Responses of growth performance,antioxidant function,small intestinal morphology and mRNA expression of jejunal tight junction protein to dietary iron in yellow-feathered broilers
Kaiwen Lei ,Hao Wu ,Jerry W Spears ,Xi Lin ,Xi Wang,Xue Bai,Yanling Huang#
1 Key Laboratory of Animal Science of State Ethnic Affairs Commission,Southwest Minzu University,Chengdu 610041,China
2 Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization,Southwest Minzu University,Chengdu 610041,China
3 Department of Animal Science,North Carolina State University,Raleigh,NC 27695-7621,USA
Abstract This study aimed to investigate the dose-effect of iron on growth performance,antioxidant function,intestinal morphology,and mRNA expression of jejunal tight junction protein in 1-to 21-d-old yellow-feathered broilers.A total of 720 1-d-old yellow-feathered male broilers were allocated to 9 treatments with 8 replicate cages of 10 birds per cage.The dietary treatments were consisted of a basal diet (contained 79.6 mg Fe kg-1) supplemented with 0,20,40,60,80,160,320,640,and 1,280 mg Fe kg-1 in the form of FeSO4·7H2O.Compared with the birds in the control group,birds supplemented with 20 mg Fe kg-1 had higher average daily gain (ADG) (P<0.0001).Adding 640 and 1,280 mg Fe kg-1 significantly decreased ADG (P<0.0001) and average daily feed intake (ADFI) (P<0.0001) compared with supplementation of 20 mg Fe kg-1.Malondialdehyde (MDA) concentration in plasma and duodenum increased linearly (P<0.0001),but MDA concentration in liver and jejunum increased linearly (P<0.05) or quadratically (P<0.05) with increased dietary Fe concentration.The villus height (VH) in duodenum and jejunum,and the ratio of villus height to crypt depth (V/C) in duodenum decreased linearly (P<0.05) as dietary Fe increased.As dietary Fe increased,the jejunal relative mRNA abundance of claudin-1 decreased linearly (P=0.001),but the jejunal relative mRNA abundance of zona occludens-1 (ZO-1) and occludin decreased linearly (P<0.05) or quadratically (P<0.05).Compared with the supplementation of 20 mg Fe kg-1,the supplementation of 640 mg Fe kg-1 or higher increased (P<0.05) MDA concentrations in plasma,duodenum,and jejunum,decreased VH in the duodenum and jejunum,and the addition of 1,280 mg Fe kg-1 reduced (P<0.05) the jejunal tight junction protein (claudin-1,ZO-1,occludin) mRNA abundance.In summary,640 mg of supplemental Fe kg-1 or greater was associated with decreased growth performance,increased oxidative stress,disrupted intestinal morphology,andreduced mRNA expression of jejunal tight junction protein.
Keywords: iron,yellow-feathered broiler,antioxidant function,intestinal morphology,tight junction protein
1.Introduction
Iron is an essential trace element and plays a vital role in animal growth processes,including oxygen transport,DNA synthesis,and energy metabolism (Wanget al.2007;Muckenthaleret al.2017).Iron deficiency usually leads to anemia and associated malfunction,which in turn affects the health and growth of animals (Bhattarai and Nielsen 2015).Therefore,in broiler production,Fe is often added as a feed additive to broiler feed to prevent Fe deficiency (Luet al.2018).However,feedstuffs (corn and soybean meal) that are commonly used in broiler diets generally supply above NRC (1994) requirements for Fe (non-heme Fe;Gouet al.2018).Several mineral feed ingredients also contain high Fe concentration,such as dicalcium phosphate (2,410-12,600 mg Fe kg-1) (Sullivanet al.1994).Sun (2008) reported the Fe concentration was as high as 178 mg kg-1or more in practical broiler diets in China.Excessive Fe intake in broilers may cause oxidative stress (Chandravathyet al.2011),impair intestinal integrity,and interfere with intestinal barrier function (Gouet al.2018).The conflict between iron-deficiency anemia and the health issue of overdose from iron supplementation has spurned greater interest in the role of dietary iron intake on intestinal health of broilers.Furthermore,Fe absorption decreases with age (Forbes and Reina 1972),suggesting that high Fe concentration may have more serious effect on intestinal health of younger animals.
