花生茎源茉莉炭疽菌的分离鉴定及生物学特性研究


摘 要: 旨在探究首次从花生茎中分离出茉莉炭疽菌(Colletotrichum jasminigenum)的生物学特性。通过三点接种法,经马铃薯葡萄糖琼脂(potato dextrose agar, PDA)培养基分离培养,分别用乳酸酚棉蓝染色和扫描电镜观察其形态,提取分离真菌DNA,用PCR方法扩增ITS区并测序,比对该基因同源性及遗传进化关系。将孢子悬液以5×107 CFU·mL-1剂量感染昆明小鼠,并观察小鼠的临床症状、血清生化指标和剖检病变。用K-B纸片法探究其耐药表型。结果显示,分离株分生孢子长29.6 μm± 0.87 μm,呈月牙形;菌丝透明无性,有隔和分枝,无刚毛;附着孢呈棍棒状或者卵球形。PCR扩增得到该菌ITS区序列长度为586 bp,序列提交GenBank(OR472966)。根据形态学结合ITS区序列分析结果确定分离株为茉莉炭疽菌,命名为SLY01。用SLY01菌株攻毒小鼠14 d后导致血清碱性磷酸酶含量极显著升高(P<0.01),肝脏器指数显著下降(P<0.05),并从感染小鼠体内再次分离鉴定出茉莉炭疽菌。组织病理学结果显示,试验组小鼠肺泡壁水肿伴有大量淋巴细胞浸润;肝细胞出现大面积颗粒变性,肝小叶内见出血和肝细胞坏死;肾可见肾小管上皮发生颗粒变性。药敏试验显示,SLY01菌株对卡泊芬净和伏立康唑耐药,对两性霉素B敏感,对伊曲康唑中度敏感。本研究分离鉴定得到茉莉炭疽菌,感染小鼠主要损伤靶器官为肝、肺,并且对多种药物耐药。这为进一步研究茉莉炭疽菌的防治提供理论依据。
关键词: 茉莉炭疽菌;分离鉴定;致病性;耐药性
中图分类号: S852.661
文献标志码:A""" 文章编号:0366-6964(2024)05-2206-08
收稿日期:2023-08-15
基金项目:四川省科技厅科研基金项目(2023YFQ0077);四川省肉羊创新团队防疫岗位(sccxtd-2020-14);西南民族大学2023年中央高校优秀学生培养工程项目(2023NYXXS116)
作者简介:郑 芮(1999-),女,云南昆明人,硕士生,主要从事动物病理学研究,E-mail:zryjsyx@163.com
*通信作者: 王 利,主要从事动物病理学研究,E-mail:qinxin916@aliyun.com;魏 勇,主要从事动物疫病防控,E-mail:veishangyan@163.com
Isolation, Identification and Biological Characterization of Colletotrichum jasminigenum
in Stems of Peanuts
ZHENG" Rui1, LIU" Zishi1, ZHANG" Kangyou2, YAN" Yong2, WEI" Ling2, ZEREN" Wengmu2,
DINGZE" Demi2, HUANG" Jianjun2, WANG" Li1*, WEI" Yong3*
(1.Southwest University for Nationalities, Qinghai Tibet Plateau Animal Genetic
Resources Protection and Utilization Key Laboratory of the Ministry of Education and Sichuan
Province, Chengdu 610041," China;
2.Agriculture, Animal Husbandry, Rural Affairs and
Science and Technology Bureau of Yajiang County, Ganzi 626700," China;
3.Sichuan Academy of Animal Husbandry Sciences, Chengdu 610066," China)
Abstract:" The objective of this study was to investigate the biological properties of Colletotrichum jasminigenum. The fungi were cultivated in PDA using the three-spot inoculation method, followed by observation of their morphology through lactophenol cotton blue staining and scanning electron microscopy. Fungal DNA was extracted via PCR to amplify the ITS region of the gene and sequenced for comparison of gene homology and genetic evolutionary relationships. The concentration of the spore suspension was adjusted to 5×107 CFU·mL-1 for mouse infection, and clinical symptoms, serum biochemical indexes, and anatomical lesions were observed. The Kirby-Bauer method was employed to investigate fungal drug resistance phenotypes. The results showed that the isolated conidia were 29.6 μm± 0.87 μm in length and crescent-shaped. The mycelium was hyaline, asexual, septate, and branched without bristles. Additionally, the adherent spores were clavate or ovoid in shape. The PCR amplification yielded a sequence length of 586 bp for the ITS region of the fungi, which was subsequently submitted to GenBank (OR472966). Based on both morphology and sequence analysis of the ITS region, it was determined that the isolate belonged to Colletotrichum jasminigenum and was named SLY01. Attacking mice with strain SLY01 for 14 days resulted in a highly significant increase in serum alkaline phosphatase levels (Plt;0.01) and a significant decrease in liver organ index (Plt;0.05). Colletotrichum jasminigenum was reisolated from the infected mice. Histopathological results showed edema of the alveolar walls with massive lymphocytic infiltration, extensive granular degeneration with hemorrhage and hepatocellular necrosis in the lobules of hepatocytes, and granular degeneration of the renal tubular epithelium in the kidneys. The results of the drug sensitivity test showed that SLY01 was resistant to caspofungin and voriconazole, sensitive to amphotericin B and moderately sensitive to itraconazole. In this study, Colletotrichum jasminigenum was isolated and characterized. The liver and lungs were identified as the main target organs affected in infected mice, and the fungus showed resistance to various drugs. These findings provide a theoretical basis for further research on fungal infections originating from food sources.
