木薯MeAHL17基因的克隆及表达分析

摘""要:AT-hook核定位蛋白(AT-hook"nuclear"localized"proteins,"AHLs)是一种小的DNA结合蛋白基序,在植物的生长发育、器官构建、逆境胁迫与激素信号应答等方面发挥重要作用。本研究从华南8号木薯(SC8)中克隆获得MeAHL17基因,利用生物信息学方法对MeAHL17基因进行启动子分析、蛋白理化性质分析、保守功能域的预测和序列比对。结果表明:MeAHL17基因的开放阅读框长度为939"bp,编码一个具有312个氨基酸的蛋白,其理论等电点为6.89,分子式为C1420H2215N415O451S11,分子量为32.669"38"kDa,带正电荷氨基酸残基总数(Lys+Arg)为23,带负电荷氨基酸残基总数(Asp+Glu)为24,脂肪系数为57.47,总平均亲水性系数(GRAVY)为–0.491,不稳定系数为58.06;该蛋白不含有信号肽,位于细胞膜内,无跨膜结构,含有5个糖基化位点和45个磷酸化位点,是一个不稳定的亲水性酸性蛋白;MeAHL17蛋白包含AT-hook保守核心序列和PPC结构域的保守核心序列,符合AT-hook家族的典型结构特征。通过亚细胞定位和转录活性分析实验证明MeAHL17是一个定位于细胞核且具有转录活性的转录因子。组织表达模式分析表明,MeAHL17基因主要在体胚、须根和愈伤组织中表达。不同激素处理表明,MeAHL17基因受到乙烯(ACC)、茉莉酸甲酯(JA)和生长素(IAA)等激素的诱导表达,推测其可能参与了乙烯、茉莉酸甲酯和生长素信号途径。非生物胁迫处理表明,MeAHL17基因响应干旱和盐胁迫。本研究初步确定了木薯MeAHL17基因在生长发育、激素信号和逆境胁迫等方面具有重要的作用,为进一步研究其功能提供理论依据和参考。
关键词:木薯;MeAHL17;基因克隆;亚细胞定位;转录活性分析;表达模式分析中图分类号:S31""""""文献标志码:A
Cloning"and"Expression"Analysis"of"MeAHL17"Gene"in"Cassava
ZHANG"Yawen1,2,3,"WANG"Xiaotong1,2,3,"ZHANG"Xinglong1,2,3,"TANG"Xiangning1,2,3,"LIU"Jiao2,3*,"GUO"Jianchun2,3*
1."School"of"Life"and"Health"Sciences,"Hainan"University,"Haikou,"Hainan"570228,"China;"2."Institute"of"Tropical"Bioscience"and"Biotechnology,"Chinese"Academy"of"Tropical"Agricultural"Sciences"/"Key"Laboratory"of"Biology"and"Genetic"Resources"of"Tropical"Crops,"Ministry"of"Agriculture"and"Rural"Affairs"/"Hainan"Institute"for"Tropical"Agricultural"Resources"/"Key"Laboratory"for"Biology"and"Genetic"Resources"of"Tropical"Crops"of"Hainan"Province,"Haikou,"Hainan"571101,"China;"3."Sanya"Research"Institute,"Chinese"Academy"of"Tropical"Agricultural"Sciences,"Sanya,"Hainan"572000,"China
Abstract:"AT-hook"nuclear"localized"proteins"(AHLs)"are"small"DNA-binding"protein"motifs"that"play"an"important"role"in"plant"growth"and"development,"organ"construction,"stress"and"hormone"signaling"response."In"this"study,"MeAHL17"was"cloned"from"cassava"cultivar"SC8."Bioinformatics"methods"were"used"to"analyze"MeAHL17"promoter,"protein"physicochemical"properties,"prediction"of"conserved"functional"domain"and"sequence"comparison."The"results"showed"that"the"open"reading"frame"length"of"MeAHL17"gene"was"939"bp,"encoding"a"protein"with"312"amino"acids,"its"theoretical"isoelectric"point"was"6.89,"its"molecular"formula"was"C1420H2215N415O451S11,"and"its"molecular"weight"was"32.669"38"kDa."The"total"number"of"positively"charged"amino"acid"residues"(Lys+Arg)"was"23,"the"total"number"of"negatively"charged"amino"acid"residues"(Asp+Glu)"was"24,"the"fat"coefficient"was"57.47,"the"total"average"hydrophilic"coefficient"(GRAVY)"was"-0.491"and"the"instability"coefficient"was"58.06."The"protein"contains"no"signal"peptide,"is"located"in"the"cell"membrane,"has"no"transmembrane"structure,"contains"5"glycosylation"sites"and"45"phosphorylation"sites,"and"is"an"unstable"hydrophilic"acidic"protein"MeAHL17"contained"AT-hook"conserved"core"sequences"and"PPC"domain"conserved"core"sequences,"which"was"consistent"with"the"typical"structural"characteristics"of"AT-hook"family."Through"subcellular"localization"and"transcriptional"activity"analysis"experiments,"MeAHL17"was"proved"to"be"a"nucleus"localized"transcription"factor"with"transcriptional"activity."Tissue"expression"pattern"analysis"showed"that"MeAHL17"was"mainly"expressed"in"somatic"embryo,"fibrous"root"and"callus."Different"hormone"treatments"showed"that"MeAHL17"was"induced"by"ethylene"(ACC),"methyl"jasmonate"(JA)"and"growth"hormone"(IAA),"which"suggested"that"MeAHL17"might"be"involved"in"ethylene,"methyl"jasmonate"and"growth"hormone"signaling"pathway."Abiotic"stress"treatment"showed"that"MeAHL17"was"responsive"to"drought"and"salt"stress."This"study"preliminarily"identified"the"important"role"of"MeAHL17"in"growth"and"development,"hormone"signaling"and"stress,"and"provided"theoretical"basis"and"reference"for"further"study"of"its"function.
