 shows the approximate phylogenetic position of the strain in. It was found to be similar toby analysis of the ITS-5.8S rDNA sequence (GenBank accession No. EU586043) and phylogenetic relationships inferred through the alignment and cladistic analysis of homologous nucleotidesequences of known. Based on the morphological characteristics and the sequencing result, strain L2 was identified as a strain of, and named.L2. It is now stored in the China General Microbiological Culture Collection Center (CGMCC), with the strain number 1146.

Figure 1 Microscopic imaging of.in slant and liquid cultures
Figure 2 The phylogenetic dendrogram for.L2 and related strains based on the ITS-5.8S rDNA sequence
(Numbers in parentheses are accession numbers of published sequences. Bootstrap values were based on 1000 replicates.was used as the outgroup)
3.2 Effect of carbon and nitrogen source
The effects of carbon and nitrogen sources on the mycelial growth of.are presented in Fig. 3. Among the eight carbon sources tested, sucrose and glucose were good candidates for the carbon source owing to their ease-of-use and low cost. Since growth on PSA was better than that on potato dextrose agar (PDA), sucrose was finally selected as the carbon source in the following experiments. Also, growth was detected with dextrin and corn flour, but the dry mass of mycelium was hard to calculate owing to their insolubility. Very weak growth was observed on starch or maltose. This result is not in accordance with the nutritional requirements of other species of cordyceps in submerged cultures. Kim. [17] observed a high level of mycelial growth ofC738 in media containing maltose in submerged cultures.
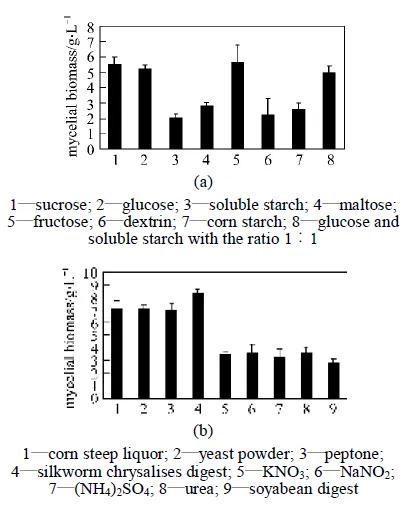
Figure 3 The effects of the carbon sources and the nitrogen sources on the mycelial growth of.(All experimental data are mean ± SD of triple determinations)
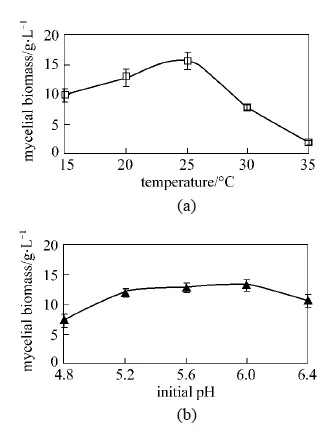
Figure 4 Effects of temperature and initial pH on mycelial growth in shake flask culture of.(All experimental data are mean ± SD of triple determinations)
Among nine nitrogen sources examined, the corn steep liquor, silkworm chrysalises digest, yeast powder, or peptone produced a high level of biomass. As seen in Fig. 3 (b), no significant difference was discovered between any two of these organic nitrogen sources, but the inorganic nitrogen sources produced relatively lower mycelial growth. This is consistent with the results from previous studies that a single amino acid was not a good nitrogen source for the mycelial growth in several genera of fungi [17, 18]. Yeast powder and silkworm chrysalises digest were selected in the following experiments for the low cost and the high biomass.
3.3 Effect of temperature and initial pH
To find out the optimal temperature for mycelial growth,.L2 was cultivated at various temperatures ranging from 15 to 35°C, and the maximum mycelial biomass (15.6 g·L-1) was observed at 25°C [Fig. 4 (a)]. The very low biomass during growth at 30-35°C indicated that.may be a relatively low temperature propensity fungus, and this is consistent with its underground growth environment. The effects of initial pH on the mycelia biomass are given in Fig. 4 (b). It can be seen that the initial pH of the medium ranging from 5 to 6 was favored for biomass (12.0 g·L-1 to 13.0 g·L-1). The optimal initial pH of 5.6 was found in this experiment. The favor of acidic pH may also relate to its natural growing environment. The medium pH may affect cell membrane function, cell morphology and structure, the uptake of various nutrients, and product biosynthesis [19]. It has been reported that acidic pH was more suitable for mycelial growth for various kinds of ascomycetes, includingsp [20].
