长枝木霉代谢物对极细链格孢产毒抑制机制解析
邵学辉 张树武 徐秉良

收稿日期:2023-09-15 接受日期:2023-11-23
基金项目:甘肃省重点研发计划项目(23YFNA0020);甘肃省科技重大专项(22ZD6NA045);甘肃农业大学“伏羲杰出人才培育计划”项目(Gaufx-03J03);兰州市科技计划项目(2021-1-39)
作者简介:邵学辉,男,在读硕士研究生,研究方向为资源利用与植物保护。E-mail:1030372086@qq.com
*通信作者 Author for correspondence. E-mail:xubl@gsau.edu.cn;E-mail:zhangsw704@126.com
DOI:10.13925/j.cnki.gsxb.20230369
摘 要:【目的】明确长枝木霉(SC5)代谢粗提物对极细链格孢(ABL2)产毒抑制机制。【方法】以富士苹果的叶片为供试材料,通过生长速率法、LC-MS和RT-qPCR技术测定了SC5代谢粗提物对ABL2菌落生长、6种非寄主选择性毒素产生及产毒相关基因表达的抑制作用。【结果】质量浓度为0.5 mg·mL-1的SC5代谢粗提物对ABL2菌落生长和致病力具有显著的抑制作用,第6天时抑制率分别为38.08%和76.96%;同时,0.5 mg·mL-1 SC5代谢粗提物对ABL2菌株产生的非寄主选择性毒素TEN、ALT和TeA均具有显著抑制作用,其中处理2 d后对毒素的抑制作用最显著,其含量分别降低69.39%、98.51%和48.99%,并且其合成相关基因TES、TES(1)、PksA和PksJ的表达量显著下调,分别降低89.02%、98.20%、46.49%和40.13%,但是对ABL2菌株非寄主选择性毒素ATX-I含量和参与编码其合成的PksF基因表达量具有提升作用。【结论】质量浓度为0.5 mg·mL-1的SC5代谢粗提物对ABL2菌株生长和致病力具有抑制作用,可能通过调控ABL2菌株TES、TES(1)、PksA和PksJ基因下调表达,进而降低TEN、ALT和TeA的毒素的产生量及致病力。
关键词:苹果叶片;极细链格孢;长枝木霉;毒素;基因表达;生物防治
中图分类号:S661.1 S436.661 文献标志码:A 文章编号:1009-9980(2024)01-0133-10
Identification and mechanism of Trichoderma longibrachiatum metabolites in inhibiting the Alternaria tenuissima toxin production
SHAO Xuehui, ZHANG Shuwu*, XU Bingliang*
(College of Plant Protection, Gansu Agricultural University/Engineering Laboratory for Biological Control of Crop Diseases and Pests, Lanzhou, 730070, Gansu, China)
Abstract: 【Objective】 Alternaria spp. can cause a variety of apple leaf diseases, which occur in all major apple producing areas in the world. It can lead to brown disease spots in apple leaves and even result in early defoliation, which seriously affects the development of apple industry and causes huge economic losses. Apple leaf blight caused by Alternaria tenuissima was found for the first time in apple producing areas of Gansu province. This fungus can damage apple leaves and petioles, causing leaves to die and fall off. A. alternata mainly damages plants by producing Alternaria toxins. The Alternaria toxins that have been found can be divided into five categories, i. e, diphenyl-α-pyrones and their derivatives, perylenequinones and their derivatives, tetraamino acids and their derivatives, long-chain amino polyols of glycerol tricarboxylate compounds, and hybrid structures. All of them have obvious toxicity, which can cause serious harm to plants and