Characterization of Domeless receptors and the role of BdDomeless3 in anti-symbiont-like virus defense in Bactrocera dorsalis
Wei Zhang ,Shaoyang Li ,Rong Li ,Jinzhi Niu ,Jinjun Wang#
1 Key Laboratory of Entomology and Pest Control Engineering,College of Plant Protection,Southwest University,Chongqing 400715,China
2 Key Laboratory of Agricultural Biosafety and Green Production of Upper Yangtze River,Ministry of Education/International Joint Laboratory of China-Belgium on Sustainable Crop Pest Control,Academy of Agricultural Sciences,Southwest University,Chongqing 400715,China
Abstract The Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway play a pivotal role in innate immunity.Among invertebrates,Domeless receptors serve as the key upstream regulators of this pathway.In our study on Bactrocera dorsalis,we identified three cytokine receptors: BdDomeless1,BdDomeless2,and BdDomeless3.Each receptor encompasses five fibronectin-type-III-like (FN III) extracellular domains and a transmembrane domain.Furthermore,these receptors exhibit the increased responsiveness to diverse pathogenic challenges.Notably,only BdDomeless3 is upregulated during symbiont-like viral infections.Moreover,silencing BdDomeless3 enhanced the infectivity of Bactrocera dorsalis cripavirus (BdCV) and B.dorsalis picorna-like virus (BdPLV),underscoring BdDomeless3’s crucial role in antiviral defense of B.dorsalis.Following the suppression of Domeless3 expression,six antimicrobial peptide genes displayed decreased expression,potentially correlating with the rise in viral infectivity.To our knowledge,this is the first study identifying cytokine receptors associated with the JAK/STAT pathway in tephritid flies,shedding light on the immune mechanisms of B.dorsalis.
Keywords: Bactrocera dorsalis,JAK/STAT pathway,Domeless receptors,antiviral immunity,symbiont-like virus
1.Introduction
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway regulates cellular and organismal development,proliferation,metabolism,infection,inflammation,and cancer (Philipset al.2022).Furthermore,the JAK/STAT pathway is a crucial component of the innate immune system that contributes to various immune responses in organisms,particularly the induction of interferon-stimulated genes for exerting antiviral effects (Huet al.2021).In insects such asDrosophila melanogaster,Aedes aegypti,andTribolium castaneum,the JAK/STAT pathway is relatively conserved (Bang 2019).Although the composition of the JAK/STAT pathway in invertebrates is simpler compared to vertebrates,it is still sufficient to provide similar signal transduction functions.InD.melanogaster,the key components of the JAK/STAT signaling pathway include unpaired ligands (Upd1,Upd2 and Upd3),a unique cytokine receptor (Domeless),an associated JAK tyrosine kinase (Hopscotch),and the substrate known as Stat92E (Sabinet al.2010).Additionally,the suppressors of cytokine signaling (SOCS) protein family,including Socs36E,Socs44A,and Socs16D,along with the protein inhibitors of activated STAT (PIAS),play crucial roles as negative regulators in the JAK/STAT pathway.
Although existing evidence suggests the JAK/STAT pathway is a crucial mechanism in modulating innate immunity in insects,most studies have primarily focused on model insect species such asD.melanogaster,A.aegypti,Anopheles gambiae,andBombyx mori.Previous research indicates that the JAK/STAT system is involved in regulating various aspects of insect biology,including growth,stem cell homeostasis,immune responses,and development (Bang 2019).Notably,the JAK/STAT pathway also plays a vital role in insect defense against parasitoids (Yanget al.2015).The first Domeless receptor discovered in invertebrates was found inD.melanogaster(Brownet al.2001).It is a transmembrane protein that shares structural and functional similarities to the interleukin 6 (IL-6) receptortype family,although its sequence has diverged significantly.InDrosophila,the Domeless receptor interacts with Upd,a known JAK/STAT ligand,to activate the highly conserved JAK/STAT signaling pathway.Furthermore,the interaction between the Domeless receptor and the SOCS family of proteins impedes signal transduction,potentially affecting the activation of the JAK/STAT pathway (Yasukawaet al.2003).These findings emphasize the critical role of the Domeless receptor in the activation of the JAK/STAT pathway.