Key words: Colletotrichum jasminigenum; isolation and identification; pathogenicity; drug resistance
*Corresponding authors:WANG Li, E-mail:qinxin916@aliyun.com;WEI Yong,E-mail: veishangyan@163.com
茉莉炭疽菌是炭疽菌属(Colletotrichum Corda)真菌,由Agarwal和Sahni[1]在1965年从茉莉叶斑中第一次分离。在越南,该种炭疽病曾对人工林造成大面积损失,感染树叶和花朵,导致落叶和枯死[2]。炭疽菌属作为一种世界性的真菌属,现存超过351个公认的真菌种,归于菌物界(Fungi),真菌门(Eumycota),半知菌亚门(Deuteromycotina),腔孢纲(Coelomycetes),黑盘孢目(Melanconiales),主要分布在全球热带和温带地区[3-5]。炭疽菌属真菌被认为是植物致病菌前十位的病原体之一[6],可引起严重的炭疽病和果腐病[7-8],殃及多种经济类作物如花生果腐病,甘蔗红腐病、咖啡浆果病、草莓和香蕉冠腐病以及豇豆褐斑[3,9-11]。目前,已报道炭疽菌属真菌的次生代谢物超过109种,主要包括氮代谢物、甾醇、萜烯、吡喃酮、酚类和脂肪酸[12-13]。并且炭疽菌属真菌毒素普遍具有热稳定、抗酸碱的特性,因此在草料中难以去除[14-15]。
炭疽菌属真菌主要通过吸入孢子或接触皮肤伤口的方式感染动物。Winter等[16]报道炭疽菌属真菌接触性感染猫后会引起皮下肿块、溃疡和红斑,嗜酸性粒细胞增多症,肺实质性病变,腹部超声显示双肾结构异常。尖孢炭疽感染的昆虫体内有大量的菌核肿块[17],肯普氏海龟感染后肺部和肾发生了弥散性真菌侵袭[18]。人类炭疽菌属感染发生在一些创伤事件后,如自然损伤或白内障手术。Guarro等[19]报道一例有外伤史的糖尿病患者,由胶孢炭疽菌引起皮下感染,并在脓肿灶中分离鉴定到胶孢炭疽菌。炭疽菌属真菌引起角膜炎的病原菌通常包括辣椒炭疽病菌、黑线炭疽菌和禾生炭疽菌[20-23]。这些报道证明炭疽菌属真菌作为条件致病菌可能引起全身病理损伤并且可以在机体定植。目前,国内外鲜有炭疽菌属真菌对动物致病性的研究,基于此本研究在草料花生茎分离、鉴定得到茉莉炭疽菌,对分离菌的病原特征、致病性和耐药性开展研究,为家畜感染炭疽杆菌属真菌疾病的用药和进一步研究提供理论基础。
1 材料与方法
1.1 样品来源和实验动物
真菌分离样品来源于四川省资阳市某羊场草料中的花生茎。试验动物为10只健康状况良好,体况相近的SPF级雌性KM小鼠,体重21 g±3 g,5周龄,购自四川省成都市中医药研究所。
1.2 主要仪器和试剂