The yellow-feathered broilers are known for their good meat quality.In China,the production of yellowfeathered broilers is comparable to that of whitefeathered broilers,approximately 4 billion per year (Jianget al.2019).However,there is limited information available on the effect of dietary Fe concentration on intestinal health of yellow-feathered broilers,especially the dose-effect.We hypothesized that the supplementation of suitable Fe to the diet of yellowfeathered broilers would affect their growth and intestinal health positively.However,dietary high Fe would have negative effects.Therefore,this study aimed to determine the effect of different dietary Fe concentration on growth performance,antioxidant function,intestinal morphology and mRNA expression of jejunal tight junction protein using yellow-feathered broilers to test the above hypothesis.
2.Materials and methods
2.1.Animals,diets and experimental design
A total of 720 1-d-old yellow-feathered male broiler chickens were randomly assigned to 9 treatments.Each treatment group consisted of 8 cages,each containing 10 broilers.All the birds were housed in a temperaturecontrolled room with fiberglass feeders and stainlesssteel cages coated with plastic.They had free access to food and Fe-free water,and the light regimen was 24 h.The corn-soybean meal basal diet was formulated to meet or exceed the nutritional requirements for yellowfeathered broilers except for Fe (MAPRC 2020).The feed ingredients and dietary nutrient compositions are presented in Table 1.The treatment diets included a basal diet and the basal diets supplemented with 20,40,60,80,160,320,640,and 1,280 mg Fe kg-1from FeSO4·7H2O (Shanghai Yuanye Bio-Technology Co.,Shanghai,China).The dietary Fe concentrations by analysis on an as-fed basis were 79.6,97.6,122,154,163,236,393,723,and 1,354 mg kg-1,respectively.Dead chicks were recorded daily.Body weight and feed intake per cage measured at 21 d of age to calculate the average daily gain (ADG),average daily feed intake (ADFI) and feed conversion ratio (F/G) as well as mortality during d 1 to 21.

Table 1 Ingredients and nutrient composition of the base diets (as-fed basis)
2.2.Sample collection
On d 21,1 broiler chicken close to the average weight was selected from each cage for sample collection.Blood samples were collectedviathe wing vein and then centrifuged at 860×g for 10 min at 4°C.The plasma was obtained and stored at -80°C for later analysis.After the blood samples were collected,the bird was harvested by cervical dislocation.Segments (2-3 cm) of duodenum and jejunum were collected and fixed in 4% paraformaldehyde solution for histometric analysis.Then,the liver,duodenum,and jejunum mucosa were immediately obtained,rinsed with PBS,frozen in liquid nitrogen,and stored at -80°C for later analysis.
2.3.Measurements of Fe,Ca and CP concentration
The concentration of Fe in the diets was measured by flame atomic absorption spectrometry (Contr AA 700,Analytik Jena,Germany) after wet digestions with HNO3.The concentration of Ca and CP in diets were determined as described by AOAC (1990).
2.4.Enzyme activity
The activity of total superoxide dismutase (T-SOD),glutathione peroxidase (GSH-Px),and concentration of malondialdehyde (MDA) were determined using available commercial kits (Nanjing Jiancheng Bioengineering Institute,Nanjing,China) following the instructions.
2.5.Morphological analysis
After being fixed for about 24 h,the duodenum and jejunum were dehydrated,embedded in paraffin,sliced (5 μm),and stained with hematoxylin and eosin.Then,the histologic sections were observed with an optical microscope (DM1000,Leica,Germany).A 10 welloriented villi were measured per section for their villus height (VH) and crypt depth (CD),and the ratio of villus height to crypt depth (V/C) was calculated.