endanger the food safety of agricultural products. At present, chemical control is still the main means to prevent and control the diseases caused by Alternaria fungi. However, due to the influence of Alternaria resistance and environmental pollution, it is of great significance to find a series of biocontrol agents with higher controlling effect on Alternaria. Biocontrol of Trichoderma is safer and greener than the traditional chemical control methods. Trichoderma metabolites are also good antifungal substances, with broad-spectrum and efficient antibacterial activity, inhibiting the growth and metabolism of pathogenic fungi. The growth and metabolism of A. tenuissima ABL2 strain were inhibited by Trichoderma longibrachiatum SC5 metabolites. The content of non-host selective toxins in the metabolites of A. tenuissima ABL2 strain and the relative expression of genes related to the synthesis of A. tenuissima toxin were determined. The inhibition mechanism of T. longibrachiatum SC5 metabolites on A. tenuissima ABL2 strain production was clarified. The aim was to provide a reference and theoretical basis for the prevention and control of apple leaf blight caused by A. tenuissima. 【Methods】 T. longibrachiatum SC5 and A. tenuissima ABL2 strains were cultured on PDA medium for 7 days. The PDB liquid medium was made, and the SC5 strain was cultured on the medium for 15 days. The fermentation broth was filtered with filter paper and the broth was retained. The liquid was added with 3-fold-volume-ethyl acetate and oscillated for 1 hour. After standing extraction, the organic phase was evaporated in a rotary evaporator, and 1 ml of methanol was added to dissolve and evaporate. The SC5 metabolites were made into an orginal liquid with a concentration of 200.00 mg·mL-1, and added to the PDA medium to make a drug-containing medium with different concentrations of SC5 metabolites (0.01, 0.05, 0.10, 0.25, 0.50, 1.00 and 2.00 mg·mL-1). The ABL2 strain was inoculated on the drug-containing medium and cultured for 24 hours in a light incubator. The diameter of ABL2 colonies was measured and the colony inhibition rate was calculated on the 2, 4, 6, 8 and 10 days by cross method. On the 6th day, 5 mycelial plugs were prepared with a sterile puncher and placed in a 10 mL of EP tube, with 