马铃薯葡萄糖琼脂培养基(potato dextrose agar,PDA)和马铃薯液体培养基(potato dextrose broth,PDB)均购自成都联智创思生物技术有限公司;乳酸酚棉蓝染色液(R20704)购自上海源叶生物科技有限公司;扫描电镜(JSM-IT700HR)购自日本电子株式会社;CTAB抽提液(NE0011)购自北京雷根生物技术有限公司;PCR仪购自Eppendorf Germany公司;谷草转氨酶(glutamic-oxaloacetic transaminase,AST)测定试剂盒、谷丙转氨酶(glutamic-pyruvic transaminase,ALT)测定试剂盒、碱性磷酸酶(alkaline phosphatase,ALP)测定试剂盒购自深圳迈瑞生物医疗电子股份有限公司;药敏纸片(antibiotic disc)购自意大利Liofilchem公司。
1.3 真菌分离培养和形态观察
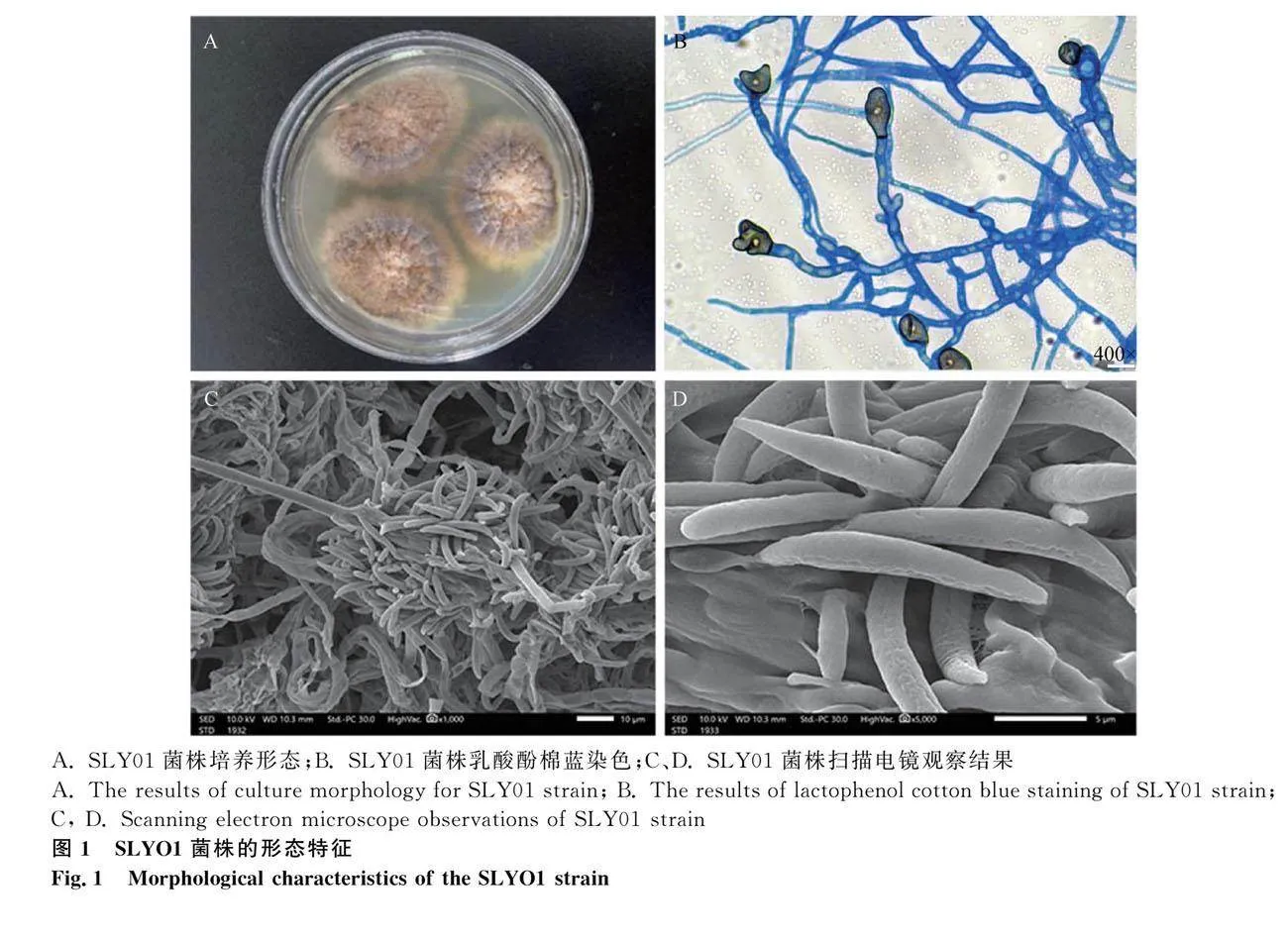
花生茎加入10 mL无菌生理盐水震荡混匀,取200 μL菌液涂布于PDA培养基(28 ℃)培养。待长出肉眼可见菌落后分三点接种至新的PDA培养基。反复分离纯化三代后得到单一菌落。纯化后的真菌在(28 ℃)恒温培养箱中培养并观察其形态。分离株培养7 d后,进行乳酸酚棉蓝染色。
1.4 扫描电镜样品准备及观察
将单菌落置于PDA培养基培养6 d后,用无菌手术刮刀取直径为0.5 cm的菌饼,用无菌生理盐水清洗杂质和表面附着物。加入3%的戊二醛固定(4 ℃,24 h)。随后将固定好的样本经超纯水(ultrapure water,UP)清洗3次,每次10 min。再用1%锇酸固定(1 h),UP水清洗3次,每次10 min。用30%~100%梯度酒精逐级脱水,每次15 min。将样品放入离子溅射仪进行喷镀处理。并置于扫描电镜下,选择适当的区域进行观察与拍照。
1.5 ITS基因鉴定及进化树构建
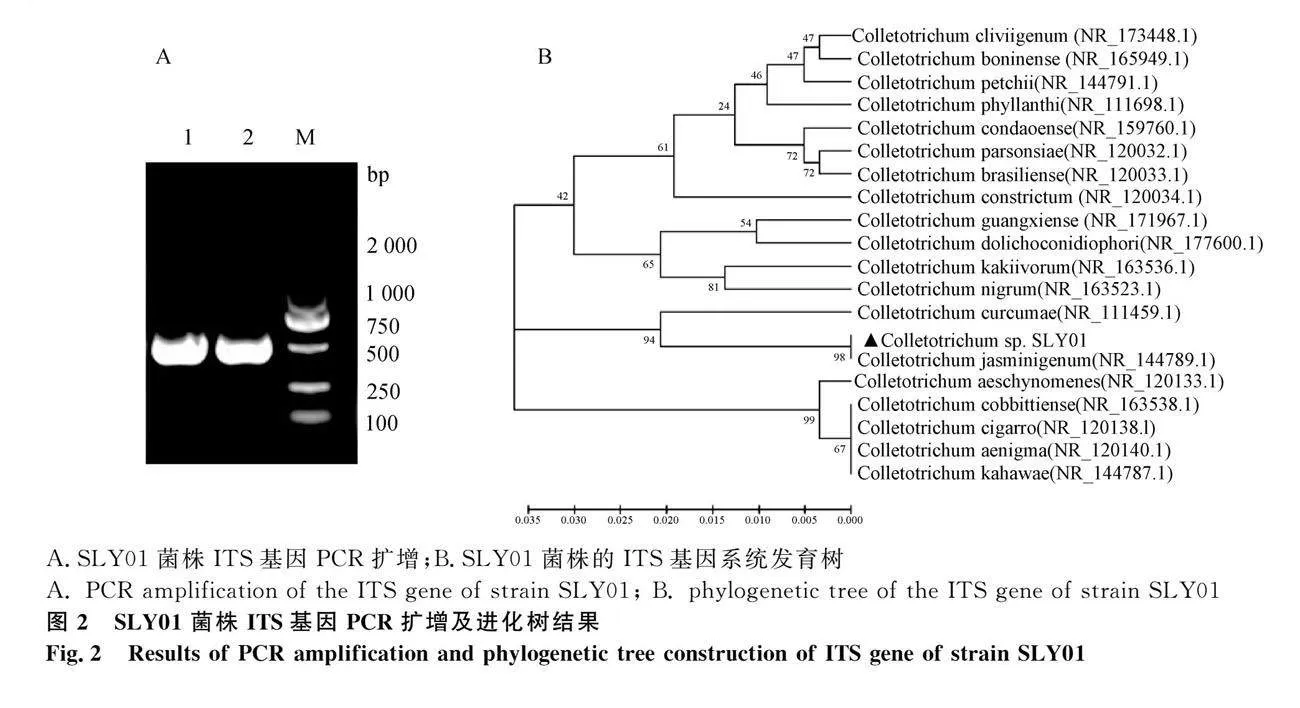
挑取SLY01菌株单菌落接种于PDA培养基,28 ℃恒温培养5 d后,参照CTAB说明书提取总DNA。以提取的总DNA为模板,用真菌rDNA通用引物ITS1:5′-TCCGTAGGTGAACCTGCGG-3′和ITS4:5′-TCCTCCGCTTATTGATATGC-3′为上下游引物[24]。对核糖体rDNA-ITS基因PCR扩增,总体系为50 μL,反应条件:98 ℃ 2 min;98 ℃ 10 s,56 ℃ 10 s,72 ℃ 30 s,35个循环;72 ℃ 2 min。扩增结果通过1%琼脂糖凝胶电泳检测后,于凝胶成像系统下观察并拍照。上海生工生物工程股份有限公司对PCR产物进行双向测序并拼接,测序结果经Blast比对后,用Mega5.0软件采用近邻相接法(Neighbor-joining method)对SLY01菌株 ITS序列进行同源性分析,构建进化树。
1.6 小鼠致病性试验