The oriental fruit fly,Bactrocera dorsalis(Hendel) (Diptera: Tephritidae),is globally recognized as the most notorious and destructive horticultural pest (Zhang Yet al.2022).The indiscriminate and extensive use of chemical pesticides has led to widespread pesticide resistance and poses significant environmental concerns.Considering these issues,pathogen-mediated biological control ofB.dorsalishas emerged as a more natural and environmentally-friendly strategy.For example,Metarhizium anisopliae(Ma04) exhibits strong toxicity towardsB.dorsalis,resulting in pupal mortality and adversely affecting the survival of emerged adults (Wanget al.2021).The combined application ofBeauveria bassianaandHeterorhabditis bacteriophorahas also shown effective control againstB.dorsalis(Wakilet al.2022),although further exploration is required for field application.Therefore,an in-depth understanding of the innate immune system of this species is imperative for the development of microbial community-based biological control strategies.The immune deficiency (Imd) and Toll signaling pathways,which are known to regulate immune responses,have been studied extensively inB.dorsalis(Donget al.2016;Liet al.2017;Weiet al.2019;Yaoet al.2022).However,limited attention has been given to the JAK/STAT signaling pathway,particularly in agricultural insects.Consequently,to advance our understanding of the immunological mechanisms inB.dorsalis,we focused on the critical upstream receptor Domeless in the JAK/STAT signaling pathway,and unraveled the essential role of BdDomeless3 in antiviral immunity inB.dorsalis.
2.Materials and methods
2.1.Insect rearing
The laboratory colony ofB.dorsaliswas initially sourced from Hainan Province,China,and has been maintained long-term in an incubator at 27°C,with 70% relative humidity and a 14 h L:10 h D photoperiod,using an artificial diet.The formulations of both larval and adult artificial diets are based on the work of Chenet al.(2013).
2.2.Bioinformatics analysis
The nucleotide and amino acid sequences ofD.melanogaster Domeless(DmDomeless) were obtained from FlyBase (http://flybase.org).Based on theDmDomelesssequence,the homologous sequences ofB.dorsaliswere generated by BLAST software against local databases of theB.dorsalisgenome.The conserved domains in the proteins were predicted using online analytic tools (http://smart.embl-heidelberg.de/) and the Conserved Domain Database (https://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml) with an e-value threshold of 1×10-5.The MUSCLE program was used to analyze the multiple sequence alignments (https://www.ebi.ac.uk/Tools/msa/muscle/),and the alignment results were visualized using GeneDoc software.The prediction of transmembrane domains was carried out using the online software TMHMM-2.0 (https://services.healthtech.dtu.dk/service.php?TMHMM-2.0).
For phylogenetic analysis,the amino acid sequences of Domeless from four tephritid flies (B.dorsalis,B.tryoni,Zeugodacus cucuribitaeandCeratitis capitata) and representative species from nine insect orders were aligned using the Mafft software (https://www.ebi.ac.uk/Tools/msa/mafft/) with the auto model.Subsequently,a phylogenetic tree was constructed employing the maximum likelihood method with 1,000 bootstrap replicates in IQ-TREE (Nguyenet al.2015),utilizing the most suitable substitution model that was determined.The resulting phylogenetic tree was visualized using Figtree v.1.4.0 (http://tree.bio.ed.ac.uk/software/figtree/).