2.6.Determinations of mRNA expression levels by real-time quantitative PCR
Based on the results of growth performance,antioxidant function and intestinal morphology in the present study,the samples collected from the 0,20,80,320 and 1,280 mg Fe kg-1supplementation groups were chosen for analyzing the relative mRNA abundance of the jejunal tight junction proteins.Total RNA in the jejunum mucosa was extracted using Trizol reagent (TaKaRa Biotechnology Co.,Ltd.,Dalian,China).The purity of total RNA was measured by using a Nanodrop ND-1000 (Thermo Fisher,Waltham,USA) for the determination of OD260and OD280(OD260/280≥1.8).The integrity of RNA was confirmed by electrophoretic analysis.Reverse transcription was conducted with PrimeScript™ RT Reagent Kit (TaKaRa Biotechnology Co.,Ltd.,Dalian,China) according to the manufacturer’s instructions.The resulting firststrand cDNA was diluted to 1:8 with ddH2O and used as a template for real-time quantitative PCR.Real-timeqPCR assay was carried out in the CFX ConnectTMReal-Time PCR Detection System (Bio-Red Laboratories,Inc.,Hercules,CA) using TB Green Premix Ex Taq II (TaKaRa Biotechnology Co.,Ltd.,Dalian,China).The reaction volume was 25 μL,containing 12.5 μL TB Green PremixExTaqII,2 μL of cDNA,1 μL of each primer and 8.5 μL ddH2O.All primers (Table 2) were synthesized by Sangon (Shanghai,China).The thermal cycling parameters were as follows: 30 s at 95°C,40 cycles at 95°C for 5 s,and 60°C for 30 s.All reactions were performed in triplicate.The relative mRNA abundance were determined by 2-ΔΔCTmethod normalized withβ-actinlevel.
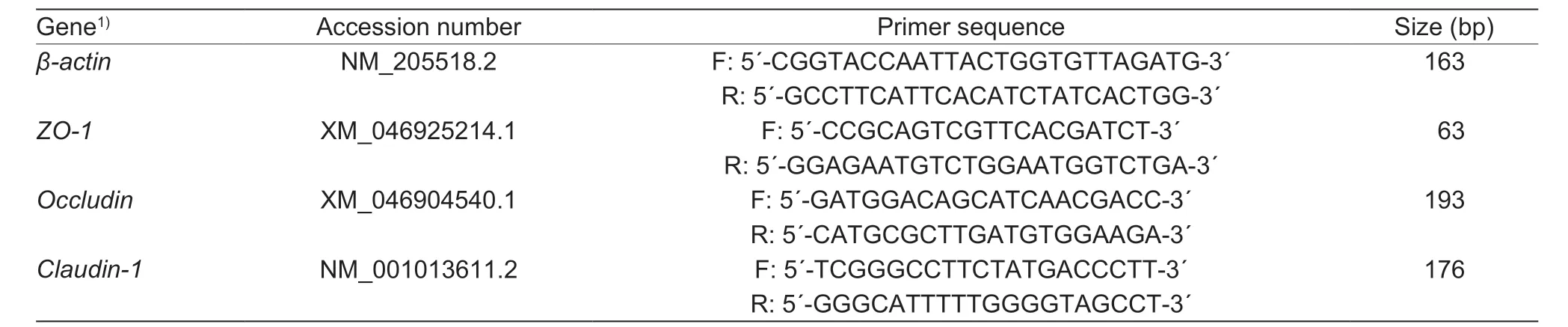
Table 2 Primer sequences of real-time fluorescence quantitative PCR1)
2.7.Statistical analysis
Data analysis was performed by ANOVA using the MIXED procedure of SAS (SAS Inst.Inc.,Cary,NC) with cage (n=8) as the experimental unit.Data from mortality of broilers were transformed using arcsin before statistical analysis.Orthogonal comparisons (unequally spaced) were applied for linear and quadratic responses.P≤0.05 was considered to be statistically significant.Differences among treatment means were determined using the LSD test.