3 replicates per concentration. After adding 8 mL of ethyl acetate, ultrasonic oscillation was performed for 1.5 hours, centrifuged and filtered. After the ethyl acetate phase was evaporated to dryness, 1 mL of methanol was added to dissolve it, and 5 mm circular sterile filter paper was placed in it for later use. Inoculate healthy leaves after disinfection and rinsing using stab inoculation method. A circular filter paper soaked in ABL2 metabolites was placed at each wound, and placed in an artificial climate box for moisturizing culture for 7 days. The lesion size was measured and the pathogenic activity of the metabolites was calculated. The optimal inhibitory concentration of SC5 metabolites on the growth of ABL2 strain was screened. The total RNA and the metabolites of ABL2 strain growing in the optimal concentration of drug-containing medium was extracted on 2, 4, 6, 8 and 10 days, and the quantitative standard curves of six non-host-selective toxins were made respectively. Six toxin-producing related gene primers were designed and verified for specificity. Liquid Chromatograph Mass Spectrometer and Real Time Quantitative PCR were used to determine the content of toxins in ABL2 metabolites and the relative expression of toxin-producing related genes. 【Results】 The crude extract of SC5 metabolism at a concentration of 0.5 mg·mL-1 had a significant inhibitory effect on the growth and pathogenicity of ABL2, and the inhibition rates were 38.08% and 76.96% on the 6th day, respectively. 0.5 mg·mL-1 SC5 metabolic crude extract had a significant inhibitory effect on the non-host selective toxins TEN, ALT and TeA produced by ABL2 strain. The inhibitory effect was the most significant after 2 days treatment, and its content was reduced by 69.39%, 98.51% and 48.99%, respectively. The expression levels of TES, TES (1), PksA and PksJ were significantly down-regulated by 89.02%, 98.20%, 46.49% and 40.13%, respectively. However, the content of non-host selective toxin ATX-I of ABL2 strain and the expression of PksF gene involved in its synthesis increased. 【Conclusion】 The SC5 metabolites of T. longibrachiatum at a concentration of 0.5 mg·mL-1 had an inhibitory effect on the growth and pathogenicity of A. tenuissima ABL2 strain. It could reduce and inhibit the content of TEN, ALT and TeA by down-regulating the expression of TES, TES (1), PksA and PksJ genes in ABL2 strain, thereby reducing its pathogenicity. This study can provide a theoretical basis for the biological control of ABL2 strain.