Keywords:"cassava;"MeAHL17;"gene"cloning;"subcellular"localization;"transcriptional"activity"analysis;"expression"pattern"analysis
DOI:"10.3969/j.issn.1000-2561.2024.07.001
木薯(Manihot"esculenta"Crantz)是大戟科(Euphorbiaceae)、木薯属(Manihot)植物,是世界三大薯类(甘薯、马铃薯、木薯)和六大粮食作物(小麦、水稻、玉米、马铃薯、大麦、木薯)之一,广泛种植于非洲、美洲和亚洲100多个国家的热带和亚热带地区[1-2]。木薯虽然具有许多生物学优势,比如耐贫瘠、抗旱和产量高等[3],但持续干旱、盐毒害和低温等不利条件均可能导致木薯长势不佳和低产[4-5]。AT-hook核定位蛋白(AT-hook"nuclear"localized"proteins,"AHLs)在植物的生长发育、器官构建、逆境胁迫和激素信号应答等方面发挥重要作用[6]。因此,研究AT-hook基因对木薯品种改良具有重要意义。
目前,AHL基因家族已在多个物种中进行了全基因组鉴定和分析,从拟南芥(Arabidopsis"thaliana)中鉴定出29个AHLs[7],水稻(Oryza"sativa)有20个AHLs[8],高粱(Sorghum"bicolor)有22个AHLs[9],玉米(Zea"mays)有37个AHLs[10],分别从雷蒙德氏棉(Gossypium"raimondii)、亚洲棉(Gossypium"arboreum)和陆地棉(Gossypium"hirsutum)中各鉴定出48、51和99个AHLs[11],大豆(Glycine"max)基因组中鉴定出63个AHLs[12],葡萄(Vitis"vinifera)中鉴定出14个AHLs[13],花生(Arachis"hypogaea)中鉴定出64个AHLs[14],胡萝卜(Daucus"carota)中鉴定出47个AHLs[15],毛果杨(Populus"trichocarpa)中鉴定出37个AHLs[16],甘蓝型油菜(Brassica"napus)中鉴定出122个AHLs[17],近一年来又相继在核桃(Juglans"regia)、鹅掌楸(Liriodendron"chinense)、芜菁(Brassica"rapa)和番茄(Solanum"lycopersicum)中各鉴定出37、21、42和18个AHLs[18-21];随着对植物中AHLs基因的研究不断积累,AHLs已被证明可以调控多种生长发育过程,包括花发育[22]、下胚轴伸长[23-24]、花粉发育和育性[25-26]、玉米穗发育[27]、维管组织分化[28],调节开花时间[29-31]以及叶片衰老调节[32]等。AHLs也参与了植物对生物和非生物胁迫的反应,包括增强抗旱性[33-34]和调节植物抵抗病原微生物入侵的能力[35-36]。AHLs还参与调节初级代谢[37]、调节赤霉素[38]的稳态以及调控茉莉酸和生长素相关基因的表达[33,"39-40]。尽管AHL基因家族有如此广泛的作用,但对其功能的了解还不够全面和系统化,依旧值得继续探索。
虽然已有研究表明MeAHL17在抗木薯细菌性枯萎病(cassava"bacterial"blight,"CBB)中具有积极作用[41],但是该基因在其他激素信号应答与逆境胁迫等方面作用的机制还未知,依旧值得继续探索。本研究通过克隆木薯MeAHL17基因,并对其进行生物信息学分析、亚细胞定位、转录活性分析和表达模式分析,为进一步研究MeAHL17参与木薯生长发育、逆境胁迫和激素信号应答等方面的功能提供理论依据和参考。
1""材料与方法
1.1""材料
植物材料:华南8号(SC8)木薯采自国家木薯种质资源圃;本生烟由本实验室提供。
质粒和菌株:pCAMBIA1300-35S-GFP和pGBKT7载体质粒由本实验室提供;大肠杆菌菌株DH5α、根癌农杆菌菌株GV3101和酵母菌株AH109购自上海唯地生物技术有限公司。
试剂:多糖多酚植物总RNA提取试剂盒(Plant"Total"RNA"Isolation"Kit"Plus)购自成都福际生物技术有限公司;RNA反转录试剂盒(MonScriptTM"RTIII"ALL-in-One"Mix)和qPCR试剂(MonAmpTM"ChemoHS"qPCR"Mix)购自莫纳生物科技有限公司;LA"Taq®高保真酶和pMDTM"19-vector购自TaKaRa公司;质粒DNA小量抽提试剂盒、PCR产物纯化试剂盒和T4"DNA连接酶购自生工生物工程(上海)股份有限公司;限制性内切酶购自Thermo"Fisher"Scientific公司;MES和乙酰丁香酮(AS)购自北京索莱宝科技有限公司;六水合氯化镁购自西陇化工科技有限公司。
引物:本研究引物均由北京擎科生物科技有限公司合成,引物信息见表1。
1.2""方法