3.Results
3.1.Growth performance
Dietary Fe concentration did not affect F/G (P=0.946) and mortality (P=0.682),but affected (P<0.0001) ADG and ADFI (Table 3).The ADG decreased linearly (P<0.0001) and ADFI decreased linearly (P<0.0001) or quadratically (P=0.050) as dietary Fe concentration increased.Compared with the birds in the control group,birds fed 20 mg Fe kg-1had higher ADG (P<0.0001).However,adding 640 and 1,280 mg Fe kg-1decreased ADG (P<0.0001) and ADFI (P<0.0001) compared with supplementation of 20 mg Fe kg-1.The results suggest dietary 20 mg Fe kg-1supplementation is suitable for the growth of yellow-feathered broilers.
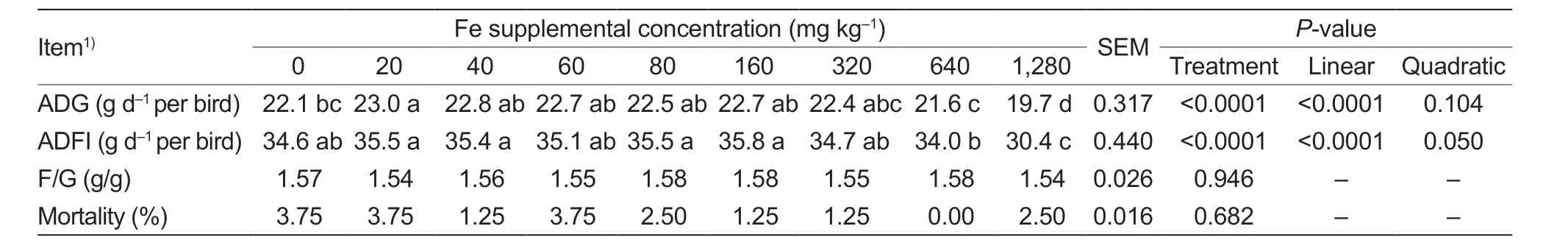
Table 3 Effect of dietary supplemental iron on growth performance of yellow-feathered broilers at d 1 to 21
3.2.Antioxidant function
No differences were detected (P>0.528) in GSH-Px activity in plasma and liver,or T-SOD activity in plasma,liver,duodenum,and jejunum (Table 4).As dietary Fe increased,concentrations of MDA in plasma and duodenum increased linearly (P<0.0001),but concentrations of MDA in liver and jejunum increased linearly (P<0.05) or quadratically (P<0.05).Supplementation of 320 mg Fe kg-1or higher to the control diet increased (P=0.012) MDA concentrations in jejunum.The addition of 640 mg Fe kg-1or greater increased (P<0.0001) MDA concentrations in plasma,liver and duodenum compared to the supplementation of 20 mg Fe kg-1.
3.3.Intestinal morphology
Crypt depth was not affected (P>0.241) by dietary Fe (Table 5).Villus height in the duodenum and jejunum decreased linearly (P<0.05) with increased dietary Fe.Increased dietary Fe also linearly reduced (P=0.001) V/C in the duodenum but not (P=0.088) in the jejunum.Supplementation of 640 mg Fe kg-1or greater significantly decreased (P<0.05) V/C in duodenum and VH in jejunum.The addition of 1,280 mg Fe kg-1decreased (P=0.028) VH in duodenum compared to the control group.
3.4.Relative mRNA abundance of tight junction proteins in jejunum
As dietary Fe increased,the jejunal relative mRNA abundance ofclaudin-1decreased linearly (P=0.001),and the jejunal relative mRNA abundance of zona occludens-1 (ZO-1) andoccludindecreased linearly (P<0.05) or quadratically (P<0.05) (Table 6).Compared to the supplementation of 20 mg Fe kg-1,the supplementation of 320 mg Fe kg-1or greater to the control diet decreased (P=0.020) the relative mRNA expression ofoccludin,and the addition of 1,280 mg Fe kg-1decreased (P<0.05) the relative mRNA expression ofZO-1andclaudin-1.