收集SLY01菌株孢子悬液,用血球计数法,将孢子悬液浓度调整为5×107 CFU·mL-1[25-26]。筛选体况相近的10只雌性SPF级KM小鼠置于西南民族大学动物实验中心,光照/暗循环12 h,温度22~26 ℃,湿度40%~70%。小鼠适应环境3 d,可自由饮水和进食,然后进行下一步试验。小鼠随机分组为试验组、对照组,每组5只。试验组小鼠每隔1 d于上午08:00腹腔注射0.3 mL,浓度为5×107 CFU·mL-1[25-26]的SLY01菌株孢子悬液。对照组腹腔注射等量灭菌生理盐水,试验周期为14 d。试验结束后所有小鼠眼眶采血,血清分离后保存于-20 ℃用于检测生化指标。处死并剖检小鼠,取心、肝、脾、肺、肾等组织用吸水纸吸干表面水分后称重,用于计算脏器指数。将组织放置于4%多聚甲醛固定,用于制作病理切片。无菌操作取小鼠各器官,通过形态学观察并分析菌株ITS区序列,分离鉴定病原。
1.7 血清生化指标检测
采用兽用生化分析仪检测对照组和试验组小鼠血清生化指标,包括AST、ALP和ALT。
1.8 组织切片制作和HE染色
取“1.6”中经过4%多聚甲醛固定的小鼠组织进行常规脱水、透明、包埋、切片和HE染色,置于光学显微镜下观察各组织病变情况。
1.9 药敏试验
按照真菌药敏试验检测说明书,收集 SLY01菌株孢子悬液,用血球计数法,将孢子悬液浓度调整为6.5×105 CFU·mL-1。采用K-B纸片法测定菌株对两性霉素B、伊曲康唑、卡泊芬净和伏立康唑四种药敏纸片的敏感性。结果判定参照意大利Liofilchem公司的真菌药敏纸片判读标准。
1.10 数据处理
用IBM SPSS Statistics 26.0对数据进行单因素方差分析,P<0.01表示差异极显著;P<0.05表示差异显著;P>0.05表示差异不显著。
2 结 果
2.1 分离株PDA培养基生长和扫描电镜形态观察
SLY01菌株在PDA培养基(28 ℃)恒温培养7 d后生长情况:菌落表面干燥呈凸起,边缘向内呈灰色至橙色,边缘有黑色斑点,边缘形成深裂,反面有褐色斑点,中间有褐色带,边缘呈现流苏状。乳酸酚棉蓝染色和扫描电镜结果显示:菌株分生孢子长29.6 μm±0.87 μm,月牙形,中间较宽;透明的无性菌丝,有隔和分枝,无刚毛,未观察到衣孢子;孢子新月形,单室,壁光滑,中央宽,透明,有沟,附着孢呈棍棒状或者卵球形,主要由菌丝体形成。初步鉴定为茉莉炭疽菌 (图1)。
2.2 ITS基因鉴定及进化树分析
提取SLY01菌株的总DNA,PCR扩增菌株ITS序列,得到大小约500 bp的目的片段。测序后,发现分离株的ITS序列长度为586 bp,与预期相符。系统发育树构建结果显示,SLY01菌株ITS区序列与茉莉炭疽菌相应基因序列相似性达98%,但与同属18种其他菌株存在较大差异。ITS区序列提交到NCBI中的GenBank编号为OR472966。结合形态学和序列对比结果综合判定,分离株为茉莉炭疽菌(图2)。
2.3 小鼠致病性试验
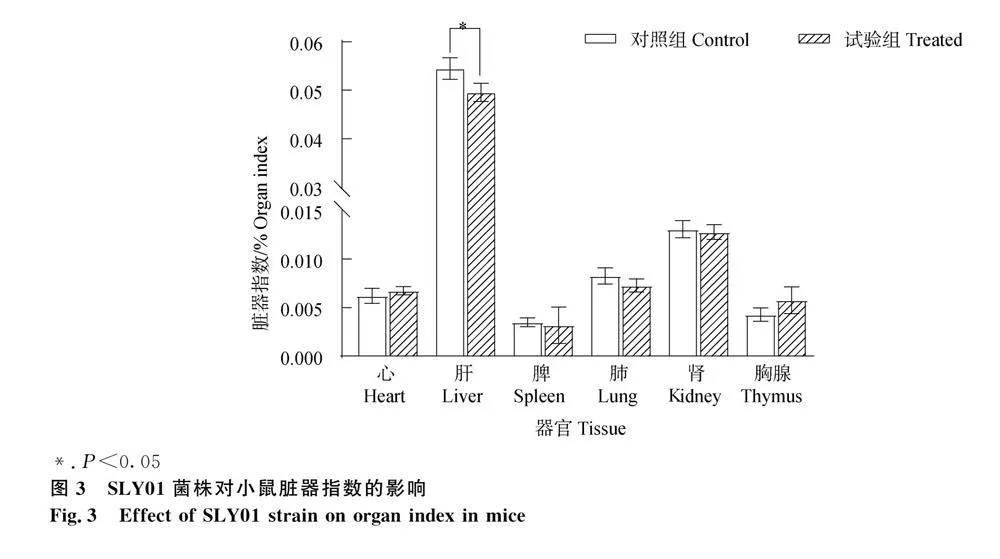