1.2.1""MeAHL17基因的克隆""以SC8组培苗作为提取RNA的材料,用多糖多酚植物总RNA提取试剂盒提取木薯总RNA,取4~6"μL总RNA样品用1%浓度的琼脂糖胶检测其质量,用超微量分光光度计检测其浓度。用MonScriptTM"RTIII"ALL-in-One"Mix试剂盒进行反转录获得cDNA,用于基因扩增。利用phytozome网站(https://phy tozome.jgi.doe.gov)查找到MeAHL17序列(Manes."02G145600)后,于NCBI数据库Primer-BLAST设计MeAHL17的扩增引物(MeAHL17-F/Me AHL17-R,"表1),以反转录后所得cDNA为模板,PCR扩增MeAHL17编码区片段。反应体系:LA"Taq"0.5"μL,10×LA"PCR"Buffer"5"μL,dNTP"Mixture"8"μL,上下游引物各1"μL,模板2"μL,加ddH2O补至50"μL。反应程序为:94"℃预变性5"min;94"℃"30nbsp;s,54"℃"30"s,72"℃"1"min,循环35次;72"℃延伸10"min;16"℃保存。取3"μL"PCR产物,用1%的琼脂糖胶检测目的条带,再进行产物纯化,用pMDTM"19-vector试剂将pMD19-T载体和MeAHL17基因片段连接,按照唯地生物的DH5α"Chemically"Competent"Cell的说明书进行转化,随机挑取多个菌斑进行PCR检测并扩大培养,选取3个阳性克隆送至楠山生物技术有限公司进行测序验证后,用质粒小量快速提取试剂盒提取pMD19T-MeAHL17质粒。
1.2.2""生物信息学分析""使用如表2所示的在线分析软件对MeAHL17进行生物信息学分析。
1.2.3""亚细胞定位""以pMD19T-MeAHL17载体质粒为模板,通过设计引物AHL17-F(Sal"Ⅰ)/AHL17-R(BamH"I)对其进行PCR扩增;pCAMBIA1300-GFP载体质粒用Sal"I和BamH"I限制性内切酶对其进行双酶切;纯化PCR产物和酶切后的载体质粒;利用T4"DNA连接酶将pCAMBIA1300-GFP载体与MeAHL17基因片段连接,转化DH5α并扩大培养后,提取质粒,取4"μL"pCAMBIA1300-MeAHL17-GFP质粒和pCA MBIA1300-GFP空载质粒转化GV3101感受态细胞,随机挑取多个菌斑进行PCR检测(引物为1300-F/1300-R)并扩大培养,将含有pCAMBIA"1300-MeAHL17-GFP质粒和pCAMBIA1300-GFP空载质粒的农杆菌注射至烟草叶片中瞬时表达,培养48~72"h后用DAPI染料染色10"min,用清水清洗5~8次后,于激光共聚焦显微镜下观察荧光。
1.2.4""转录活性分析""设计同源重组引物BD-"AHL17-F/BD-AHL17-R,并以pMD19T-MeAHL17载体质粒为模板进行扩增;利用限制性内切酶BamH"I和Sal"I对pGBKT7载体进行双酶切,纯化PCR产物和酶切后的载体后,利用同源重组酶将pGBKT7载体与MeAHL17基因片段连接,转化DH5α并扩大培养后,提取质粒,取4"μL"pGB KT7-MeAHL17、pGBKT7-p53+pGADT7-largeT(阳性对照)和pGBKT7(阴性对照)转化AH109感受态细胞,PCR检测(引物为BD-F/BD-R)后,扩大培养至OD600约0.7,稀释后各取5"μL菌液点到SD/-Trp/X-α-gal固体缺陷培养基上,观察酵母菌的生长情况。
1.2.5""MeAHL17基因表达模式分析""(1)MeAHL17在不同组织器官的表达模式分析。将SC8的愈伤、体胚、嫩叶、成熟叶、茎、须根、块根、块根韧皮部和块根木质部等9个组织进行转录组测序,根据测序结果提取MeAHL17的FPKM值(每千个碱基的转录每百万映射读取的片段)进行不同组织的表达模式分析。
(2)MeAHL17在不同块根发育时期的表达模式分析。将SC8的块根形成期(种植后80"d)、块根膨大期(种植后130、180"d)和块根成熟期(种植后230、280"d)的块根进行转录组测序,根据测序结果提取MeAHL17的FPKM值进行木薯块根不同发育时期的表达模式分析。
(3)MeAHL17在不同激素处理下的表达模式分析。以MS培养基上生长8周左右的生长状态良好且一致的木薯组培苗为对象,用100"µmol/L脱落酸(ABA)、100"µmol/L茉莉酸甲酯(MeJA)、100"µmol/L乙烯前体(ACC)和100"µmol/L生长素(IAA)溶液喷洒木薯的根、茎、叶,进行外源激素处理0、6、12、24"h,每个样品3个重复。提取样品RNA并反转录为cDNA,将cDNA稀释10倍作为模板,以β-Tublin为内参基因,对MeAHL17基因进行实时荧光定量PCR。反应体系:MonAmpTM"ChemoHS"qPCR"Mix"5"μL,上、下游引物各0.2"μL,DNA模板1"μL,Nuclease-Free"Water"3.6"μL;反应程序:95"℃"10"min;95"℃"10"s,58"℃"10"s,72"℃"30"s,共40个循环;95"℃"15"s,60"℃"15"s,95"℃"15"s作溶解曲线。每个样品设置3个重复,表达量用2–ΔΔCT方法计算相对表达量。
(4)MeAHL17在不同胁迫处理下的表达模式分析。用20%的聚乙二醇(polyethylene"glycol,"PEG)溶液模拟干旱胁迫处理;用300"mmol/L的氯化钠(NaCl)溶液进行盐胁迫处理;将木薯放置到4"℃的培养房中进行低温胁迫处理。分别处理0、12、24、48"h,每个样品3个重复,后续实验操作同1.2.5-(3)。
2""结果与分析
2.1""MeAHL17基因的克隆