2.3.Sample collection of various developmental stages
To determine the differential expression levels of theDomelessgenes during various developmental stages,samples from the egg,larval,pupal,and adult periods were gathered.Fresh eggs were specifically collected and incubated in the previously described larval artificial diet.After 12 h,a random selection of 100 eggs was earmarked as the egg samples.At the 36-h mark posthatching,a group of 100 1st-instar larvae were randomly procured as representative samples of that stage.This process was repeated at 24 h following the first larval molt with the selection of 50 2nd-instar larvae,and then again at 48 h after the second molting occasion with the selection of 20 3rd-instar larvae.At 2 h post-pupation,20 pupae at the white puparium state were obtained randomly as pre-pupal samples;and at 148 h following pupation,20 pupae were gathered as late pupal samples.On the 1st,5th,and 9th days post-emergence,we acquired 10 adults (split evenly between the sexes) as samples denoting those respective stages.All samples were promptly frozen in liquid nitrogen and stored at -80°C for the ensuing examination.To ensure insect physiological uniformity,each specimen gathering event was conducted within 2 h and independently replicated four times.
2.4.Sample collection of various tissues
Nine-day-old adult flies,with males and females separated,were dissected in 0.01 mol L-1PBS buffer (pH=7.0) under an optical microscope (Chongqing Ott optical instruments,Chongqing,China).For RNA isolation,samples of the fat body,hemolymph,midgut,Malpighian tubules,ovaries,and testes were collected separately.Each sample was suspended in 1 mL of Trizol reagent (n=3) and then rapidly frozen using liquid nitrogen.
2.5.cDNA synthesis and RT-qPCR
Total RNA was extracted using a Trizol reagent (Life Technologies,Carlsbad,CA,USA) following the manufacturer’s instructions.Genomic DNA was subsequently removed using RQ-Free DNase (Promega,Madison,WI,USA).The resulting RNA was then utilized for cDNA synthesis using a first-strand cDNA synthesis kit (TaKaRa,Dalian,China).For reverse transcriptionquantitative PCR (RT-qPCR),the SYBR super mix (Novoprotein,Suzhou,China) was employed.All primers for RT-qPCR were designed using Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome).The evaluation of relative gene expression levels was performed using qBase+software (http://www.qbaseplus.com/),usingα-tubulinandribosomal protein subunit 3(rps3) as the reference genes (Shenet al.2010).
2.6.Microorganism infection
In our previous study (Liet al.2023),we provided details on the strain source and culture methods for grampositive bacteriumEnterococcus faecalis,gram-negative bacteriumPseudomonas aeruginosa,and fungusB.bassiana.For bacterial infection,four-day-old adults were injected with 200 nL of bacterial suspension (with a final A600 of 0.3 forE.faecalisand 0.1 forP.aeruginosa),while 0.01 mol L-1PBS was used as the negative control.Samples were collected at 4,8,and 12 h after infection for gene expression analysis.
Regarding fungal infection,four-day-old adults were subjected to a 1.5 mL spraying of the conidia suspension,which had a final concentration of 1.0×107conidia per mL.A sterile 0.05% Tween 80 solution was used as the negative control.Samples were collected at 48,60,and 72 h after treatment for subsequent RNA isolation and RTqPCR analysis.
The method outlined by Zhanget al.(2020) was used for viral infection,using RNA viruses obtained fromB.dorsalisadults.The RNA of the viral suspension was extracted to identify the virus,and virus-specific primers (Appendix A) were employed for RT-PCR.After confirming the viral composition,four-day-old adults were subjected to injection with 200 μL of the viral suspension,while an equivalent volume of 0.01 mol L-1PBS buffer served as the negative control.For RNA isolation and RT-qPCR,four biological samples (each consisting of three females and three males) were collected at 8,16,and 24 h after infection.The relative expression levels of threeBdDomelessgenes and three RNAi pathway core genes (Dicer2,Ago2,andR2D2) were subsequently determined through RT-qPCR analysis.The primer sequences used for RT-qPCR are provided in Appendix A.
2.7.Dynamics of virus replication-based RNAi
Double-stranded RNA (dsRNA) targetingBdDomeless3andGFPwas synthesizedin vitrousing the TranscriptAid T7 High Yield Transcription Kit (Thermo Fisher Scientific,Waltham,MA,USA),and the set of primers for dsRNA template generation are provided in Appendix A.