Key words: Fuji apple leaves; Alternaria tenuissima; Trichoderma longibrachiatum; Toxin; Gene expression; Biological control
植物病原链格孢(Alternaria spp.)引起的苹果病害,在世界各大产区普遍发生[1]。如苹果链格孢专化型(A. alternata f. sp. mail)引起的苹果斑点落叶病,主要危害苹果叶片,使叶部出现褐色病斑,最终导致提前落叶[2]。同时,也可危害嫩枝和果实,导致树势衰弱,影响花芽形成和果实正常生长[3]。但是,近年来笔者课题组在甘肃省苹果产区首次发现由极细链格孢(A. tenuissima)引起的苹果叶枯病,发生后严重导致苹果叶片枯死脱落。据报道,链格孢菌可通过产生链格孢毒素危害植物。已发现的链格孢毒素可分为5类,分别为二苯-α-吡喃酮类及其衍生物、苝醌类及其衍生物、四氨基酸及其衍生物、长链氨基多元醇的丙三羧酸酯类化合物、混杂多样结构[4-5]。目前,研究最多的链格孢毒素有交链孢酚(AOH:Alternariol)、交链孢单甲醚(AME:Alternariol monomethyl ether)、交链孢烯(ALT:Altenuene)、交链孢毒素(ATX-Ⅰ、Ⅱ、Ⅲ:Altertoxin)、腾毒素(TEN:Tentoxin)、细交链孢菌酮酸(TeA:Tenuazonic Acid),其不仅对植物产生严重危害[6-7],而且危及农产品食品安全[8-9]。同时,相关研究发现链格孢毒素的生物合成受相关基因调控。Wenderoth等[10]鉴定了AOH、AME生物合成相关的基因簇PksI、omtI、moxI、aohR、sdrI、doxI并确定其功能;Liu等[11]发现聚酮合酶基因Pks参与链格孢代谢途径且参与次生代谢物的合成,进而影响链格孢毒素含量;Li等[12]研究发现和验证了TES和TES(1)基因是链格孢合成TEN毒素所需的两个基因簇。目前,化学防治依旧是防控链格孢属真菌引起的病害的主要手段[13],但是长期大量使用可导致链格孢菌抗药性产生和污染环境[14],因此,寻找一类对链格孢具有较好防治效果的生防制剂对该病害的防控具有重要意义。
木霉作为一种优良的生防菌,被广泛应用于农业生产中的病害防治[15]。前期Abdel-Lateff等[16]从长枝木霉代谢物中分离出一种吡喃酮衍生物,发现其具有清除自由基、抗氧化、抑菌活性;Mironenka等[17]发现哈茨木霉(Trichoderma harzianum)代谢物可抑制黄色镰孢菌产孢,同时减少其色素和主要毒素的含量。但是,目前有关木霉代谢物对极细链格孢产毒抑制机制尚未见报道。因此,笔者在本试验中评价了长枝木霉代谢粗提物对极细链格孢的抑菌效果和毒素产生的影响,旨在为防治链格孢引起的苹果叶枯病提供参考依据和理论基础。
1 材料和方法
1.1 材料
1.1.1 供试菌株 极细链格孢(A. tenuissima)ABL2(序列号:MZ222271)和长枝木霉(T. longibrachiatum)SC5(序列号:ON786721)菌株由甘肃农业大学植物保护学院植物病毒学和分子生物学实验室分离、鉴定并保存。
1.1.2 供试叶片 供试叶片采自甘肃农业大学苹果种植园,品种为富士。
1.1.3 标准溶液与试剂 链格孢毒素标准溶液(AOH、AME、ALT、TEN、TeA、ATX-Ⅰ)购自青岛普瑞邦生物工程有限公司;乙酸乙酯(分析纯)、甲酸(色谱纯)、乙醇(分析纯)、甲醇(色谱纯)和乙腈(色谱纯)均购自天津市大茂化学试剂厂;TRNzol Universal 总RNA提取试剂购自TIANGEN公司;cDNA合成试剂盒、RT-qPCR试剂盒和DNA Marker均购自TaKaRa Bio公司;上下游引物由北京擎科生物科技有限公司西安分公司合成。
1.2 方法
1.2.1 长枝木霉SC5和极细链格孢ABL2菌株活化 将低温保存的SC5和ABL2菌株分别接种于PDA培养基活化培养7 d后,并再次制取菌饼接种于新的PDA培养基活化备用。
1.2.2 长枝木霉SC5代谢粗提物制备 SC5代谢粗提物制备参照Liu等[18]的方法并稍作修改。利用无菌打孔器制取活化3 d且直径为5 mm的SC5菌饼,并接种于200 mL PDB培养基(3个菌饼),然后置于光照、28 ℃、150 r·min-1的恒温振荡培养箱中振荡培养。待培养15 d后,利用中速定性滤纸抽滤去除菌丝,并将滤液经4 ℃、10 000 r·min-1离心30 min后,利用3倍体积的乙酸乙酯充分振荡并萃取。将乙酸乙酯相置于旋转蒸发仪蒸干浓缩并经1 mL甲醇溶解和0.22 μm滤器过滤,再次浓缩获得SC5代谢粗提物。