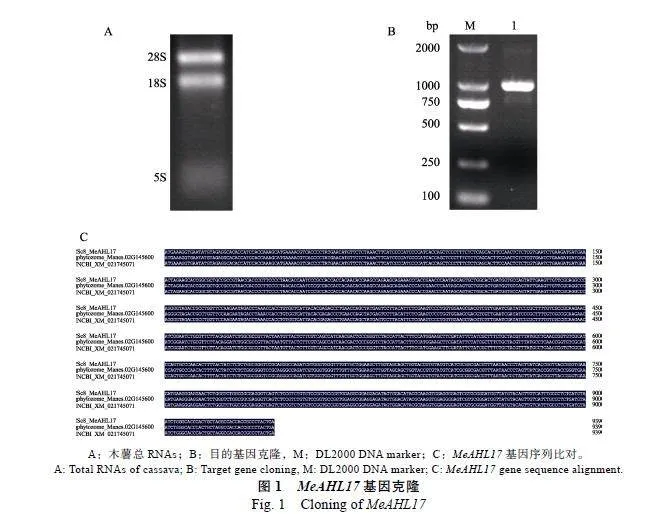
本研究从SC8组培苗中提取总RNA,并用1%浓度的琼脂糖胶检测其质量,胶图显示质量良好(图1A)。反转录成cDNA后,以其为模板PCR扩增出MeAHL17编码区序列,获得全长939"bp的目的基因(图1B)。为验证得到的MeAHL17序列的准确性,将扩增的MeAHL17编码区序列与将pMD19-T载体进行连接并测序,测序结果发现,扩增获得的基因序列与NCBI数据库中LOC110606315对应的基因序列和木薯数据库中Manes.02G145600对应的基因序列完全一致,说明成功得到目的基因MeAHL17(图1C)。
2.2""生物信息学分析
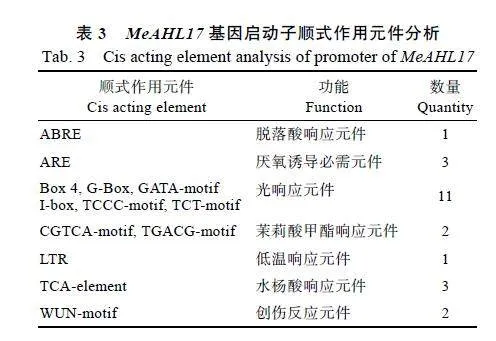
2.2.1""MeAHL17基因启动子分析""通过在线网站Plantcare对MeAHL17的启动子进行顺式作用元件分析发现,MeAHL17基因启动子的顺式作用元件除了核心元件TATA-box与CAAT-box外,还包括脱落酸、水杨酸和茉莉酸甲酯等激素响应元件,低温响应元件和光响应元件等(表3)。
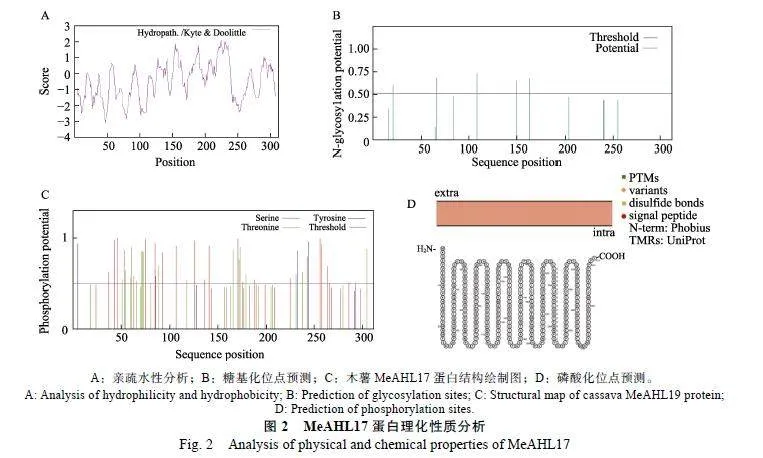
2.2.2""MeAHL17蛋白理化性质分析""通过在线网站ProtParam对MeAHL17蛋白理化性质进行分析,结果发现,MeAHL17蛋白由312个氨基酸组成,理论等电点为6.89,分子式为C1420H2215N415O451S11,分子量为32"669.38"kDa,带正电荷氨基酸残基总数(Lys+Arg)为23,带负电荷氨基酸残基总数(Asp+Glu)为24,脂肪系数为57.47,总平均亲水性系数(GRAVY)为–0.491,不稳定系数为58.06,结合亲水性预测结果(图2A)表明,MeAHL17蛋白是一个亲水性的、不稳定的酸性蛋白。
对MeAHL17蛋白进行糖基化位点和磷酸化位点预测,结果显示,MeAHL17蛋白有5个糖基化位点(图2B)和45个磷酸化位点(图2C),包括Tyr(18个)、Ser(24个)、Thr(3个),说明木薯MeAHL17蛋白活性的调控可能与磷酸化修饰有关。通过Protter软件对MeAHL17进行蛋白结构分析,发现MeAHL17不含有信号肽,位于细胞膜内,无跨膜结构(图2D)。
2.2.3""MeAHL17蛋白保守功能域的预测和序列比对""通过NCBI网站对MeAHL17蛋白的保守功能域进行预测发现,该蛋白包含AHL基因家族特有的DUF296保守结构域,即PPC结构域(图3);将木薯MeAHL17与其他物种中同源性较高的AHL蛋白进行序列比对,发现所有蛋白序列具有高度保守性,且包含AT-hook保守核心序列和包含PPC结构域的保守核心序列(图4),说明MeAHL17是一个AHL基因。
2.3""亚细胞定位
将GV3101/pCAMBIA1300-MeAHL17-GFP和GV3101/pCAMBIA1300-GFP活化集菌后,利用侵染液将菌体重悬,然后注射烟草叶片进行瞬时表达。结果如图5所示,MeAHL17定位在细胞核。
2.4""转录活性分析
将pGBKT7-MeAHL17、pGBKT7-p53+pGADT7-"largeT(阳性对照)和pGBKT7(阴性对照)质粒分别转化至AH109酵母感受态后,点到SD/-Trp/"X-α-gal固体缺陷培养基上,发现pGBKT7-MeAH L17在SD/-Trp/X-α-gal固体缺陷培养基上与阳性对照一样变蓝,而阴性对照未变蓝(图6),说明MeAHL17是转录因子且具有转录活性。
2.5""MeAHL17基因表达模式分析
2.5.1""MeAHL17在不同组织器官的表达模式分析""根据实验室已有的SC8木薯的转录组数据,对MeAHL17进行表达模式分析,发现该基因在木薯不同组织表达量差异显著,其中,MeAHL17在体胚的表达量最高,其次是须根、愈伤和块根韧皮部,在块根和茎中表达量较低,其余组织几乎不表达(图7)。
2.5.2""MeAHL17在块根发育时期的表达模式分析""在SC8木薯块根发育过程中,MeAHL17在木薯种植后180"d的块根中表达量最高,在其他时间段表达量差异不显著(图8)。
2.5.3""MeAHL17在各激素处理下的表达模式分析""由于MeAHL17基因启动子含有ABA、MeJA等响应元件,为验证MeAHL17是否在木薯中响应激素胁迫,通用实时荧光定量PCR法分析MeAHL17在各激素处理下的响应情况。如图9所示,MeAHL17可以被外源乙烯前体、茉莉酸甲酯、和生长素诱导,并且在根、茎和叶中的表达模式略有不同:施加乙烯前体(ACC)后,MeAHL17在茎中的相对表达量随着处理时间的延长逐渐增加,该基因在根、茎和叶中的相对表达量均在处理后24"h达到最高水平,其相对表达量分别约为0"h的9倍、5倍和3倍;施加脱落酸后,MeAHL17的相对表达量在根、茎和叶中略有变化;施加茉莉酸甲酯后,MeAHL17在根和茎中的相对表达量随着处理时间的延长逐渐增加,且在处理后24"h达到最高水平,其相对表达量分别约为0"h的10倍和6倍,但MeAHL17在叶中不响应外源茉莉酸甲酯的诱导;施加生长素后,MeAHL17在根、茎和叶中的相对表达量随着处理时间的延长逐渐增加,且其在茎和叶中的相对表达量在处理后24"h达到最高水平,分别约为0"h的8倍和10倍,但MeAHL17在根中的相对表达量在处理后12"h达到最高水平,约为0"h对照的27倍。结果表明,MeAHL17可能参与了乙烯、茉莉酸甲酯、和生长素信号途径。