For the RNAi experiment,three-day-old adults were subjected to injection with 1 μg dsRNA,followed by another injection of the same dsRNA dose after a 24 h interval.DsGFPwas used as the negative control.Four samples were collected at 12,24,and 48 h after the second injection to assess RNAi efficiency.RNA extraction and cDNA synthesis were carried out in the same manner as described above.In addition,as potential downstream effectors of the JAK-STAT pathway,we also assessed the expression of six antimicrobial peptides (AMPs) genes (Diptericin A,Cecropin-1,Cecropin-like,Attacin-B,Attacin-CandAttacin-like) upon downregulation ofBdDomeless3,and the specific sequence information and primer details are presented in the Appendix A.Virus infections were initiated 24 h after the dsRNA treatment to examine the dynamics of virus replication.Four independent biological samples,each comprising three females and three males,were collected after infection for subsequent RNA isolation and RT-qPCR analysis.Subsequently,the relative viral titers were determined using qBase+software,relative to an appropriate reference gene.
2.8.Statistical analyses
All statistical analyses were performed using IBM SPSS 25.0 software (Armonk,NY,USA).One-way ANOVA was used to assess the significance of gene expression levels across different developmental stages and tissues.Posthoc comparisons were conducted using the Tukey test for cases with homogeneous variances and the Tamhane T2 test for cases with inhomogeneous variances.The statistical significance of gene expression levels and viral titers was determined by conducting independent samplet-tests following normality testing (*,P<0.05,**,P<0.01,and***,P<0.001).GraphPad Prism 8 software (GraphPad Software,San Diego,USA) was used for data visualization and figure plotting.
3.Results
3.1.Conserved domains and phylogenetic analysis
ThreeDomelessgenes,namelyBdDomeless1,BdDomeless2,andBdDomeless3,were identified based on genome annotations ofB.dorsalis(Yanget al.2023).The open reading frame (ORF) ofBdDomeless1spans 4,278 bp,encoding a protein of 1,425 residues (GenBank accession no.OQ560053).The ORF ofBdDomeless2,on the other hand,covers 3,459 bp and encodes a protein of 1,152 residues (GenBank accession no.OQ560054).Lastly,the ORF ofBdDomeless3measures 4,575 bp,encoding a protein of 1,524 residues (GenBank accession no.OQ560055).
The conserved domains of the three BdDomeless proteins and DmDomeless were predicted in order to investigate the core functional domain of the three BdDomeless proteins inB.dorsalis,as depicted in Fig.1-A.BdDomeless1,BdDomeless2,and DmDomeless all feature a signal peptide,five fibronectin-type-III-like (FN III) extracellular domains,and a transmembrane domain located to the right of FN III.Notably,BdDomeless3 is unique in that it lacks a signal peptide,and its transmembrane domain is positioned to the left of FN III.Sequence analysis of the CBM I domain revealed that DmDomeless,BdDomeless1,and BdDomeless2 all possess four conserved cysteine residues (Appendix B,indicated by red arrows),whereas BdDomeless3 lacks the fourth cysteine residue.Further analysis of the transmembrane domain reveals that BdDomeless1,BdDomeless2,and DmDomeless each exhibit both intra-and extra-membrane sections,located on the left and right sides of the transmembrane domain,respectively.In contrast,BdDomeless3 consists exclusively of the extramembrane section (Appendix C).

Fig.1 Conserved domain and phylogenetic analysis of three Domeless receptors in Bactrocera dorsalis.A,domain diagrams of Domeless proteins from B.dorsalis and Drosophila melanogaster.Red rectangles represent signal peptides,while blue rectangles represent the transmembrane regions.FN3 and Blast FN3 are fibronectin type 3 domains.B,phylogenetic tree of Domeless receptors in the representative insects.The tree was constructed using IQtree based on the maximum likelihood method according to the amino acid sequences.The accession numbers in NCBI are shown in the figure.CR,cytokine receptor.
The phylogenetic tree was constructed using the maximum likelihood method to investigate the evolutionary relations of the threeBdDomelessgenes among different insect species (Fig.1-B).In summary,theDomelessgenes ofB.dorsaliscluster together with those of the four tephritid flies and twoDrosophilaspecies.Notably,Domeless3from the four tephritid flies,includingB.dorsalis,diverged early from the otherDomelessgenes within this lineage.