1.2.3 长枝木霉SC5代谢粗提物对极细链格孢ABL2生长的抑制作用 将浓缩后的SC5代谢粗提物利用甲醇配制质量浓度为200 mg·mL-1母液。同时,分别取1.000、0.500、0.250、0.125、0.050、0.025和0.005 mL的母液用甲醇制成1 mL的工作液,并加入99 mL灭菌后待凝固的PDA中,制得含SC5代谢粗提物质量浓度为2.00、1.00、0.50、0.25、0.10、0.05和0.01 mg·mL-1含药培养基,以加入1 mL甲醇的培养基作为对照。然后,将直径为5 mm的ABL2菌饼接种于不同浓度含药培养基中央,并置于温度为25 ℃全光照培养箱中培养,每个处理和对照均设10次重复,分别在接种培养第2、4、6、8和10 d时,采用十字交叉法测量菌落直径,并计算SC5代谢粗提物对ABL2生长抑制率。
[生长抑制率/%=对照菌落直径-处理菌落直径对照菌落直径-5 mm×100]。
1.2.4 长枝木霉SC5代谢粗提物对极细链格孢ABL2致病力的影响 将1.2.3中经不同浓度SC5代谢粗提物和甲醇处理培养6 d时的ABL2菌株作为供试处理菌株和对照菌株,参照Desrochers等[19]的方法提取ABL2菌株代谢产物,并测定其致病力。利用直径为7 mm打孔器制取5个不同浓度处理菌株和对照菌株菌饼,并分别置于10 mL EP管中,每个处理3次重复。然后,分别加入8 mL乙酸乙酯,并经涡旋振荡和超声提取后离心(8000 r·min-1)去除菌饼、菌丝。待静置1 h后将乙酸乙酯相置于旋转蒸发仪蒸干浓缩并经1 mL甲醇溶解和0.22 μm滤器过滤,收集获得不同浓度处理菌株和对照菌株代谢产物。然后,将无菌滤纸片(直径5 mm)置于不同浓度处理菌株和对照菌株代谢产物浸泡1 h,制备含有ABL2代谢产物的滤纸片备用。利用75%乙醇将健康的苹果叶片消毒后,经无菌水清洗3次,然后,利用吸水纸吸干叶片表面水分,采用针刺接种法刺破叶片表面,并将含有不同浓度处理菌株和对照菌株ABL2代谢产物(阳性对照)的滤纸片接种于刺伤部位,试验以1 mL甲醇浸泡等时间的滤纸片作为阴性对照,每组5个重复。接种后置于人工气候箱,于第5天时利用十字交叉法测量其病斑大小。
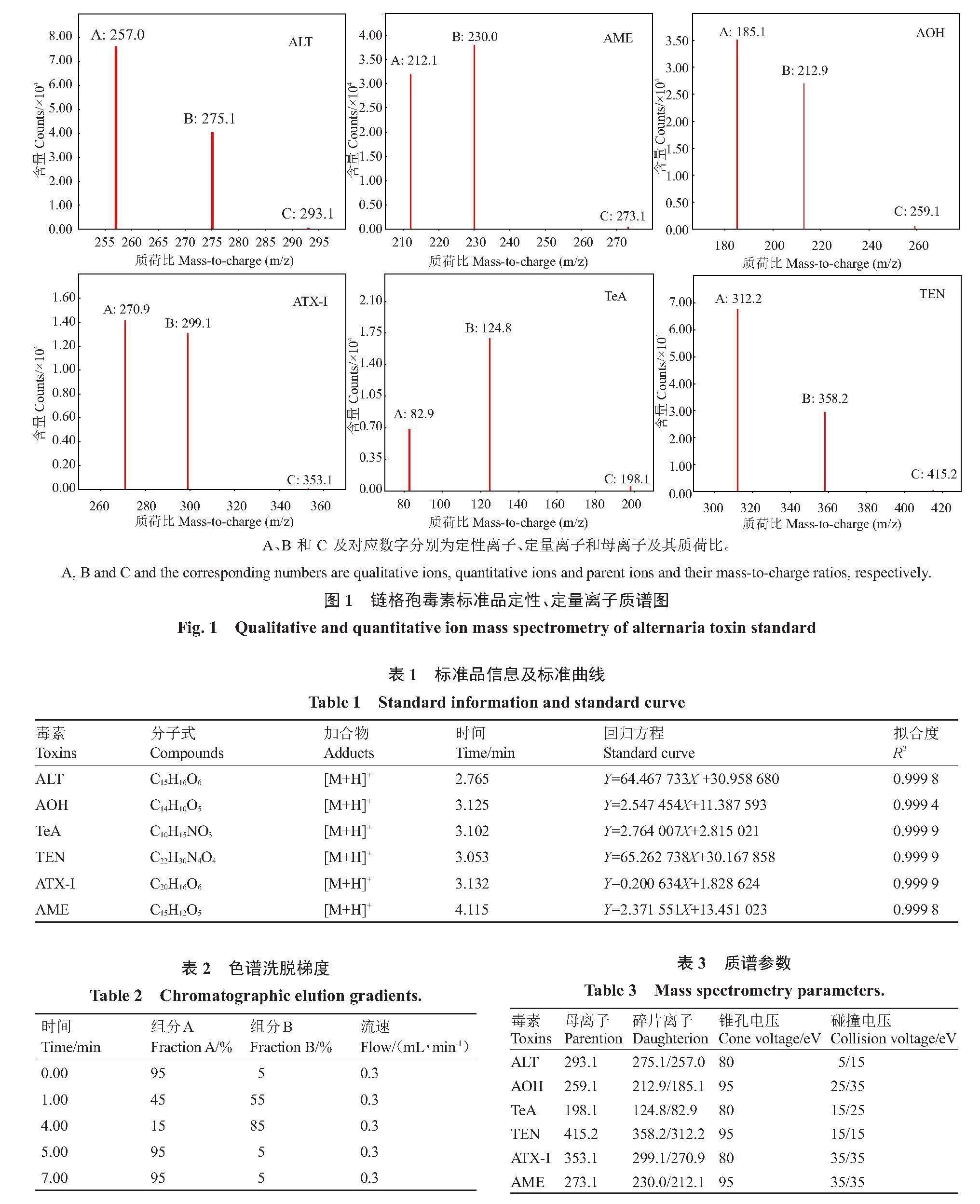
1.2.5 长枝木霉SC5代谢粗提物对极细链格孢ABL2毒素含量的影响 将SC5代谢粗提物对ABL2菌落和致病力抑制最佳浓度作为供试浓度,测定SC5代谢粗提物对ABL2毒素含量的影响。试验以1.2.3 中SC5代谢粗提物对ABL2菌落生长抑制作用最佳浓度培养基培养的ABL2菌株作为处理,并以加入等体积甲醇的培养基培养的ABL2菌株作为对照,分别于培养2、4、6、8和10 d后制备处理和对照的菌饼,并参考1.2.4方法提取和制备处理和对照菌株代谢产物。待制备获得处理和对照菌株代谢产物后,利用LC-MS检测其AOH、AME、ALT、TEN、TeA和ATX-Ⅰ含量。试验利用甲醇作为溶剂配制不同质量浓度(1000、500、250、100、50、25、10、5和1 ng·mL-1)链格孢毒素AOH、AME、ALT、TEN、TeA和ATX-Ⅰ标准品的混标溶液,并利用LC-MS测定后制作标准曲线(图1、表1)。<\\LENOVO-FAN\fapai\果树学报\1期-果树学报-定版\1-2024-1-学报-飞翔\Image\image11.png>
然后,利用LC-MS检测处理和对照菌株代谢产物AOH、AME、ALT、TEN、TeA和ATX-Ⅰ含量。超高效液相色谱条件为:Agilent 1290超高效液相色谱仪配备Elipse Plus C18 (规格1.8 μm,2.1 mm×150 mm)色谱柱;柱温设置为30 ℃;流动相A为0.2%的甲酸水,B 为加入0.2%氨水的乙腈;进样量为5 μL;流速设置0.3 mL·min-1(表2);质谱条件为:Agilent 6460三重四级杆质谱检测器配备电喷雾离子源,采用正离子模式(ESI+);扫描范围(m·z-1)设置为100~900;锥孔电压分别设置为80、95 eV:碰撞电压为15、25、35 eV;NMR数据监测模式;锥孔气流150 L·h-1;离子源温度350 ℃;喷雾电压7500 V(表3)。同时,利用Agilent MassHunter Quantitative Analysis(For QQQ)分析并计算处理和对照菌株代谢产物AOH、AME、ALT、TEN、TeA和ATX-Ⅰ含量。
1.2.6 长枝木霉SC5代谢粗提物对极细链格孢ABL2毒素合成相关基因表达的影响 (1)样品采集及总RNA的提取和cDNA第一链合成。将SC5代谢粗提物对ABL2菌落和致病力抑制最佳浓度作为供试浓度,测定SC5代谢粗提物对ABL2毒素合成相关基因表达的影响。试验以1.2.3中SC5代谢粗提物对ABL2菌落生长抑制作用最佳浓度培养基培养的ABL2菌株作为处理,并以加入等体积甲醇的培养基培养的ABL2菌株作为对照,待处理2、4、6、8和10 d后收集处理和对照ABL2菌株菌丝。菌丝处理方法参照郑朋飞等[20]的,将处理和对照菌丝置于液氮中并充分研磨破碎,每个处理和对照均3个重复。然后,根据TRNzol Universal 总RNA提取试剂说明书提取RNA,并进行浓度及A260/A280 比值测定。利用PrimeScript? RT reagent Kit (Perfect Real Time) 试剂盒进行反转录,合成处理和对照样品cDNA第一链。
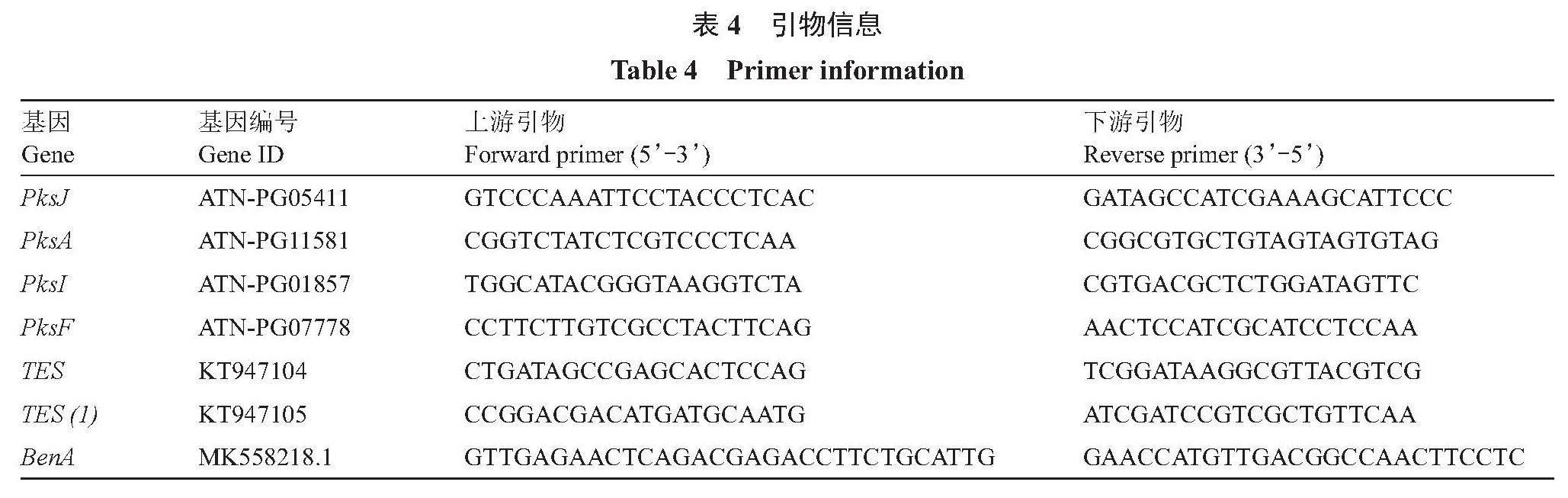