2.5.4""MeAHL17在不同胁迫处理下的表达模式分析""为验证MeAHL17是否在木薯中响应逆境胁迫,通过实时荧光定量PCR法分析MeAHL17在不同胁迫处理下的响应情况。如图10所示,在干旱处理下,MeAHL17相对表达量在12"h达到最高水平,约为0"h的2倍,而随着时间的延长,相对表达量回到正常水平;在盐胁迫处理下,MeAHL17相对表达量在12"h达到最高水平,约为0"h的2倍,而随着时间的延长,相对表达量逐渐降低;在低温处理下,MeAHL17相对表达量在0~24"h无明显变化,但是在处理48"h后MeAHL17相对表达量降低。结果表明,MeAHL17响应干旱和盐胁迫。
3""讨论
AT-hook核定位蛋白是一种小的DNA结合蛋白基序,在生物界中广泛存在[42],最先是由GOODWIN等[43]在哺乳动物非组蛋白的染色体高迁移率蛋白HMG-I"(Y)中发现。AT-hook核定位蛋白主要包含2个特殊的保守功能结构域,AT-hook基序和植物与原核生物保守基序(plant"and"Prokaryote"conservative,"PPC)结构域,后者也称为domain"of"unknown"function#296(DUF296)结构域[44-45]。AT-hook基序是AT-hook蛋白结合DNA和核定位的重要结构域[46-47],其核心序列由Arg(R)-Gly(G)-Arg(R)-Pro(P)构成,其中脯氨酸主要负责该序列的稳定性,而精氨酸则是深入到双链DNA内部并和DNA中富含AT碱基的区域相互作用[46,"48];PPC结构域与AT-hook蛋白在细胞核中的定位有关,该结构域长度约为120个氨基酸,位于AT-hook基序的羧基末端,PPC结构域包含保守的Gly(G)-Arg(R)-Phe(F)-Glu(E)-Ile(I)-"Leu(L)序列[9,"44,"49]。
本研究成功从华南8号木薯中克隆获得MeAHL17基因,其编码区序列长939"bp,编码312个氨基酸。通过对MeAHL17蛋白理化性质分析发现,MeAHL17蛋白是一个亲水性的、不稳定的酸性蛋白且不含有信号肽和跨膜结构,与胡冬秀等[14]和王晓彤等[50]的研究结果一致。对MeAHL17蛋白进行糖基化位点和磷酸化位点预测,结果发现MeAHL17蛋白有5个糖基化位点和45个磷酸化位点,已有研究表明AtAHL10的S314位点磷酸化能在干旱胁迫下调节发育和激素相关基因的表达,从而对植物的生长发育具有重要的调控作用[33],因此推测MeAHL17蛋白的磷酸化修饰对木薯的生长发育具有重要的调控作用。通过MeAHL17蛋白保守功能域的预测和与其他物种中已鉴定的且同源性较高的AHL蛋白进行序列比对发现,该蛋白包含AHL基因家族特有的PPC保守结构域,且包含AT-hook保守核心序列和PPC结构域的保守核心序列,符合AT-hook基因家族结构特征[7]。通过亚细胞定位和转录活性分析实验证明,MeAHL17是一个定位于细胞核且具有转录活性的转录因子。
已有研究表明,AT-hook基因在植物的生长发育、器官构建、逆境胁迫和激素信号应答等方面发挥重要的作用[6],如,AHL18通过调节根尖分生组织的伸长和细胞分裂从而参与主根生长和侧根发育[51];AHL22通过调控FT和PIF4的表达来控制开花和下胚轴伸长[24,"31],AHL转录因子通过降低PIFs与激素信号通路相关基因的结合从而抑制其转录激活来影响叶柄的生长[52];ORE7/ESC基因可以延缓茉莉酸甲酯、脱落酸和乙烯激素参与的叶片衰老进程[32];LcAHL基因参与了抗干旱胁迫和体细胞胚的发育,且在体细胞胚发生过程中呈高表达[19]。在本研究中,结合MeAHL17在各激素处理下的表达模式分析和不同组织器官的表达模式分析,推测MeAHL17可能通过参与乙烯、茉莉酸甲酯、和生长素信号途径,从而对植物的生长发育,尤其是体胚和根的发育发挥重要的调控作用;MeAHL17在不同胁迫处理下的表达模式分析结果表明MeAHL17对干旱和盐胁迫有所响应。以上研究结果为进一步了解MeAHL17基因在木薯生长发育、器官构建及逆境胁迫与激素信号应答等方面的功能奠定基础。
参考文献
- TEMBO"M,"MATAA"M,"LEGG"J"P,"CHIKOTI"P"C,"NTAWURUHUNGA"P."Cassava"mosaic"disease:"incidence"and"yield"performance"of"cassava"cultivars"in"Zambia[J]."Journal"of"Plant"Pathology,"2017,"99(3):"681-689.
- LEBOT"V,"ATHERTON"J,"REES"A."Tropical"root"and"tuber"crops:"cassava,"sweet"potato,"yams"and"aroids[M]."Beijing:"CABI,"2020.
- OKOGBENIN"E,"SETTER"T"L,"FERGUSON"M,"MUTEGI"R,"CEBALLOS"H,"OLASANMI"B,"FREGENE"M."Phenotypic"approaches"to"drought"in"cassava:"review[J]."Frontiers"in"Physiology,"2013,"4:"93.