3.2.Temporal-spatial expression patterns of Domeless in B.dorsalis
The relative expression patterns of the threeBdDomelessgenes were assessed in different developmental stages and tissues ofB.dorsalis.Throughout the entire developmental process,all threeBdDomelessgenes are expressed.Of these,BdDomeless1manifests the highest expression level during the egg stage,whileBdDomeless2andBdDomeless3reach their peak expression levels in the 9th day adults (Fig.2-A).The spatial expression of the threeBdDomelessgenes was evaluated using various tissues ofB.dorsalis,including fat body,hemolymph,midgut,Malpighian tubule,ovary,and testis.BdDomeless1was found to be highly expressed in the hemolymph and testis,BdDomeless2was significantly highly expressed in the Malpighian tubule.BdDomeless3exhibits high expression levels in the fat body and Malpighian tubules,with significantly higher expression observed in females compared to males in both tissues (Fig.2-B).
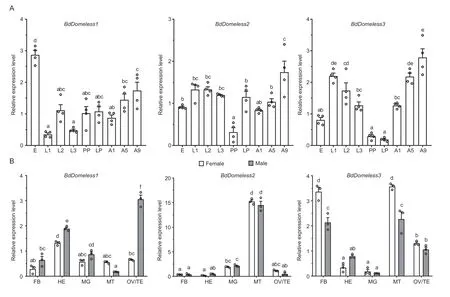
Fig.2 Expression profiles of three Domeless genes in Bactrocera dorsalis.A,relative expression levels of the three Domeless genes during the developmental stages of B.dorsalis.E,eggs;L1,first-instar larvae;L2,second-instar larvae;L3,third-instar larvae;PP,pre-pupae;LP,late pupae;A1,1-day-old adults;A5,5-day-old adults;A9,9-day-old adults.Error bars are mean±SE (n=4).B,relative expression of the three Domeless genes in different tissues of 5-d-old adults.FB,fat body;HE,hemolymph;MG,midgut;MT,Malpighian tubules;OV,ovary;TE,testis.Error bars are mean±SE (n=3).Statistical significances were calculated using one-way ANOVA with the Tukey test.Different lower case letters above columns indicate statistical differences at P<0.05.
3.3.Immune-induced responses of Domeless genes in B.dorsalis
The expression profiles of the threeBdDomelessgenes in response to four pathogens were examined using RTqPCR.The four pathogens wereEnterococcus faecium(a gram-positive bacterium),Pseudomonas aeruginosa(a gram-negative bacterium),B.bassiana(Bb-07 strain) (a fungus),and symbiont-like RNA viruses isolated fromB.dorsalis.UponE.faecalisinfection,the expressions ofBdDomeless1andBdDomeless2were induced at 4 and 12 h post-injection,whileBdDomeless3was induced at 8 and 12 h post-injection (Fig.3-A).Similarly,P.aeruginosainfection resulted in significant upregulation ofBdDomeless1at 12 h,BdDomeless2at 8 and 12 h,andBdDomeless3at 4,8,and 12 h post-injection (Fig.3-B).However,the effects ofB.bassianainfection on the expression levels of the threeBdDomelessgenes were not significant (Fig.3-C).When infected with purified virions,ten types of RNA viruses were identified (Appendix D):Bactrocera dorsaliscripavirus,Bactrocera dorsalisnegev-like virus,Bactrocera dorsalispicornalike virus,Bactrocera dorsalisnarnavirus,Bactrocera dorsalissigmavirus,Bactrocera dorsalisborna-like virus,Bactrocera dorsalistoti-like virus 1,Batrocera dorsalisorbivirus,Batrocera dorsalispartiti-like virus 1,andBatrocera dorsalispartiti-like virus 2.Among these,onlyBdDomeless3displayed significant upregulation following the multiple infection with these RNA viruses (Fig.3-D).Furthermore,the expression of the RNAi antiviral pathway core genesDicer2,Ago2,andR2D2was assessed following virus infection,but showed that none of these genes were activated (Appendix E-a).