小鼠攻毒14 d后,试验组均表现出精神沉郁、少动并且伴随腹式呼吸,采食量下降,但未出现死亡,对照组未发病。试验结束剖检小鼠,试验组小鼠肝表面出现白色斑块,肺充血肿胀,肾轻微肿胀,其他脏器与对照组相比未见明显变化,之后从感染小鼠体内再次分离鉴定到茉莉炭疽菌。称量各器官湿重计算脏器指数,结果显示,试验组肝脏器指数与对"" 照组相比显著降低(P<0.05)(图3),其余器官脏器指数均无明显变化。
2.4 血清生化指标检测
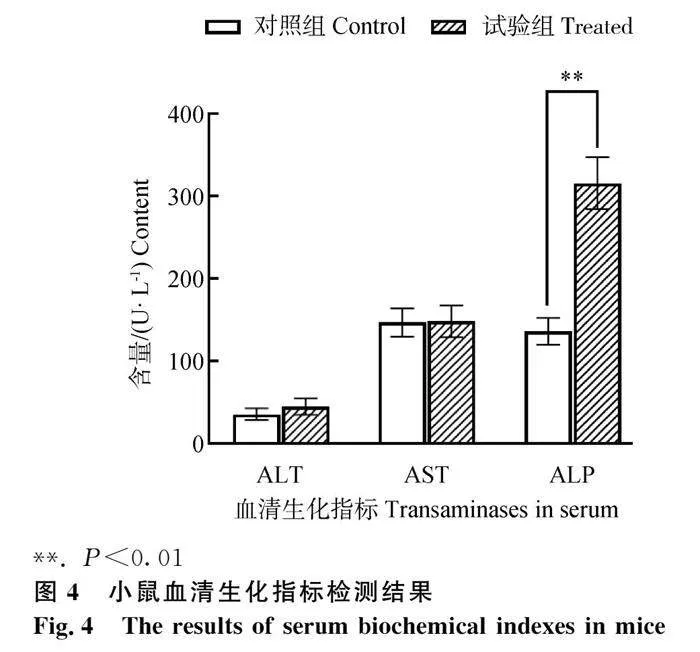
肝损伤的三个生化指标检测结果显示,与对照组相比,试验组小鼠血清ALP含量极显著升高(P<0.01)(图4),血清中ALT和AST没有明显变化。这提示肝组织发生炎症反应。
2.5 小鼠病理切片观察
小鼠各组织切片经HE染色后,在显微镜下观察。结果显示,试验组小鼠心动脉充血;肺支气管充血并有组织液渗出,肺泡壁水肿伴有大量淋巴细胞浸润,肺泡轻微出血;肝中央静脉淤血,肝细胞有大面积颗粒变性,肝小叶结构不清晰,肝小叶内见出血和肝细胞坏死,炎性细胞浸润;肾见肾小管上皮细胞发生颗粒变性,肾小球毛细血管内皮细胞和系膜细胞水肿(图5)。
2.6 耐药性试验
用K-B纸片法评价SLY01菌株对4种常见抗真菌药物的敏感性,结果表明:SLY01菌株对卡泊芬净和伏立康唑耐药;对两性霉素B敏感;对伊曲康唑中度敏感(表1)。
3 讨 论
炭疽杆菌属真菌已是世界公认的植物致病菌[27]。其主要分布在全球湿润和半湿润热带地区[10]。关于炭疽杆菌属真菌对动物致病的报道包括感染昆虫、幼年肯普雷氏海龟和人类[16]。Wikee等[28]在2011年从茉莉花病叶和花中分离鉴定一株茉莉炭疽菌,PDA上观察到菌落呈火山口状,气生菌丝白色,边缘流苏状,反面乳白色;麦芽浸粉琼脂培养基上形成白色菌落,火山口状,气生菌丝白色,边缘和反面呈乳白色;显微镜观察到透明的无性菌丝,有隔有分枝,宽20~28 μm;分生胞透明,长23~39 μm[2,28]。与本研究分离得到的SLY01菌株形态相似。在核糖体密码子区域中,内部转录间隔区(ITS)成功鉴定真菌的概率最高,种间和种内变异之间的条形码差距最明显[29]。所以将分离株TIS区PCR 扩增并测序,测序结果用 BLAST进行同源性比对,结果与表型鉴定结果相吻合,证实SLY01菌株为茉莉炭疽菌。
炭疽菌属真菌感染通常通过皮肤受损的地方进入机体,而且多数感染是在免疫系统受损的患者中发生[30]。血清内源性酶升高提示肝细胞损伤到了细胞器的水平[19-20]。血清中ALP主要来自肝胆管上皮细胞和骨骼,ALP升高提示胆管阻塞或者肝细胞受损,其他真菌感染时该指标也显示异常[31]。Manire等[18]报道的尖孢炭疽感染海龟后出现AST升高,组织病理学检查结果包括严重的肾炎和肺炎,中度肝炎,并从肾和肺中分离到尖孢炭疽。猫感染炭疽杆菌属真菌出现真菌定植皮下引起溃疡和红斑,嗜酸性粒细胞增多症,肺实质病变,腹部超声显示双肾结构异常[16]。在人感染炭疽杆菌属真菌的致死病例中,尸检显示右肺出现混合性炎症反应和大面积坏死灶,并伴有分枝菌丝浸润到血管间隙[30]。本研究血清生化结果显示,SLY01菌株感染小鼠后试验组血清ALP极显著高于对照组(P<0.01),提示肝发生炎症反应。进一步通过小鼠的组织病理学结果证实,分离株与同属真菌的致病性报告均出现肺部的混合性炎症反应,主要靶器官为肝、肺。但本研究试验组小鼠肺部未出现大面积坏死,可能是炭疽菌属亚种间的毒力和致病机制不同所致。