- EL-SHARKAWY"M"A."Cassava"biology"and"physiology"cassava:"a"crop"for"sustainable"agriculture"and"food"security"in"developing"countries[J]."Plant"Molecular"Biology,"2004,"56:"481-501.
- 王惠君,"王文泉,"李文彬,"陈新,"卢诚,"黎明,"陈友."木薯的抗寒性及北移栽培技术研究进展综述[J]."热带作物学报,"2016,"37(7):"1437-1443."WANG"H"J,"WANG"W"Q,"LI"W"B,"CHEN"X,"LU"C,"LI"M,"CHEN"Y."Research"progress"on"cold"resistance"of"cassava"and"northward"migration"cultivation"techniques[J]."Chinese"Journal"of"Tropical"Crops,"2016,"37(7):"1437-1443."(in"Chinese)
- 李津璇,"郭敏,"王加峰,"杨瑰丽."AT-hook基因及其在植物开花调控中的研究进展[J]."中国农学通报,"2021,"37(27):"77-81.LI"J"X,"GUO"M","WANG"J"F,"YANG"G"L."Research"progress"of"AT-hook"genes"and"their"role"in"plant"flowering"regulation[J]."Chinese"Agricultural"Science"Bulletin,"2021,"37(27):"77-81."(in"Chinese)
- 肖朝文."拟南芥AT-hook蛋白的功能研究[D]."北京:"中国农业科学院,"2009.XIAO"C"W."Functional"study"of"AT-hook"protein"in"Arabidopsis"Thaliana[D]."Beijing:"Chinese"Academy"of"Agricultural"Sciences,"2009."(in"Chinese)
- KIM"H"B,"OH"C"J,"PARK"Y"C,"LEE"Y,"CHOE"S,"AN"C"S,"CHOI"S"B."Comprehensive"analysis"of"AHL"homologous"genes"encoding"AT-hook"motif"nuclear"localized"protein"in"rice[J]."Bmb"Reports,"2011,"44(10):"680.
- ZHAO"J,"FAVERO"D"S,"QIU"J,"ROALSON"E"H,"NEFF"M"M."Insights"into"the"evolution"and"diversification"of"the"AT-hook"motif"nuclear"localized"gene"family"in"land"plants[J]."Bmc"Plant"Biology,"2014,"14(1):"266.
- BISHOP"E"H,"KUMAR"R,"LUO"F,"SASKI"C,"SEKHON"R"S."Genome-wide"identification,"expression"profiling,"and"network"analysis"of"AT-hook"gene"family"in"maize[J]."Genomics,"2019,"112(2):"1233-1244.
- ZHAO"L,"LU"Y,"CHEN"W,"YAO"J,"LI"Y,"LI"Q,"PAN"J,"FANG"S,"SUN"J,"ZHANG"Y."Genome-wide"identification"and"analyses"of"the"AHL"gene"family"in"cotton"(Gossypium)[J]."BMC"Genomics,"2020,"21(1):"69.
- WANG"M,"CHEN"B,"ZHOU"W,"XIE"L,"WANG"L,"ZHANG"Y,"ZHANG"Q."Genome-wide"identification"and"expression"analysis"of"the"AT-hook"motif"nuclear"localized"gene"family"in"soybean[J]."BMC"Genomics,"2021,"22(1):"361.
- LI"X,"HE"H,"WANG"H,"WU"X,"MAO"J."Identification"and"expression"analysis"of"the"AHL"gene"family"in"grape"(Vitix"vinifera)[J]."Plant"Gene,"2021,"26:"100285.
- 胡冬秀,"刘浩,"梁炫强,"吴自明,"方加海."花生AT-hook家族基因的生物信息学分析[J]."热带作物学报,"2021,"42(3):"649-659.HU"D"X,"LIU"H","LIANG"X"Q,"WU"Z"M,"FANG"J"H."Bioinformatics"analysis"of"AT-hook"genes"in"peanut[J]."Chinese"Journal"of"Tropical"Crops,"2021,"42(3):"649-659."(in"Chinese)
- MACHAJ"G,"GRZEBELUS"D."Characteristics"of"the"AT-Hook"motif"Containing"Nuclear"Localized"(AHL)"genes"in"carrot"provides"insight"into"their"role"in"plant"growth"and"storage"root"development[J]."Genes,"2021,"12(5):"764.
- WANG"H,"LENG"X,"YANG"J,"ZHANG"M,"ZENG"M,"XU"X,"WANG"F,"LI"C."Comprehensive"analysis"of"AHL"gene"family"and"their"expression"under"drought"stress"and"ABA"treatment"in"Populus"trichocarpa[J]."PeerJ,"2021,"9:"e10932.
- ZHANG"W"M,"FANG"D,"CHENG"X"Z,"CAO"J,"TAN"X"L."Insights"into"the"molecular"evolution"of"AT-Hook"motif"nuclear"localization"genes"in"Brassica"napus[J]."Frontiers"in"Plant"Science,"2021,"12:"714305.
- JIA"P,"LIU"J,"YAN"R,"YANG"K,"DONG"Q,"LUAN"H,"ZHANG"X,"LI"H,"GUO"S,"QI"G."Systematical"characterization"of"the"AT-Hook"gene"family"in"Juglans"regia"L."and"the"functional"analysis"of"the"JrAHL2"in"flower"induction"and"hypocotyl"elongation[J]."International"Journal"of"Molecular"Sciences,"2023,"24(8):"7244.
- TANG"Y,"WU"W,"ZHENG"X,"LU"L,"CHEN"X,"HAO"Z,"LIU"S,"CHEN"Y."AT-Hook"transcription"factors"show"functions"in"Liriodendron"chinense"under"drought"stress"and"somatic"embryogenesis[J]."Plants,"2023,"12(6):"1353.
- ZHANG"X,"LI"J,"CAO"Y,"HUANG"J,"DUAN"Q."Genome-wide"identification"and"expression"analysis"under"abiotic"stress"of"BrAHL"genes"in"Brassica"rapa[J]."International"Journal"of"Molecular"Sciences,"2023,"24(15):"12447."