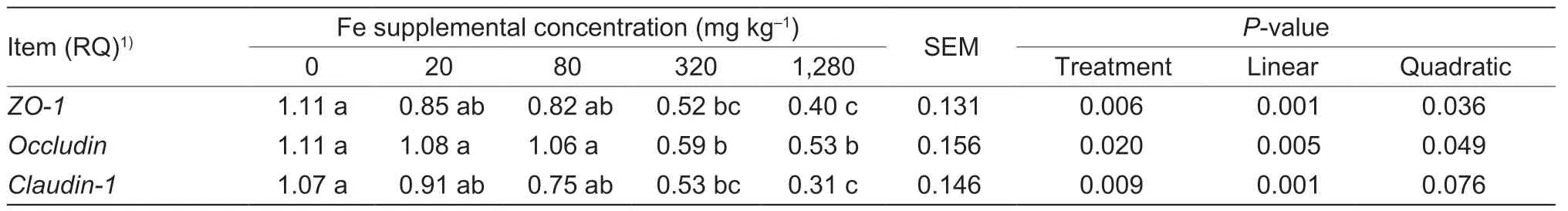
Table 6 Effect of dietary supplemental iron on relative mRNA abundance of tight junction protein in jejunum mucosa of yellowfeathered broilers at 21 d of age
4.Discussion
We hypothesized that suitable and high Fe supplementation would have positive and negative effect on the growth and intestinal health of yellow-feathered broilersrespectively.The results of the present study have partly supported the hypothesis.Dietary 20 mg Fe kg-1supplementation improved the growth performance of yellow-feathered broilers which means dietary 20 mg Fe kg-1supplementation is suitable for the growth of yellowfeathered broilers in this study,however no positive effects of dietary 20 mg Fe kg-1supplementation on intestinal health were observed.It may be due to the relative high Fe concentration (79.6 mg Fe kg-1) in the basal diet.The concentration of Fe in the basal diet is not low enough to cause the intestinal health problems.Compared with the supplementation of 20 mg Fe kg-1to the basal diet,the addition of 640 mg Fe kg-1or greater resulted in decreased growth performance,increased oxidative stress,disrupted intestinal morphology,and the addition of 1,280 mg Fe kg-1reduced mRNA expression of jejunal tight junction protein.These findings provide a theoretical basis for the rational and effective use of Fe in yellow-feathered broilers production.
Previous studies showed that supplementation of broiler diets with appropriate amounts of Fe improved growth performance from d 1 to 21 (Vahl and Klooster 1987;Maet al.2016).Iron supplementation (20 mg Fe kg-1) to a basal diet containing 107 mg Fe kg-1increased ADG in broilers from d 1 to 21 (Vahl and Klooster 1987).Maet al.(2016) also reported that supplementation of 40 or 60 mg Fe kg-1in broiler diets significantly increased ADG from d 1 to 21 compared to the basal diet without Fe addition (67 mg Fe kg-1in the basal diet).Our results demonstrated a similar increase in ADG in yellowfeathered broilers when 20 mg Fe kg-1was added to a basal diet containing 79.6 mg Fe kg-1from d 1 to 21.
Feed intake is one of the critical factors that affect the growth performance of poultry (Mataret al.2020).Observations from several studies have implied that decreased ADG and ADFI of broilers may be detected when high dietary Fe concentrations were presented.Caoet al.(1996) reported that the supplementation of Fe (400,600,and 800 mg kg-1) in a basal diet containing 188 mg Fe kg-1decreased ADG and ADFI in Ross broilers from d 1 to 21.Supplementation of 500 mg Fe kg-1to a basal diet containing 109 mg Fe kg-1also decreased the ADG and ADFI of Ross broilers from d 1 to 21 (Baiet al.2021).The addition of 540,or 1,620 mg Fe kg-1to a control diet containing 107 mg Fe kg-1also decreased ADG in broilers from d 1 to 21 (Vahl and Klooster 1987).In the present study supplementation of 640 mg Fe kg-1or higher decreased ADG and ADFI compared with supplementation of 20 mg Fe kg-1.Excessive Fe may augment reactive oxygen species (ROS) production (Galariset al.2019),leading to intestinal oxidative damage,lipid peroxidation,and inflammation (Liet al.2016),which may ultimately lead to decreased feed intake (Salah 2013).The supplementation of high Fe to the diet of broilers decreased ADG,which could be attributed to the reduced availability of nutrients due to low feed intake.