3.4 Effect of medium capacity and agitation rate
As seen in Fig. 5, the mycelial biomass (about 12.0 g·L-1) displayed no evident changes in various medium capacities ranging from 100 to 200 ml. Simultaneously, the mycelial biomass (about 15.0 g·L-1) showed no significant differences under the range of 120 to 180 r·min-1. Medium capacity and agitation rate are the parameters closely related to oxygen supply, which is an important factor for controlling the growth ofin a more compact pellet form [21, 22]. These results are in accordance with the filamentous form of mycelia of..

Figure 5 Effects of medium capacity and agitation rate on mycelial growth in shake flask cultures of.(All experimental data are mean± SD of triple determinations)
3.5 Effect of seed age and inoculum size
To determine the effect of seed age and inoculum size,.L2 was cultivated in the optimal medium for four different time periods (2, 4, 6, and 8 days) while varying the inoculum size (3%, 6%, 9% and 12%). As seen in Table 1, the inoculum size appeared to have little or no visible effect on the mycelial growth. In contrast, the seed age is more important for the mycelial biomass. There was no obvious progress in 6-8 days, therefore, we chose 6 days age of seed and 4%-6% (by volume) inoculum size for the mycelial growth.
3.6 Fractional factorial design and central composite design
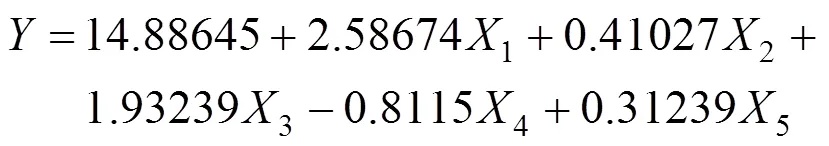


Table 1 Effects of seed age and inoculum size on mycelial growth of C. ophioglossoides
①.was cultured in shake flask cultures for 4 days at 25°C with initial pH 5.5; values are mean ± SD of triple determinations and values followed by the same letters are not significantly different by Duncan’s multiple range test (<0.05).

Table 2 Experimental design and results of FFD for mycelial biomass of C. ophioglossoides
The effects of two important factors, sucrose (1) and yeast powder(3) were further evaluated by CCD as shown in Table 4. Tables 5 and 6 shows the analysis of variance and the regression analysis for the experiment. The polynomial model for biomass yieldbiomasswas regressed by mainly considering the significant terms and was expressed by Eq. (4).
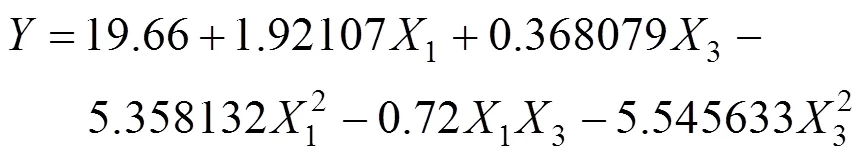
The result indicated that2 (98.09%) of the variability in the response could be explained by the model. The model predicted the maximum biomass yield of 19.84 g·L-1 that appeared at sucrose 65.34 g·L-1, yeast powder 10.11 g·L-1, respectively. To illustrate this result in more detail, we obtained the three-dimensional response surface curve and the corresponding contour plot by the statistically significant model (Fig. 6). Verification of the calculated optimum of the model [Eq. (4)] for biomass production was done with the optimal medium (g·L-1) consisting of sucrose 66.0, yeast powder 10.0, and silkworm chrysalises digest 30.0, MgSO4·7H2O 0.4, and KH2PO40.4. The maximum biomass 20.0 g·L-1(average of three repeats) was more than three times higher than that at the basal medium.