- WANG"L,"LI"T,"LIU"N,"LIU"X."Identification"of"tomato"AHL"gene"families"and"functional"analysis"their"roles"in"fruit"development"and"abiotic"stress"response[J]."Plant"Physiol"Biochem,"2023,"202:"107931.
- JIN"Y,"LUO"Q,"TONG"H,"WANG"A,"CHENG"Z,"TANG"J,"LI"D,"ZHAO"X,"LI"X,"WAN"J,"JIAO"Y,"CHU"C,"ZHU"L."An"AT-hook"gene"is"required"for"palea"formation"and"floral"organ"number"control"in"rice[J]."Developmental"Biology,"2011,"359(2):"277-288.
- STREET"I"H,"SHAH"P"K,"SMITH"A"M,"AVERY"N,"NEFF"M"M.The"AT-hook-containing"proteins"SOB3/AHL29"and"ESC/AHL27"are"negative"modulators"of"hypocotyl"growth"in"Arabidopsis[J]."The"Plant"Journal,"2010,"54(1):"1-14.
- XIAO"C,"CHEN"F,"YU"X,"LIN"C,"FU"Y"F."Over-expression"of"an"AT-hook"gene,"AHL22,"delays"flowering"and"inhibits"the"elongation"of"the"hypocotyl"in"Arabidopsis"thaliana[J]."Plant"Molecular"Biology,"2009,"71(1):"39-50.
- LOU"Y,"XU"X"F,"ZHU"J,"GU"J"N,"BLACKMORE"S,"YANG"Z"N."The"tapetal"AHL"family"protein"TEK"determines"nexine"formation"in"the"pollen"wall[J]."Nature"Communications,"2014,"5(1):"3855.
- JIA"Q"S,"ZHU"J,"XU"X"F,"LOU"Y,"ZHANG"Z"L,"ZHANG"Z"P,"YANG"Z"N."Arabidopsis"AT-hook"protein"TEK"positively"regulates"the"expression"of"arabinogalactan"proteins"for"Nexine"formation[J]."Molecular"Plant,"2015,"8(2):"251-260.
- GALLAVOTTI"A,"MALCOMBER"S,"GAINES"C,"STANFI ELD"S,"WHIPPLE"C,"KELLOGG"E,"SCHMIDT"R"J."BARREN"STALK"FASTIGIATE1"is"an"AT-hook"protein"required"for"the"formation"of"maize"ears[J]."The"Plant"Cell,"2011,"23(5):"1756-1771.
- ZHOU"J,"WANG"X,"LEE"J"Y,"LEE"J"Y."Cell-to-cell"movement"of"two"interacting"AT-hook"factors"in"Arabidopsis"root"vascular"tissue"patterning[J]."The"Plant"Cell,"2013,"25(1):"187-201.
- XU"Y,"WANG"Y,"STROUD"H,"GU"X,"SUN"B,"GAN"E"S,"NG"K"H,"JACOBSEN"S"E,"He"Y,"ITO"T."A"matrix"protein"silences"transposons"and"repeats"through"interaction"with"retinoblastoma-associated"proteins[J]."Current"Biology,"2013,"23(4):"345-350.
- XU"Y,"GAN"E"S,"ITO"T."The"AT-hook/PPC"domain"protein"TEK"negatively"regulates"floral"repressors"including"MAF4"and"MAF5[J]."Plant"Signaling"amp;"Behavior,"2013,"8(8):"e25006.
- YUN"J,"KIM"Y"S,"JUNG"J"H,"SEO"P"J,"PARK"C"M."The"AT-hook"motif-containing"protein"AHL22"regulates"flowering"initiation"by"modifying"FLOWERING"LOCUS"T"chromatin"in"Arabidopsis[J]."Journal"of"Biological"Chemistry,"2012,"287(19):"15307-15316.
- LIM"P"O,"KIM"Y,"BREEZE"E,"KOO"J"C,"WOO"H"R,"RYU"J"S,"PARK"D"H,"BEYNON"J,"TABRETT"A,"BUCHANAN-"WOLLASTON"V,"NAM"H"G."Overexpression"of"a"chromatin"architecture-controlling"AT-hook"protein"extends"leaf"longevity"and"increases"the"post-harvest"storage"life"of"plants[J]."Plant"Journal"for"Cell"amp;"Molecular"Biology,"2010,"52(6):"1140-1153.
- WONG"M"M,"BHASKARA"G"B,"WEN"T"N,"LIN"W"D,"NGUYEN"T"T,"CHONG"G"L,"VERSLUES"P"E."Phosphoproteomics"of"Arabidopsis"highly"ABA-Induced1"identifies"AT-hook-LIke10"phosphorylation"required"for"stress"growth"regulation[J]."Proceedings"of"the"National"Academy,"2019,"116(6):"2354-"2363.
- ZHOU"L,"LIU"Z,"LIU"Y,"KONG"D,"LI"T,"YU"S,"MEI"H,"XU"X,"LIU"H,"CHEN"L,"LUO"L."A"novel"gene"OsAHL1"improves"both"drought"avoidance"and"drought"tolerance"in"rice[J]."Scientific"Reports,"2016,"6:"30264.
- LU"H,"ZOU"Y,"FENG"N."Overexpression"of"AHL20"negatively"regulates"defenses"in"Arabidopsis[J]."Journal"of"Integrative"Plant"Biology,"2010,"52(9):"801-808.
- KUMAR"K,"PURAYANNUR"S,"KALADHAR"V"C,"PARIDA"S"K,"VERMA"P"K."mQTL-seq"and"classical"mapping"implicates"the"role"of"an"AT-HOOK"MOTIF"CONT AINING"NUCLEAR"LOCALIZED"(AHL)"family"gene"in"Ascochyta"blight"resistance"of"chickpea[J]."Plant,"Cell"amp;"Environment,"2018,"41(9):"2128-2140.
- LI"B,"KLIEBENSTEIN"D"J."The"AT-hook"motif-encoding"gene"METABOLIC"NETWORK"MODULATOR"1"underlies"natural"variation"in"Arabidopsis"primary"metabolism[J]."Frontiers"in"Plant"Science,"2014,"5:"415.