药敏试验对指导真菌感染临床用药有重要意义,治疗抗真菌药物的唑类药物有伏立康唑、伊曲康唑;棘白素类药物有卡伯芬净;多烯类药物有两性霉素B[32]。病例报道感染炭疽属真菌的猫用伊曲康唑治疗后其症状得到改善[16];而人感染胶孢炭疽菌可用两性霉素B和伊曲康唑治疗[19];这与SLY01菌株药敏试验结果相同。这说明炭疽杆菌属真菌普遍对两性霉素B和伊曲康唑敏感,临床动物感染茉莉炭疽菌可使用多烯类和唑类药物进行治疗。
4 结 论
本研究从花生茎中分离得到SLY01菌株,鉴定为炭疽杆菌属茉莉炭疽菌。菌株感染小鼠可导致血清ALP升高,肝、肺出现病理损伤。药敏试验显示菌株对卡泊芬净和伏立康唑耐药,对两性霉素B敏感,对伊曲康唑中度敏感。这为进一步研究饲草源真菌感染提供理论依据。
参考文献(References):
[1] AGARWAL G P, SAHNI V P. Fungi causing plant diseases at Jabalpur (M.P.) XI[J]. Mycopathol Mycol Appl, 1965, 27(1):136-144.
[2] SUN M Y, LI C N, LI X M, et al. First report of Colletotrichum siamense causing leaf spot on Jasminum sambac in China[J]. Plant Dis, 2023, 107(7):2225.
[3] SHI X C, WANG S Y, DUAN X C, et al. First report of Colletotrichum brevisporum causing soybean anthracnose in China[J]. Plant Dis, 2021, 105(3):707.
[4] FREEMAN S, KATAN T. Identification of Colletotrichum species responsible for anthracnose and root necrosis of strawberry in Israel[J]. Phytopathology, 1997, 87(5):516-521.
[5] WEIR B S, JOHNSTON P R, DAMM U. The Colletotrichum gloeosporioides species complex[J]. Stud Mycol, 2012, 73(1):115-180.