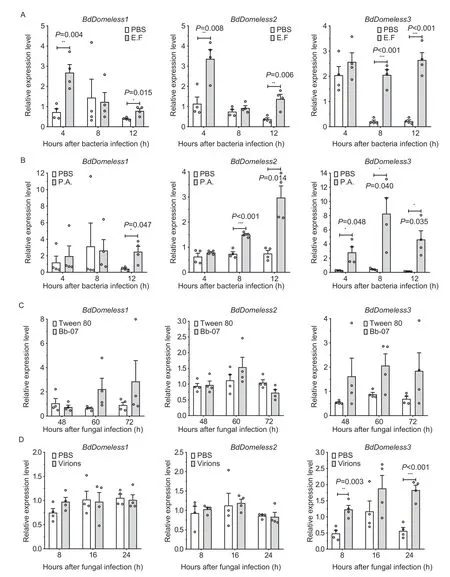
Fig.3 Expression pattern of the three Domeless genes in 4-d-old adults in response to injections of Enterococcus faecalis (A;E.F),Pseudomonas aeruginosa (B;P.A).Beauveria bassiana (C;Bb-07) and virons (D).Samples were collected at 4,8,and 12 h after bacterial infection;48,60,and 72 h after fungal infection;and 8,16,and 24 h after virual infection.Sterile 0.01 mol L-1 PBS and Tween 80 (0.05%) served as the negative control.Statistical significances were calculated using an independent sample t-test (*,P<0.05,**,P<0.01,and ***,P<0.001).Error bars are mean±SE (n=4).
3.4.Virus infection-based RNAi targeting of BdDomeless3 in B.dorsalis
A series of RNAi and virus infection studies were conducted to investigate the role ofBdDomeless3in antiviral immunity.The detailed experimental workflow is presented in Fig.4-A.The knockdown efficiency of specific dsRNA onBdDomeless3expression was assessed using RT-qPCR.Compared to the dsGFPcontrol group,BdDomeless3expression decreased by 33.8% at 12 h,21.9% at 24 h,and 51.0% at 48 h post dsBdDomeless3 injection (Fig.4-B).To exclude compensatory effects ofBdDomeless3deficiency,the expression ofBdDomeless1andBdDomeless2was also evaluated.The results showed thatBdDomeless1expression was upregulated in response toBdDomeless3knockdown (Fig.4-B).Additionally,we assessed the expression of core genes involved in the RNAi pathway followingBdDomeless3knockdown.Interestingly,onlyBdDicer2showed an increase after 12 h ofBdDomeless3knockdown (Appendix E-b).Notably,compared to the control,the expression levels of four AMP genes (Cecropin-1,Cecropin-like,Attacin-BandAttacin-like) were each significantly downregulated after 12 h of dsDomeless3treatment (Appendix F).Virus activation and propagation were examined,and the results are presented in Fig.4-C and Appendix G.The viral titers of BdCV and BdPLV increased significantly withBdDomeless3knockdown,while there were no significant differences observed in the titers of the other eight viruses.

Fig.4 RNAi targeting of BdDomeless3 and virus infection.A,experimental design for RNAi and virus infection.B,relative expressions of the three Domeless genes under dsDomeless3 injection.C,effect of BdCV and BdPLV infection after knocking down BdDomeless3 expression.The mean±SE expression levels were calculated using four biological replicates,represented by the number of scatter points.Statistical significances were calculated using an independent sample t-test (*,P<0.05,**,P<0.01,and ***,P<0.001).