(2)ABL2毒素合成相关基因表达量分析。试验以处理2、4、6、8和10 d后的处理和对照ABL2菌株菌丝cDNA第一链为模板,利用TB Green? Premix Ex Taq? (Tli RNaseH Plus)试剂盒测定SC5代谢粗提物对ABL2毒素合成相关基因[PksJ、PksA、PksI、PksF、TES、TES(1)]表达的影响,以BenA作为内参基因,并利用2-??Ct法计算其相对表达量。利用Primer 5软件设计引物,引物序列见表4。
1.3 数据处理
采用MS office Excel 2021和IBM SPSS Statistics 27软件对数据进行统计分析,并运用Duncans新复极差法进行多重比较,利用OriginPro 2021作图并分析。
2 结果与分析
2.1 长枝木霉SC5代谢粗提物对极细链格孢ABL2生长的抑制作用
SC5代谢粗提物对ABL2菌落的抑制作用随着代谢粗提物浓度的增加而逐渐增强(图2)。与对照(图2-A)相比,经质量浓度为2.00、1.00、0.50、0.25、0.10、0.05和0.01 mg·mL-1的SC5代谢粗提物处理6 d的ABL2菌落直径显著小于对照(图2-B~H),且不同质量浓度之间存在显著差异。如表5所示,当培养2 d时,质量浓度为2 mg·mL-1的SC5代谢粗提物对ABL2菌落生长抑制率达到最大值,为90.42%,当SC5代谢粗提物质量浓度大于0.05 mg·mL-1时,随着培养时间的增加,抑制率趋于平稳。
2.2 长枝木霉SC5代谢粗提物对极细链格孢ABL2致病力的影响
结果如图3所示,经不同浓度的SC5代谢粗提物处理后的ABL2代谢产物致病力随着浓度的增加而减弱。接种叶片5 d后,经质量浓度为0.01~0.25 mg·mL-1(图3-A、B)SC5代谢粗提物处理后的ABL2代谢产物的致病力呈降低趋势,与其他处理相比,经质量浓度为0.50~2.00 mg·mL-1 (图3-A、B)SC5代谢粗提物处理后的ABL2代谢产物致病力显著降低,与阳性对照相比分别降低了76.96%、81.94%和80.95%。
2.3 长枝木霉SC5代谢粗提物对极细链格孢ABL2毒素产生的影响
结果表明(图4),SC5代谢粗提物对ABL2菌株ALT、AME、AOH、TeA、ATX-I和TEN毒素含量具有显著的影响,并且不同时间段的毒素含量存在明显差异。与对照相比,处理2~8 d后,SC5代谢粗提物对ABL2菌株ALT(图4-A)和TEN(图4-D)毒素含量具有显著的抑制作用,其处理时间段内平均含量分别较对照降低59.74%和84.41%。然而,处理2~8 d后,SC5代谢粗提物对ABL2菌株AME(图4-B)、AOH(图4-C)、TeA(图4-E)和ATX-I(图4-F)毒素含量具有不同程度的影响,其中在处理2 d后均表现出一定的抑制作用,但是随着处理时间的增加,其抑制作用不显著,与对照相比,处理时间段内TeA含量整体呈降低趋势,ATX-I含量整体呈上升趋势,AOH和AME含量在处理6~8 d时显著高于对照。
2.4 产毒相关基因表达分析
结果表明(图5),SC5代谢粗提物对ABL2菌株产毒相关基因TES、TES(1)、PksJ、PksA、PksF和PksI表达具有显著的影响,并且不同时间段的表达存在显著差异。与对照相比,处理2~10 d,SC5代谢粗提物对ABL2菌株TES(图5-A)、TES(1)(图5-B)、PksA(图5-D)和PksI(图5-F)基因表达量具有显著的抑制作用,其处理时间段内平均表达量较对照降低了61.35%、65.83%、56.94%和65.19%。然而,处理2~10 d,SC5代谢粗提物对ABL2菌株PksJ(图5-C)和PksF(图5-E)基因表达量具有不同程度的影响,其中在处理2~4 d时对PksF表现出一定的抑制作用,但随着处理时间的增加,抑制作用变为促进作用,而在处理2~8 d时对PksJ表现出一定的抑制作用,但在处理10 d后却由抑制变为促进。
3 讨 论
Liu等[11]发现链格孢代谢途径中与毒素合成相关的聚酮合酶Pks基因会受到肉桂醛的抑制;Yun等[21]证明TAS1可催化异亮氨酸和乙酰辅酶合成TeA;Saha等[22]研究发现PksJ下调会直接抑制AOH和AME的合成,PksB和PksI的下调并不直接影响AOH和AME的合成,而PksH则通过调节PksJ和PksI 的表达来控制AOH和AME的合成。Li等[12]研究发现两个分别编码一个非核糖体合成酶和一种细胞色素P450蛋白的基因TES和TES(1),并进一步验证TES和TES(1)参与Tentoxin合成。目前,利用长枝木霉代谢粗提物抑制极细链格孢产毒的研究尚未报道。笔者在本研究中发现0.5 mg·mL-1的SC5代谢粗提物对ABL2菌落生长和代谢产物的致病力有较好的抑制效果,并通过抑制产毒相关基因的表达减少毒素含量。