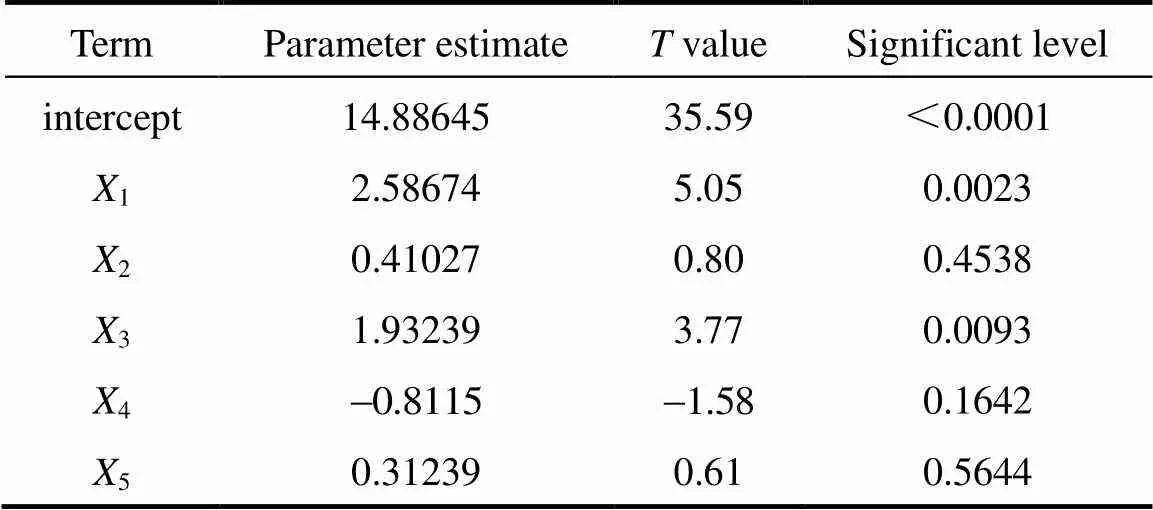
Table 3 Results of the fractional factorial design regression analysis for mycelial biomass of C. ophioglossoides
Note:1, sucrose;2, silkworm chrysalises digest;3, yeast powder;4, MgSO4·7H2O;5, KH2PO4.
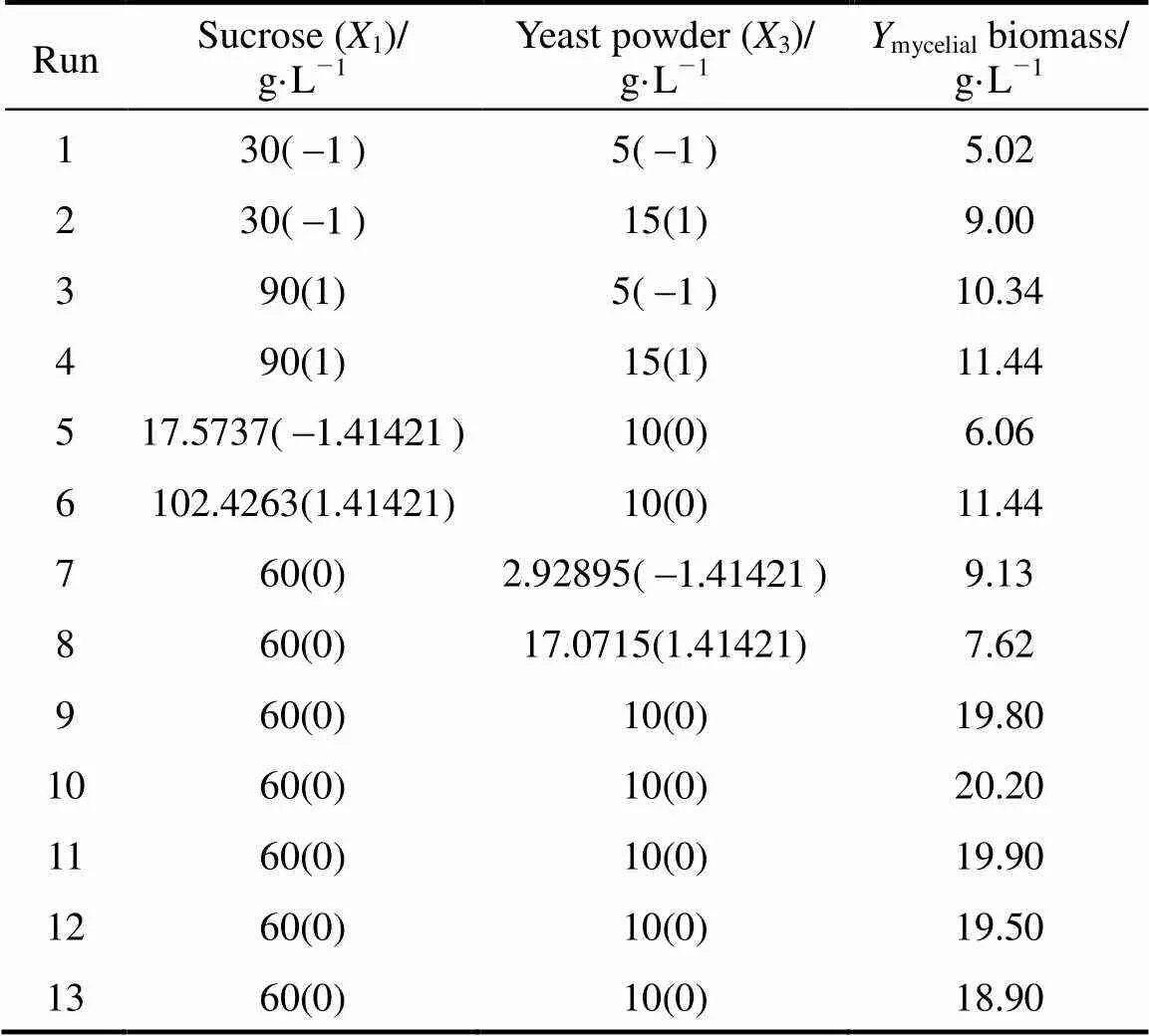
Table 4 Experimental design and results of the central composite design
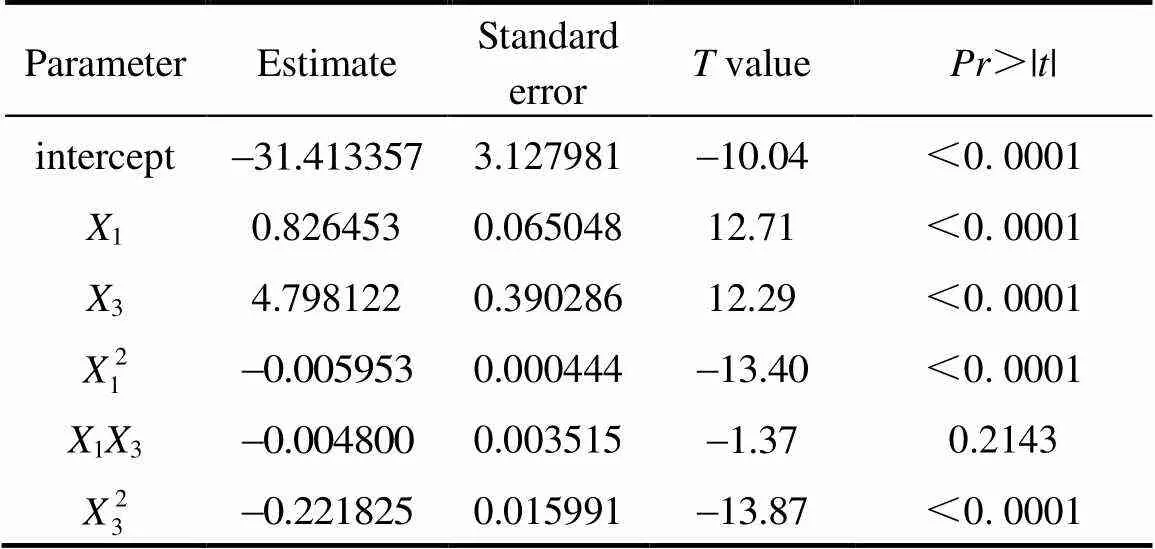
Table 5 Result of regression analysis of CCD for optimization of mycelial biomass of C. ophioglossoides
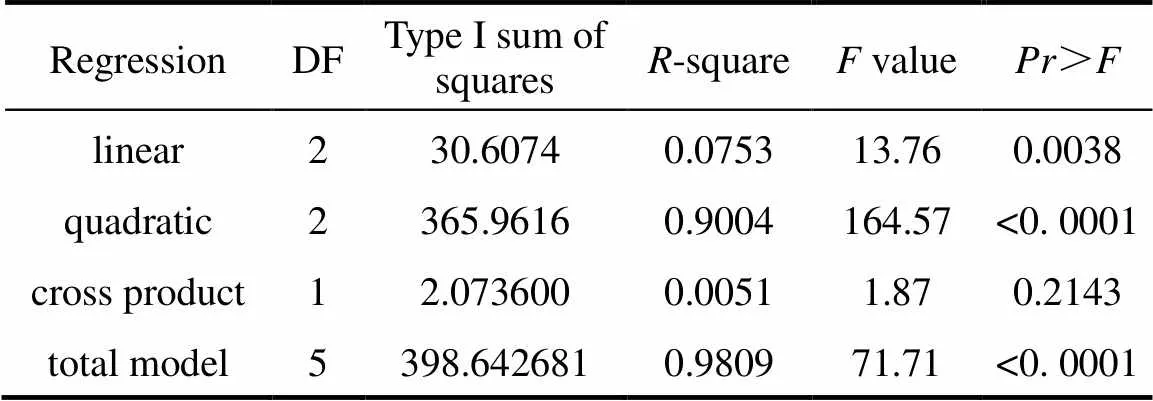
Table 6 Analysis of variance (ANOVA) for the fitted quadratic polynomial model for optimization of mycelial biomass of C. ophioglossoides

Figure 6 Three-dimensional curve indicating the effect of sucrose and yeast powder on mycelial biomass of.in the RSM-optimized medium
3.7 Batch fermentation