4.Discussion
The JAK/STAT pathway plays a critical role in the immune system as a signaling cascade.In this study,we identified Domeless,a key receptor in the JAK/STAT pathway,fromB.dorsalisfor the first time and subsequently employed an RNAi system to knock down the expression ofBdDomeless3.In tephritid flies,the efficiency of RNAi is typically diminished due to a variety of factors,such as the activity of nucleases,SID-1 deficiency,and the presence of virus-encoded silencing suppressors (Makturaet al.2021).The existing research demonstrates thatB.dorsalisexhibits distinct phenotypic changes with gene silencing efficacy of approximately 50% (Zhouet al.2022;Zhanget al.2023).In this study,we employed a double-injection approach to perpetuate the silencing ofBdDomeless3expression,aiming to achieve an efficient level.Our findings revealed that symbiont-like viral replication (e.g.,BdCV and BdPLV) is enhanced in individuals with suppressedDomeless3expression,suggesting a potential role forDomeless3in the antiviral immune response ofB.dorsalis.Additionally,a reduction in the titer of BdSV was observed following injection of dsDomeless3(Appendix F).However,while the reduction in virus titer is evident,the difference is not statistically significant.It is worth noting that,as discussed in our previous research (Zhanget al.2020),the infection frequency of this virus in our laboratory population is relatively low,and high-titer infections in some individuals could potentially skew the results.
Drosophilais known to possess oneDomeless(DmDomeless),which consists of a signal peptide,five fibronectin-type-III-like (FN III) extracellular domains,and a transmembrane domain (Fig.1-A).DmDomeless also encompasses two CBM domains,CBM1 and CBM2,which harbor four conserved cysteine residues and incomplete WSXWS motifs (NTXWS),respectively (Brownet al.2001).CBM represents the characteristic domain of a type I cytokine receptor,which is conserved in various vertebrates and acts as a molecular switch involved in receptor activation (Dagilet al.2012).In this study,we discovered threeDomelessgenes inB.dorsalis,designated asBdDomeless1,BdDomeless2,andBdDomeless3.The presence of moreDomelessgenes inB.dorsaliscompared toD.melanogastersuggests a potential gene expansion ofDomelesswithinB.dorsalis.However,based on the chromosomal positions of these three genes (data not shown),it appears unlikely that they have undergone genome duplication.Sequence and conserved domain analysis revealed that the BdDomeless1 and BdDomeless2 proteins exhibit a similar structure to DmDomeless (Fig.1-A;Appendix B),in that they all possess four conserved cysteine residues at the N-terminus.These characteristics suggest that BdDomeless1,BdDomeless2,and DmDomeless belong to the IL-6 receptor-type family and may have comparable functions.Unlike DmDomeless,BdDomeless3 lacks the signal peptide,and its transmembrane domain is situated to the left of the FN3 domains.Additionally,BdDomeless3 lacks the four conserved cysteine residues.These findings indicate that the function of BdDomeless3 may differ from DmDomeless,BdDomeless1,and BdDomeless2.
Spatiotemporal expression analysis is a crucial approach for investigating gene functions.In this study,the threeDomelessgenes inB.dorsalisexhibited high expression levels during the adult stages,implying their essential roles in adult-specific processes such as development and immunity.Interestingly,Domeless3displayed elevated expression not only in the Malpighian tubules but also in the fat body,distinguishing it fromDomeless1andDomeless2.These findings,combined with protein structural data,suggest thatBdDomeless3performs distinct functions compared toBdDomeless1andBdDomeless2.Additionally,the fat body is known to produce and release a series of AMPs into the hemolymph in response to immune challenges (Yuet al.2022).Remarkably,the downregulation ofBdDomeless3resulted in a significant reduction in the expression of specific AMPs,underscoring the crucial role ofBdDomeless3in regulating this process.Moreover,Domeless,which is expressed in the fat body,is reportedly associated with adipose metabolism and glycemic regulation (Louridoet al.2021) as well as wing development (Wanget al.2022) in insects.
The current studies on antiviral immunity in insects primarily focus on the immune response following infection with exogenous pathogens.This approach allows for better control over the timing and dosage of infection,as well as the diversity of viruses used.However,this approach tends to overlook the possibility of persistent infections or symbiont-like infections in insects.Notably,with the advancements in high-throughput sequencing and viral metagenomics technologies,it has become evident that healthy organisms can be infected by a broad range of RNA viruses (Shiet al.2016;Zhanget al.2018),many of which exhibit symbiont-like characteristics.Consequently,further research aimed at investigating the immune mechanisms employed by organisms to defend against these symbiont-like viruses is highly warranted.