通过分析基因表达量和毒素含量后发现随着培养时间的增加PksJ表达量呈下调趋势,同时TeA含量呈下降趋势,且在第2天、第4天和第6天时显著低于对照,但在第10天时PksJ表达量高于对照,同时TeA含量也高于对照,表明TeA的合成主要受PksJ调控,这与Liu等[11]的研究结果一致;而PksA的表达量先上升后下降,在第6天时达到最大值,但均显著低于对照,这与TEN毒素含量的变化趋势相同,初步推测PksA参与了TEN毒素的生物合成,同时PksI表达量也随时间的增加呈上调趋势,但显著低于对照,且AOH和AME含量随时间变化呈上升趋势,在第6天和第8天时却显著高于对照,表明PksA和PksI共同参与AOH和AME的生物合成,且PksA在第6天时开始参与调控TEN毒素的生物合成,这与Liu等[11]的研究结果一致,但与Saha等[22]的研究结果略有差异,初步判断差异的原因是抑菌物质和致病菌的不同;TES和TES(1)表达量与对照相比显著降低,同时TEN含量(ρ)在第6天达到最大值8.74 ng·mL-1,说明TES、TES(1)主要参与调控TEN的生物合成,这与Li等[12]等的研究结果相同;ATX-I含量呈上升趋势,在第4天、第6天、第8天和第10天时均显著高于对照,这与PksF表达情况相似,同时ALT含量呈上升趋势且显著低于对照,这与PksI表达情况相似,但PksF和PksI如何调控ATX-I和ALT的生物合成还有待研究。结果表明,SC5代谢粗提物在0.5 mg·mL-1的质量浓度下会抑制ABL2菌落的生长和非寄主选择性毒素TEN、ALT和TeA的生物合成,从而减少毒素含量,但有关SC5代谢粗提物抑制ABL2菌株毒素合成及其相关基因表达的分子作用机制有待深入研究。
4 结 论
(1)筛选获得了长枝木霉SC5代谢粗体物对极细链格孢ABL2菌株生长抑制作用及其代谢物质致病作用最佳质量浓度为0.5 mg·mL-1,处理6 d后,其对ABL2菌落生长和致病力抑制率分别为38.08%和76.96%。
(2)初步推测SC5代谢粗提物抑制ABL2菌株产毒的机制可能通过抑制ABL2菌株生长同时抑制其产毒相关基因如TES、TES(1)、PksA和PksJ基因的表达,进而降低ALT、TEN和TeA毒素含量,削弱ABL2菌株致病力。
参考文献References:
[1] ZHANG Q L,XU C R,WEI H Y,FAN W Q,LI T Z. Two pathogenesis-related proteins interact with leucine-rich repeat proteins to promote Alternaria leaf spot resistance in apple[J]. Horticulture Research,2021,8:219.
[2] 鄢海峰,周宗山. 异菌脲与戊唑醇、吡唑醚菌酯复配对苹果斑点落叶病菌的联合毒力[J]. 中国果树,2021(6):19-23.
YAN Haifeng,ZHOU Zongshan. Synergistic interaction of the mixtures of iprodione with pyraclostrobin or tebuconazole on Alternaria alternata f. sp. mali[J]. China Fruits,2021(6):19-23.
[3] LIU B Y,LI Z W,DU J F,ZHANG W,CHE X Z,ZHANG Z R,CHEN P,WANG Y Z,LI Y,WANG S L,DING X H. Loop-mediated isothermal amplification (LAMP) for the rapid and sensitive detection of Alternaria alternata (Fr.) Keissl in apple Alternaria blotch disease with Aapg-1 encoding the endopolygalacturonase[J]. Pathogens,2022,11(11):1221.
[4] HAN X M,XU W J,WANG L X Y,ZHANG R N,YE J,ZHANG J,XU J,WU Y. Natural occurrence of Alternaria toxins in citrus-based products collected from China in 2021[J]. Toxins,2023,15(5):325.
[5] TANG H X,HAN W,FEI S X,LI Y B,HUANG J Q,DONG M F,WANG L,WANG W M,ZHANG Y. Development of acid hydrolysis-based UPLC-MS/MS method for determination of Alternaria toxins and its application in the occurrence assessment in solanaceous vegetables and their products[J]. Toxins,2023,15(3):201.
[6] JI X F,XIAO Y P,LYU W T,LI M L,WANG W,TANG B,WANG X D,YANG H. Probabilistic risk assessment of combined exposure to deoxynivalenol and emerging Alternaria toxins in cereal-based food products for infants and young children in China[J]. Toxins,2022,14(8):509.