Figure 7 shows the time course of 15-litre batch fermentation, including dissolved oxygen (DO) and pH value recorded during the whole fermentation process in the optimized fermentation by the software of fermentor itself. The values of the parameters in the first 20 hours decreased sharply, and the logarithmic phase requiring plenty of nutrition and sufficient oxygen supplement appeared in the period from 48 h to 84 h, accompanied with rising of pH. The maximum mycelial biomass, 19.1 g·L-1 in the optimized fermentation after 84 hours cultivation, was compared with the mycelial biomass using basal culture in Fig. 8. The time profile of mycelial growth in 15-litre fermentor indicated that the first 36 h was the stage of accommodation for the new growth environment of mycelium.

Figure 7 Time profile of dissolved oxygen (DO) (■) and pH (▲) of.in 15-litre fermentor under the optimized medium and culture conditions (The initial DO of fermentation was defined as 100; all experimental data are mean ± SD of triple determinations)
Figure 8 Time profile of mycelial biomass of batch fermentation in 15-litre fermentor using the optimized medium and culture conditions (▲) and the basal culture in flask culture (■) (All experimental data are mean ± SD of triple determinations)
3.8 Estrogen-like activity of mycelia
Two models, namely immature mouse and ovariectomized mice were studied and the ratio of organ to body mass was calculated (Tables 7 and 8). The experiment result showed that mycelia of.L2-treated mice presented an activity similar with puerarin on uterus but showed nearly no change on the mass of thymus gland and spleen at low-dose. This result suggested that mycelia of.L2 from submerged culture may manifest estrogenic actions, which have potential application to therapy for gynecological conditions; these may also have application to therapy associated with illnesses in aged females including cardiovascular diseases, osteoporosis, and neuro-degenerative diseases.