Animals generate energy by reducing oxygen to water during which harmful intermediates are generated;however,antioxidant enzymes can scavenge harmful intermediates in the body under homeostatic conditions (Rao and Jagadeesan 1996).Iron is a component of numerous enzymes and oxygen-carrier proteins and has anti-oxidation functions in the body (Finazzi and Arosio 2014).Some reports demonstrated suitable dietary Fe supplementation could increase the activity of antioxidant enzymes.The addition of 80,120,or 160 mg Fe kg-1,from Fe glycine (Fe-Gly),to a control diet containing 85.81 mg Fe kg-1increased SOD activity in the liver of Ross broilers at 42 d of age (Maet al.2012).Sunet al.(2015) also found a linear increase in serum SOD activity in broilers supplemented with 40,60,80,100,120,140,and 160 mg Fe kg-1as Fe-Gly (90.31 mg Fe kg-1in the basal diet) at 21 d of age.In contrast,no differences were found in the GSH-Px activity in plasma and liver and T-SOD activity in plasma,liver,duodenum,and jejunum among treatments in the current study.The inconsistent results might be due to different forms of Fe being supplemented.Inorganic Fe (FeSO4·7H2O) was used in the present study,while an organic Fe source was used in the previous studies (Maet al.2012;Sunet al.2015).The effects of different forms of Fe supplementation on the activity of antioxidant enzymes of broilers need further investigation.
Excessive Fe disrupts the balance between ROS generation and the antioxidant system in animals,finally leading to oxidative stress (Finazzi and Arosio 2014).Absorption of Fe occurs primarily in the duodenum and upper jejunum.Divalent metal transporter 1 is the major transporter that imports Fe into the enterocyte,and ferroportin is the transporter that exports Fe from the enterocyte into the plasma (Domenicoet al.2007).Because of the ability of excess Fe to cause oxidative damage,Fe homeostasis is tightly controlled primarily by altering absorption from the small intestine.Hepcidin,a peptide hormone produced in the liver and secreted into the plasma,plays an important role in regulating Fe absorption (Domenicoet al.2007).Expression of hepcidin is increased when liver Fe stores are adequate or high,thus,downregulating Fe transporters in the small intestine.However,high dietary Fe can overwhelm homeostatic mechanisms and result in Fe accumulation in certain tissues.Supplementation of a diet containing 188 mg Fe kg-1with 400 mg Fe kg-1for 21 d increased liver Fe concentrations by 58% in Ross broilers (Caoet al.1996).In nursery pigs,increasing dietary Fe from adequate (120 mg kg-1) to high levels (620 mg kg-1) increased liver and duodenal mucosa Fe concentrations by 81 and 99%,respectively (Hansenet al.2009).The elevated tissue Fe resulted in increased MDA concentrations in liver and duodenum of pigs fed high Fe (Liet al.2016).
In the present study,the addition of 320 mg Fe kg-1or higher to the control diet increased MDA concentrations in jejunum.The addition of 640 mg Fe kg-1or greater increased MDA concentrations in plasma,liver,and duodenum compared with the supplementation of 20 mg Fe kg-1.Chandravathyet al.(2011) reported that the addition of 1,930 mg Fe kg-1to the basal diet of Cobb broiler led to significantly higher MDA concentration in the liver at 42 d of age.The addition of 700 or 1,400 mg Fe kg-1to a basal diet containing 245 mg Fe kg-1increased MDA concentration in the jejunum of 21-d-old Chinese Yellow broilers (Gouet al.2018).