Insects lack an adaptive immune system and primarily rely on the innate immune response to defend against microbial pathogens (Buchonet al.2014).The RNAi pathway is a critical antiviral defense mechanism,with the siRNA pathway playing a particularly important role in insect antiviral immunity (Bonning and Saleh 2021).However,we did not observe significant changes in the expression of RNAi core genes inB.dorsalisfollowing the injection of virions (Appendix E-a).Bactrocera dorsalisis known to harbor various types of RNA viruses that can trigger the host RNAi response (Zhanget al.2020;Zhang Wet al.2022).This may be attributed to the long-term persistent infection ofB.dorsalisby these viruses,leading to continuous activation of the RNAi pathway.The JAK/STAT pathway plays a complex,specific,and versatile role in mounting antiviral responses by inducing the synthesis of AMPs (Kingsolveret al.2013).Specific viruses,such as Flock house virus (FHV),Vesicular stomatitis virus,andDrosophilaX virus (DXV),can stimulate the expression of target genes in the JAK/STAT pathway,including Upd2 and Upd3 (Kempet al.2013).Through immunological induction and gene knockdown experiments,we discovered thatDomeless3is involved in the immunity against symbiontlike viruses (BdCV and BdPLV) inB.dorsalis.These viruses are widely distributed in various fruit flies (Sharpeet al.2021;Zhang Wet al.2022),and are believed to be associated with ecological invasiveness (Zhanget al.2020).Importantly,we observed a significant increase inBdDicer2expression following the knockdown ofBdDomeless3(Appendix E-b),suggesting the potential for cross-talk between the JAK/STAT and RNAi pathways.Similar phenomena have been reported in virual infections ofD.melanogasterandA.aegypti,where the JAK/STAT pathway ligand,vago,is upregulated and appears to be linked toDicer2(Deddoucheet al.2008;Paradkaret al.2012).Moreover,the activation of the JAK/STAT pathway byvagois independent of the Domeless receptor (Chenget al.2016;Gaoet al.2021),suggesting that the knockdown ofDomeless3may enhance communication between the RNAi pathway and the JAK/STAT pathway triggered byvagoactivation.Nevertheless,further investigation is required to elucidate the precise mechanisms involved.
5.Conclusion
Our work highlights the distinctiveness of the BdDomeless3 receptor in terms of its structure and tissue distribution.Furthermore,we elucidated the role ofBdDomeless3in the context of symbiont-like virus infections inB.dorsalis.These findings offer a novel perspective on immunity and bio-protection strategies that involveB.dorsalissymbiont-like viruses.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32202278) and the Chongqing Special Postdoctoral Science Foundation of China,and the earmarked fund for China Agricultural Research System (CARS-26).
Declaration of competing interests
The authors declare that they have no conflict of interest.
Ethical statement
All applicable international,national and institutional guidelines for the care and use of animals were followed.
Appendicesassociated with this paper are available on https://doi.org/10.1016/j.jia.2023.10.003
 Journal of Integrative Agriculture2024年4期
Journal of Integrative Agriculture2024年4期
- Journal of Integrative Agriculture的其它文章
- Invasion of fall armyworm led to the succession of maize pests in Southwest China
- Genome-wide and candidate gene association studies identify BnPAP17 as conferring the utilization of organic phosphorus in oilseed rape
- Impacts of agri-food e-commerce on traditional wholesale industry: Evidence from China
- Transcriptomic and metabolomic analysis provides insights into lignin biosynthesis and accumulation and differences in lodging resistance in hybrid wheat
- Responses of growth performance,antioxidant function,small intestinal morphology and mRNA expression of jejunal tight junction protein to dietary iron in yellow-feathered broilers
- Genome-wide identification of the CONSTANS-LIKE (COL) family and mechanism of fruit senescence regulation by PpCOL8 in sand pear (Pyrus pyrifolia)