4 CONCLUSIONS
In this study, the strain was isolated from the fruit body of. Consequently, the optimal medium consisting of (g·L-1) sucrose 66.0, yeast extract 10.0, silkworm chrysalis 30.0, MgSO4·7H2O 0.4, and KH2PO4 0.4, and the optimal fermentation conditions of a temperature of 24°C, an initial pH of 5.6, agitation rate 160 r·min-1, a liquid medium volume of 130 ml in a 500 ml flask, inoculated size of 6% (by volume), and 6 days seed age, were established for the first time. The experimental results indicated that the biomass was dependent mainly on the concentrations of sucrose and yeast powder, as well as the culture temperature. The result of the optimization culture conditions revealed that artificial culture had a close relation with its natural growth environment, which may help us to hit the suitable fermentation conditions more quickly when starting to cultivate a new strain. Under the optimal medium and conditions, the productivity of.L2 reached 20.2 g·L-1, which is about three times higher than that at the basal medium. This result revealed that fermentation of.is feasible. Study of elementary pharmacological activities showed that the mycelia from submerged culture had estrogen-like activity. Our laboratory is commencing studies on a more large-scale production using optimal fermentation conditions in a bioreactor with complex media and screening the chemicals with estrogenic activities contained in mycelia.
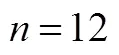
Table 7 The uterine and several organ mass of immature mice treated with mycelia of
①,② Statistical significance at P<0.05, P<0.01, respectively, compared with the value of control mice.
Note: BM, body mass; TGM, thymus gland mass; SM: spleen mass; UM: uterus mass; OM: ovary mass. The values are expressed as mean ± SEM.
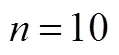
Table 8 The uterine and several organ mass of ovariectomized mice treated with mycelia of C. ophioglossoides L2 and puerarin (, )
①,② Statistical significance at P<0.05, P<0.01, respectively, compared with the value of control mice.
③,④ Statistical significance at P<0.05, P<0.01, respectively, compared with the value of SHAM.
Note: BM, body mass; TGM, thymus gland mass; SM, spleen mass; UM, uterus mass. The values are expressed as mean±SEM.
Acknowledgements
..
1 Zhu, J.S., Halpern, G.M., Jones, K., “The scientific rediscovery of an ancient Chinese herbal medicine:Part I”,...., 4, 289-303 (1998).
2 Ng, T.B., Wang, H.X., “Pharmacological actions of cordyceps, a prized folk medicine”,..., 12, 1509-19 (2005).
3 Liu, B., Medical Fungi of China, 3rd print, People’s Publisher of Shanxi, China (1984).
4 Nikoh, N., Fukatsu, T., “Interkingdom host jumping underground: Phylogenetic analysis of ntomoparasitic fungi of the genus cordyceps”,..., 17 (4), 629-638 (2000).
5 Ding, H.S., Chinese Medicinal Spore-producing Plant, Shanghai Science and Technology Publishing, Shanghai (1982).
6 Zang, M., Xu, X.R., Ji, D.Q., “Yunnan economic funus data”,, 1, 28-34 (1975).
7 Ohmori, T., Tamura, K., Tsuru, S., Nomoto, K., “Antitumor activity of protein-bound polysaccharide fromin mice”,..., 77 (12), 1256-1263 (1986).
8 Jin, D.Q., Park, B.C., Lee, J.S., Choi, H.D., Lee, Y.S., Yang, J.H., Kim, J.A., “Mycelial extract ofprevents neuronal cell death and ameliorates beta-amyloid peptide-induced memory deficits in rats”,..., 27 (7), 1126-1129 (2004).
9 Kawagishi, H., Okamura, K., Kobayashi, F., Kinjo, N., “Estrogenic substances from the mycelia of medicinal fungus(Ehrh.) Fr. (Ascomycetes)”,...., 6, 249-251 (2004).