[7] ZHU X,CHEN Y Y,TANG X Y,WANG D X,MIAO Y Q,ZHANG J,LI R R,ZHANG L S,CHEN J Y. General toxicity and genotoxicity of altertoxin I:A novel 28-day multiendpoint assessment in male Sprague-Dawley rats[J]. Journal of Applied Toxicology,2022,42(8):1310-1322.
[8] DEL FAVERO G,HOHENBICHLER J,MAYER R M,RYCHLIK M,MARKO D. Mycotoxin altertoxin Ⅱ induces lipid peroxidation connecting mitochondrial stress response to NF-κB inhibition in THP-1 macrophages[J]. Chemical Research in Toxicology,2020,33(2):492-504.
[9] WITTE T E,VILLENEUVE N,BODDY C N,OVERY D P. Accessory chromosome-acquired secondary metabolism in plant pathogenic fungi:The evolution of biotrophs into host-specific pathogens[J]. Frontiers in Microbiology,2021,12:664276.
[10] WENDEROTH M,PINECKER C,VO? B,FISCHER R. Establishment of CRISPR/Cas9 in Alternaria alternata[J]. Fungal Genetics and Biology,2017,101:55-60.
[11] LIU M,XU L C,LIU W J,YU J N,JING G X,LIU H. Cinnamaldehyde regulates the synthesis of Alternaria alternata non-host selective toxins by influencing PKS gene expression and oxidoreductase activity[J]. Industrial Crops and Products,2020,145:112074.
[12] LI Y H,HAN W J,GUI X W,WEI T,TANG S Y,JIN J M. Putative nonribosomal peptide synthetase and cytochrome P450 genes responsible for tentoxin biosynthesis in Alternaria alternata ZJ33[J]. Toxins,2016,8(8):234.
[13] LI J K,LI H,JI S F,CHEN T,TIAN S P,QIN G Z. Enhancement of biocontrol efficacy of Cryptococcus laurentii by cinnamic acid against Penicillium italicum in citrus fruit[J]. Postharvest Biology and Technology,2019,149:42-49.
[14] HOUGH J,HOWARD J D,BROWN S,PORTWOOD D E,KILBY P M,DICKMAN M J. Strategies for the production of dsRNA biocontrols as alternatives to chemical pesticides[J]. Frontiers in Bioengineering and Biotechnology,2022,10:980592.
[15] GUZM?N-GUZM?N P,KUMAR A,DE LOS SANTOS-VILLALOBOS S,PARRA-COTA F I,OROZCO-MOSQUEDA M D C,FADIJI A E,HYDER S,BABALOLA O O,SANTOYO G. Trichoderma species:Our best fungal allies in the biocontrol of plant diseases:A review[J]. Plants,2023,12(3):432.
[16] ABDEL-LATEFF A,FISCH K,WRIGHT A D. Trichopyrone and other constituents from the marine sponge-derived fungus Trichoderma sp.[J]. Zeitschrift Für Naturforschung C,2009,64(3/4):186-192.
[17] MIRONENKA J,R??ALSKA S,SOBO? A,BERNAT P. Trichoderma harzianum metabolites disturb Fusarium culmorum metabolism:Metabolomic and proteomic studies[J]. Microbiological Research,2021,249:126770.
[18] LIU S Y,LO C T,SHIBU M A,LEU Y L,JEN B Y,PENG K C. Study on the anthraquinones separated from the cultivation of Trichoderma harzianum strain Th-R16 and their biological activity[J]. Journal of Agricultural and Food Chemistry,2009,57(16):7288-7292.
[19] DESROCHERS N,WALSH J P,RENAUD J B,SEIFERT K A,YEUNG K K C,SUMARAH M W. Metabolomic profiling of fungal pathogens responsible for root rot in American ginseng[J]. Metabolites,2020,10(1):35.
[20] 郑朋飞,张学勇,孙骞,王楚堃,杨钰莹,芮麟,宋来庆,张振鲁,由春香. 苹果花脸症状相关基因差异表达的分析[J]. 果树学报,2023,40(6):1109-1120.
ZHENG Pengfei,ZHANG Xueyong,SUN Qian,WANG Chukun,YANG Yuying,RUI Lin,SONG Laiqing,ZHANG Zhenlu,YOU Chunxiang. Transcriptome sequencing analysis of differentially expressed genes involved in the formation of dapple symptoms in apple fruits[J]. Journal of Fruit Science,2023,40(6):1109-1120.
[21] YUN C S,MOTOYAMA T,OSADA H. Biosynthesis of the mycotoxin tenuazonic acid by a fungal NRPS-PKS hybrid enzyme[J]. Nature Communications,2015,6:8758.
[22] SAHA D,FETZNER R,BURKHARDT B,PODLECH J,METZLER M,DANG H,LAWRENCE C,FISCHER R. Identification of a polyketide synthase required for alternariol (AOH) and alternariol-9-methyl ether (AME) formation in Alternaria alternata[J]. PLoS One,2012,7(7):e40564.