- MATSUSHITA"A,"FURUMOTO"T,"ISHIDA"S,"TAKAHASHI"Y."AGF1,"an"AT-hook"protein,"is"necessary"for"the"negative"feedback"of"AtGA3ox1"encoding"GA"3-oxidase[J]."Plant"Physiology,"2007,"143(3):"1152-1162.
- RASHOTTE"A"M,"CARSON"S"D,"TO"J"P,"KIEBER"J"J."Expression"profiling"of"cytokinin"action"in"Arabidopsis[J]."Plant"Physiology,"2003,"132(4):1998-2011.
- VOM"ENDT"D,"SOARES"E"SILVA"M,"KIJNE"J"W,"PASQUALI"G,"MEMELINK"J."Identification"of"a"bipartite"jasmonate-responsive"promoter"element"in"the"Catharanthus"roseus"ORCA3"transcription"factor"gene"that"interacts"specifically"with"AT-hook"DNA-binding"proteins[J]."Plant"Physiology,"2007,"144(3):"1680-1689.
- HU"W,"JI"C,"LIANG"Z,"YE"J,"OU"W,"DING"Z,"ZHOU"G,"TIE"W,"YAN"Y,"YANG"J,"MA"L,"YANG"X,"WEI"Y,"JIN"Z,"XIE"J,"PENG"M,"WANG"W,"GUO"A,"XU"B,"GUO"J,"CHEN"S,"WANG"M,"ZHOU"Y,"LI"X,"LI"R,"XIAO"X,"WAN"Z,"AN"F,"ZHANG"J,"LENG"Q,"LI"Y,"SHI"H,"MING"R,"LI"K."Resequencing"of"388"cassava"accessions"identifies"valuable"loci"and"selection"for"variation"in"heterozygosity[J]."Genome"Biology,"2021,"22(1):"316.
- CHURCHILL"M"E,"TRAVERS"A"A."Protein"motifs"that"recognize"structural"features"of"DNA[J]."Trends"in"Biochemical"Sciences,"1991,"16(1):"92-97.
- GOODWIN"G"H,"SANDERS"C,"JOHNS"E"W."A"new"group"of"chromatin-associated"proteins"with"a"high"content"of"acidic"and"basic"amino"acids[J]."European"Journal"of"Biochemistry,"1973,"38(1):"14-19.
- 王元元,"刘姣,"郭育强,"王硕,"符少萍,"段瑞军,"李瑞梅,"姚远,"胡新文,"郭建春."AT-hook蛋白的最新研究进展[J]."基因组学与应用生物学,"2020,"39(2):"713-717.WANG"Y"Y,"LIU"J,"GUO"Y"Q,"WANG"S,"FU"S"P,"DUAN"R"J,"LI"R"M,"YAO"Y,"HU"X"W,"GUO"J"C."Recent"advances"in"AT-hook"proteins[J]."Genomics"and"Applied"Biology,"2020,"39(2):"713-717."(in"Chinese)
- ZHAO"J,"FAVERO"D"S,"PENG"H,"NEFF"M"M."Arabidopsis"thaliana"AHL"family"modulates"hypocotyl"growth"redundantly"by"interacting"with"each"other"via"the"PPC/DUF296"domain[J]."Proceedings"of"the"National"Academy"of"Sciences,"2013,"110(48):"E4688-E4697.
- ARAVIND"L,"LANDSMAN"D."AT-hook"motifs"identified"in"a"wide"variety"of"DNA-binding"proteins[J]."Nucleic"Acids"Research,"1998,"26(19):"4413-4421.
- DO"H"J,"SONG"H,"YANG"H"M,"KIM"D"K,"KIM"N"H,"KIM"J"H,"CHA"K"Y,"CHUNG"H"M,"KIM"J"H."Identification"of"multiple"nuclear"localization"signals"in"murine"Elf3,"an"ETS"transcription"factor[J]."FEBS"Letters,"2006,"580(7):"1865-"1871.
- DELANEY"S"K,"ORFORD"S"J,"MARTIN-HARRIS"M,"TIMMIS"J"N."The"fiber"specificity"of"the"cotton"FSltp4"gene"promoter"is"regulated"by"an"AT-rich"promoter"region"and"the"AT-hook"transcription"factor"GhAT1[J]."Plant"amp;"Cell"Physiology,"2007,"48(10):"1426.
- FUJIMOTO"S,"MATSUNAGA"S,"YONEMURA"M,"UCHI YAMA"S,"AZUMA"T,"FUKUI"K."Identification"of"a"novel"plant"MAR"DNA"binding"protein"localized"on"chromosomal"surfaces[J]."Plant"Molecular"Biology,"2004,"56(2):"225-239.
- 王晓彤,"张建禹,"耿沙,"任思杨,"毋志浩,"李瑞梅,"姚远,"郭建春,"刘姣,"胡新文."木薯MeAHL22基因的克隆及功能初步分析[J]."分子植物育种,"2022,"20(5):"1443-1451.WANG"X"T,"ZHANG"J"Y,"GENG"S,"REN"S"Y,"WU"Z"H,"LI"R"M,"YAO"Y,"GUO"J"C,"LIU"J,"HU"X"W."Cloning"and"functional"analysis"of"MeAHL22"gene"in"cassava[J]."Molecular"Plant"Breeding,"2022,"20(5):"1443-1451."(in"Chinese)
- SIRL"M,"SNAJDROVA"T,"GUTIERREZ-ALANIS"D,"DUBROVSKY"J"G,"VIELLE-CALZADA"J"P,"KULICH"I,"SOUKUP"A."AT-Hook"motif"nuclear"localised"protein"18"as"a"novel"modulator"of"root"system"architecture[J]."International"Journal"of"Molecular"Sciences,"2020,"21(5):"1886.
- FAVERO"D"S,"KAWAMURA"A,"SHIBATA"M,"TAK EBAYASHI"A,"JUNG"J"H,"SUZUKI"T,"JAEGER"K"E,"ISHIDA"T,"IWASE"A,"WIGGE"P"A,"NEFF"M"M,"SUGI MOTO"K."AT-hook"transcription"factors"restrict"petiole"growth"by"antagonizing"PIFs[J]."Current"Biology,"2020,"30(8):"1454-1466.