[6] DOS SANTOS VIEIRA W, VELOSO J S, SILVA A C D, et al. Elucidating the Colletotrichum spp." diversity responsible for papaya anthracnose in Brazil[J]. Fungal Biol, 2022, 126(10):623-630.
[7] CAMILETTI B X, LICHTEMBERG P S F, PAREDES J A, et al. Characterization of Colletotrichum isolates causing Colletotrichum dieback of citrus in California[J]. Phytopathology, 2022, 112(7):1454-1466.
[8] DA SILVA L L, MORENO H L A, CORREIA H L N, et al. Colletotrichum:species complexes, lifestyle, and peculiarities of some sources of genetic variability[J]. Appl Microbiol Biotechnol, 2020, 104(5):1891-1904.
[9] THAN P P, PRIHASTUTI H, PHOULIVONG S, et al. Chilli anthracnose disease caused by Colletotrichum species[J]. J Zhejiang Univ Sci B, 2008, 9(10):764-778.
[10] WANG Q T, LIU F, HOU C L, et al. Species of Colletotrichum on bamboos from China[J]. Mycologia, 2021, 113(2):450-458.
[11] 范腕腕, 李绍建, 桑素玲, 等. 河南省花生果腐病病原菌的分离及鉴定[J]. 中国油料作物学报, doi:10. 19802/j. issn. 1007-9084. 2022289.
FAN W W, LI S J, SANG S L, et al. Isolation and identification of the pathogens causing peanut pod rot in Henan province[J]. Chinese Journal of Oil Crop Sciences, doi:10. 19802/j. issn. 1007-9084. 2022289. (in Chinese)
[12] KIM J W, SHIM S H. The fungus Colletotrichum as a source for bioactive secondary metabolites[J]. Arch Pharm Res, 2019, 42(9):735-753.