The small intestine is the leading site of nutrient digestion and absorption in broilers,and the VH,CD,and V/C are important indicators of intestinal function (Liuet al.2020).Iron may affect intestinal stem cell activity and proliferationviaWnt/β-catenin signaling,which in turn affects intestinal development (Songet al.2011).Previous studies in weanling pigs indicated that supplementing 100 mg Fe kg-1for 28 d increased VH in the duodenum and jejunum (Zhuoet al.2018).In the present study the addition of 20 mg Fe kg-1to the control diet did not affect intestinal morphology.However,high dietary Fe resulted in a linear decrease in VH of the duodenum and jejunum,with VH in broilers fed diet supplemented with 1,280 mg Fe kg-1differing significantly from that in broilers fed the control diet.The increased lipid peroxidation within the enterocytes of duodenum and jejunum observed in the present study could have contributed to more epithelial damage and loss and thus reduced VH.In agreement with the present study,Gouet al.(2018) reported that adding 700 or 1,400 mg Fe kg-1to a broiler diet containing 245 mg Fe kg-1decreased the VH of jejunum at 21 d of age.The decreased VH of the duodenum and jejunum and C/V of the duodenum may be one of the reasons for the decreased ADG and ADFI in the yellow-feathered broiler fed high Fe concentration in the present study.
Dietary nutrient absorption in the gastrointestinal tract occurs mainly at the duodenum and proximal jejunum in broilers.Based on the results of antioxidant function and intestinal morphology in the present study,and the results of Gouet al(2018),jejunum was chosen to analyze the mRNA expression of tight junction protein.Increasing dietary Fe resulted in a linear decrease in relative mRNA expression of the tight junction proteins,ZO-1,occludin,andclaudinin the jejunum in yellow-feathered broilers.This result is similar to the findings of Dinget al.(2020) in weanling pigs and Luoet al.(2020) in mice.Dinget al.(2020) found that dietary supplementation with 3,000 mg Fe kg-1significantly decreased the relative mRNA abundance ofZO-1,occludin,andclaudin-1in the duodenum of weanling pigs compared with those fed a basal diet containing 79 mg Fe kg-1.Luoet al.(2020) showed that relative mRNA abundance ofZO-1,occludin,andclaudin-1in jejunum was markedly down-regulated in the Fe overload mice model.Tight junction proteins are important for maintaining intestinal barrier integrity and function.Collectively,down-regulation of the mRNA expression for these proteins suggests that high Fe supplementation may have damaged the jejunal mucosal barrier.
5.Conclusion
In summary,the addition of 20 mg kg-1of Fe to a basal diet containing 79.6 mg Fe kg-1improved growth performance in yellow-feathered broilers from d 1 to 21,but dietary supplementation of 640 mg Fe kg-1or greater decreased growth performance compared with the supplementation of 20 mg Fe kg-1.Compared with the supplementation of 20 mg Fe kg-1,the addition of 640 mg Fe kg-1or greater to a control diet (contained 79.6 mg Fe kg-1) resulted in decreased growth performance,increased oxidative stress,disrupted intestinal morphology,and the supplementation of 1,280 mg Fe kg-1reduced the mRNA expression of jejunal tight junction protein.These results are expected to provide a theoretical basis for the rational use of iron supplements in broiler production.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31501977),the Sichuan Provincial Key R&D Project,China (22ZDYF0194),and the Double World-Class Project of Southwest Minzu University,China (XM2023010).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Ethical approval
All experimental procedures were approved by the Animal Care and Use Committee of Southwest Minzu University,China.
 Journal of Integrative Agriculture2024年4期
Journal of Integrative Agriculture2024年4期
- Journal of Integrative Agriculture的其它文章
- Invasion of fall armyworm led to the succession of maize pests in Southwest China
- Genome-wide and candidate gene association studies identify BnPAP17 as conferring the utilization of organic phosphorus in oilseed rape
- Impacts of agri-food e-commerce on traditional wholesale industry: Evidence from China
- Transcriptomic and metabolomic analysis provides insights into lignin biosynthesis and accumulation and differences in lodging resistance in hybrid wheat
- Genome-wide identification of the CONSTANS-LIKE (COL) family and mechanism of fruit senescence regulation by PpCOL8 in sand pear (Pyrus pyrifolia)
- ldentification of S-RNase genotype and analysis of its origin and evolutionary patterns in Malus plants