10 Shih, I.L., Tsai, K.L., Hsieh, C., “Effects of culture conditions on the mycelial growth and bioactive metabolite production in submerged culture of”,..., 33, 193-201 (2007).
11 Dong, C.H., Yao, Y.J., “Nutritional requirements of mycelial growth ofin submerged culture”,.., 99 (3), 483-492 (2005).
12 Kim, S.W., Hwang, H.J., Park, J.P., Cho, Y.J., Song, C.H., Yun, J.W., “Mycelial growth and exo-biopolymer production by submerged culture of various edible mushrooms under different media”,..., 34, 56-61 (2002).
13 Li, S.P., Yang, F.Q., Tsim, K.W.K., “Quality control of, a valued traditional Chinese medicine”,...., 41 (5), 1571-1584 (2006).
14 Xiao, J.H., Chen, D.X., Liu, J.W., Liu, Z.L., Wan, W.H., Fang, N., Xiao, Y., Qi, Y., Liang, Z.Q., “Optimization of submerged culture requirements for the production of mycelial growth and exopolysaccharide byJXPJ 0109”,..., 96 (5), 1105-1116 (2004).
15 Kong, Q., He, G.Q., Chen, Q., Chen, F., “Optimization of medium composition for cultivating Clostridium butyricum with response surface methodology”,.., 69, 163-168 (2004).
16 Stévigny, C., Rolle, L., Valentini, N., Zeppa, G., “Optimization of extraction of phenolic content from hazelnut shell using response surface methodology”,..., 87, 2817-2822 (2007).
17 Kim, S.W., Hwang, H.J., Xu, C.P., Sung, J.M., Choi, J.W., Yun, J.W., “Optimization of submerged culture process for the production of biomass and exo-polysaccharides byC738”,..., 94, 120-126 (2003).
18 Zhang, Z., Li, Y., Zhang, K., “Application of statistical analysis for the optimization of mycelia and polysaccharide production by”,.., 45 (1), 45-50 (2007).
19 Shu, C.H., Lung, M.Y., “Effect of pH on the production and molecular weight distribution of exopolysaccharide byin batch cultures”,., 39, 931-937 (2004).
20 Hsieh, C., Tsai, M.J., Hsu, T.H., Chang, D.M., Lo, C.T., “Medium optimization for polysaccharide production of”,..., 120 (2), 145-157 (2005).
21 Mao, X.B., Zhong, J.J., “Hyperproduction of cordycepin by two-stage dissolved oxygen control in submerged cultivation of medicinal mushroomin bioreactors”,.., 20, 1408-1413 (2004).
22 Park, J.P., Kim, Y.M., Kim, S.W., Hwang, H.J., Cho, Y.J., Lee, Y.S., Song, C.H., Yun, J.W., “Effect of aeration rate on the mycelial morphology and exo-biopolymer production in”,., 37, 1257-1262 (2002).
2008-06-23,
2008-11-10.
the Research Project of Science and Technology of Zhejiang Province, China (2005C23027), the National High Technology Research and Development Program of China (2007AA021506) and the Natural Science Foundation of Zhejiang Province (R207609).
** To whom correspondence should be addressed. E-mail: lyq@zju.edu.cn
——基于103个裁判文书数据
 Chinese Journal of Chemical Engineering2009年2期
Chinese Journal of Chemical Engineering2009年2期
- Chinese Journal of Chemical Engineering的其它文章
- Kinetics of Reactive Extraction of Nd from Nd2O3 with TBP-HNO3Complex in Supercritical Carbon Dioxide*
- Activity Coefficient Models to Describe Vapor-Liquid Equilibrium in Ternary Hydro-Alcoholic Solutions*
- Position Group Contribution Method for Predicting the Normal Boiling Point of Organic Compounds
- Experimental Study on the Initial Position Distribution of Taylor Bubbles in Cryogenic Upward Inclined Tubes*
- Enhancement of Proton Exchange Membrane Fuel Cell Performance Using a Novel Tapered Gas Channel*
- Tert-butylation of Toluene with Tert-butyl Alcohol over Realuminated H-mordenite Zeolite*