[13] TAO H, LAUTERBACH L, BIAN G K, et al. Discovery of non-squalene triterpenes[J]. Nature, 2022, 606(7913):414-419.
[14] ZHANG G M, FANG B H, CHEN H, et al. Characteristics of the toxin extracted from liquid culture of Colletotrichum capsici F." nicotianae[J]. Appl Biochem Biotechnol, 2012, 167(1):52-61.
[15] XU D, XUE M Y, SHEN Z, et al. Phytotoxic secondary metabolites from fungi[J]. Toxins (Basel), 2021, 13(4):261.
[16] WINTER R L, LAWHON S D, HALBERT N D, et al. Subcutaneous infection of a cat by Colletotrichum species[J]. J Feline Med Surg, 2010, 12(10):828-830.
[17] MARCELINO J, GIORDANO R, GOULI S, et al. Colletotrichum acutatum var." fioriniae (teleomorph:Glomerella acutata var." fioriniae var." nov.) infection of a scale insect[J]. Mycologia, 2008, 100(3):353-374.
[18] MANIRE C A, RHINEHART H L, SUTTON D A, et al. Disseminated mycotic infection caused by Colletotrichum acutatum in a Kemp’s Ridley sea turtle (Lepidochelys kempi)[J]. J Clin Microbiol, 2002, 40(11):4273-4280.
[19] GUARRO J, SVIDZINSKI T E, ZAROR L, et al. Subcutaneous hyalohyphomycosis caused by Colletotrichum gloeosporioides[J]. J Clin Microbiol, 1998, 36(10):3060-3065.
[20] RITTERBAND D C, SHAH M, SEEDOR J A. Colletotrichum graminicola:a new corneal pathogen[J]. Cornea, 1997, 16(3):362-364.
[21] SHUKLA P K, KHAN Z A, LAL B, et al. Clinical and experimental keratitis caused by the Colletotrichum state of Glomerella cingulata and Acrophialophora fusispora[J]. Sabouraudia, 1983, 21(2):137-147.
[22] BUCHTA V, NEKOLOV J, JIRSKOV N, et al. Fungal keratitis caused by Colletotrichum dematium:case study and review[J]. Mycopathologia, 2019, 184(3):441-453.
[23] WANG L, YU H, JIANG L, et al. Fungal keratitis caused by a rare pathogen, Colletotrichum gloeosporioides, in an east coast city of China[J]. J Mycol Md, 2020, 30(1):100922.
[24] 邓 静, 刘吉华, 余伯阳. 具有生物碱转化活力的4株喜树内生真菌的鉴定[J]. 药物生物技术, 2006, 13(6):436-441.
DENG J, LIU J H, XU B Y. The identities of 4 strains of alkaloids-bioconverting endophytic fungi isolated from Camptotheca Acuminata Decne[J]. Pharmaceutical Biotechnology, 2006, 13(6):436-441. (in Chinese)
[25] 马晓平, 姜尧章, 张和民, 等. 大熊猫源杯梗孢属真菌(Cyphellophora pluriseptata)的分离鉴定与致病性研究[J]. 中国预防兽医学报, 2018, 40(11):1066-1070.
MA X P, JIANG Y Z, ZHANG H M, et al. Isolation, identification and pathogenicity of Cyphellophora pluriseptata from Giant panda[J]. Chinese Journal of Preventive Veterinary Medicine, 2018, 40(11):1066-1070. (in Chinese)
[26] MA X P, JIANG Y Z, WANG C D, et al. Identification, genotyping, and pathogenicity of Trichosporon spp. Isolated from Giant pandas (Ailuropoda melanoleuca)[J]. BMC Microbiol, 2019, 19(1):113.
[27] DEAN R, VAN KAN J A L, PRETORIUS Z A, et al. The top 10 fungal pathogens in molecular plant pathology[J]. Mol Plant Pathol, 2012, 13(4):414-430.
[28] WIKEE S, CAI L, PAIRIN N, et al. Colletotrichum species from Jasmine (Jasminum sambac)[J]. Fungal Divers, 2011, 46(1):171-182.
[29] SCHOCH C L, SEIFERT K A, HUHNDORF S, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi[J]. Proc Natl Acad Sci USA, 2012, 109(16):6241-6246.
[30] O′QUINN R P, HOFFMANN J L, BOYD A S. Colletotrichum species as emerging opportunistic fungal pathogens:a report of 3 cases of phaeohyphomycosis and review[J]. J Am Acad Dermatol, 2001, 45(1):56-61.
[31] GIANNINI E G, TESTA R, SAVARINO V. Liver enzyme alteration:a guide for clinicians[J]. Can Med Assoc J, 2005, 172(3):367-379.
[32] SHARMA U, PAL D, PRASAD R. Alkaline phosphatase: an overview[J]. Indian J Clin Biochem, 2014, 29(3):269-278.
(编辑 "白永平)

