射血分数保留性心力衰竭常见合并症的病理机制及治疗策略
张啸 刘剑刚
【摘要】射血分数保留性心力衰竭(HFpEF)的病理生理机制尚未明确,目前认为与肾素-血管紧张素-醛固酮系统、晚期糖基化终末产物及其受体、冠状动脉微血管炎症等介导的细胞自噬、氧化应激、炎症反应及心脏能量代谢障碍等相关。高血压、糖尿病、冠心病、心房颤动等均可通过各种信号途径致使左心室心肌僵硬度增加、舒张期充盈受损等病理改变,最终导致HFpEF的发生。目前研究显示能够改善HFpEF预后的药物主要为钠葡萄糖共转运蛋白2抑制剂,而射血分数降低性心力衰竭的治疗手段也并不适用于HFpEF,故通过对HFpEF合并症进行早期预防及治疗,以控制HFpEF的发生发展显得尤为重要。现从HFpEF常见合并症的病理机制及治疗等方面进行归纳,以期为HFpEF的临床治疗提供借鉴和指导。
【关键词】射血分数保留性心力衰竭;高血压;糖尿病;病理机制;治疗方针
【DOI】10.16806/j.cnki.issn.1004-3934.2024.02.000
Pathophysiology and Treatment Strategies of Comorbidities in Heart Failure with Preserved Ejection Fraction
ZHANG Xiao1,2,LIU Jiangang1
(1.Xiyuan Hospital, China Academy of Chinese Medical Sciences, National Center for Clinical Cardiovascular Disease of Traditional Chinese Medicine, Beijing 100091, China;2.Graduate School of China Academy of Chinese Medical Sciences, Beijing 100700, China)
【Abstract】The pathophysiological mechanisms of heart failure with preserved ejection fraction (HFpEF) are not yet fully understood. It is currently believed to be associated with cellular autophagy mediated by the renin-angiotensin-aldosterone system,advanced glycation end products-receptor for advanced glycation end products,coronary microvascular inflammation,oxidative stress,inflammatory response,and cardiac energy metabolism disorder.Hypertension,diabetes,coronary artery disease,and atrial fibrillation can lead to increased left ventricular myocardial stiffness and impaired diastolic filling through various pathways,promoting HFpEF.Current studies have shown that the drugs which can improve the prognosis of HFpEF are mainly sodium glucose cotransport protein 2 inhibitors,and the treatment of heart failure with reduced ejection fraction is not applicable to HFpEF,so it is important to control the development of HFpEF by early prevention and treatment of its comorbidities.This paper reviews the pathological mechanisms and treatment of the common comorbidities of HFpEF to help the clinical management of HFpEF.
【Keywords】Heart failure with preserved ejection fraction;Hypertension;Diabetes mellitus;Pathological mechanism;Treatment guidelines
目前,在大于65岁的心力衰竭(heart failure,HF)患者中,有约70%的射血分数保留性心力衰竭(heart failure with preserved ejection fraction,HFpEF)患者,且相对于射血分数降低性心力衰竭(heart failure with reduced ejection fraction,HFrEF)的发病率,HFpEF每10年约增长10%[1]。HFpEF病因复杂,精准诊断相对困难。HFpEF流行病学资料提示,高血压、糖尿病、冠心病、心房颤动等是HFpEF的常见合并症,深入研究HFpEF合并癥的病理生理机制对于HFpEF的预防治疗和药物研发具有重要的前瞻性意义。
从治疗角度,2023年美国心脏病学会颁布了关于HFpEF管理的共识,推荐使用钠葡萄糖共转运蛋白2抑制剂(sodium-dependent glucose transporters 2,SGLT2i)、血管紧张素受体脑啡肽酶抑制剂、醛固酮受体拮抗剂等用于治疗HFpEF[2]。HFpEF病因治疗与合并症管理的目标是改善症状和预后,推荐在HFpEF病因分型基础上,对病因与合并症进行针对性管理,根据相应的指南进行最佳治疗。而SGLT2i是唯一明确能改善HFpEF患者预后的药物,这提示应当重视并早期识别高血压、糖尿病、冠心病及心房颤动伴随HFpEF的情况,并积极采取有效的干预手段控制该合并症的进展。HFpEF的心血管合并症包括缺血性心脏病、瓣膜性心脏病、肺动脉高压、心房颤动和室性心律失常以及传导障碍。而非心血管合并症包括糖尿病、慢性肾病、肥胖、肺病、缺铁和贫血、睡眠呼吸暂停、情绪障碍等。现主要归纳和讨论HF的潜在病因和主要合并症的管理内容,通过指南、专家共识及大型临床流行病学研究结论阐述HFpEF合并相关疾病的病理机制及临床特征,并进行分类分析,以期总结归类HFpEF合并症的治疗方针,现综述如下。
1 HFpEF合并症的发病机制
1.1 肾素-血管紧张素-醛固酮系统
肾素-血管紧张素-醛固酮系统(renin-angiotensin-aldosterone system,RAAS)通过诱导血管重建、增加心脏后负荷等,使得心脏肥大、心肌纤维化,从而导致舒张功能下降,对于高血压、心房颤动及HF的发生发展至关重要。血管紧张素Ⅱ水平升高可增加Rac 1/2 GTPase活性,诱导氧化氮合成酶产生活性氧[3]。活性氧导致一氧化氮缺乏以造成内皮功能障碍,促进心脏成纤维细胞增殖,激活基质金属蛋白酶而导致细胞外基质重塑,造成心肌细胞的损伤,引起心脏舒张功能障碍,促进了HFpEF的发生发展[4]。
1.2 晚期糖基化终末产物及其受体
糖尿病的慢性高糖状态使得糖基化反应过度从而产生过多的晚期糖基化终末产物(advanced glycation end product,AGE)与晚期糖基化终末产物受体(receptor for advanced glycation endproducts,RAGE)结合,抑制 Na+-K+-ATP酶的活性和氧化代谢,导致心肌细胞游离脂肪酸的堆积,产生脂肪毒性,对肌浆网的钙摄取造成负向影响,使得心肌细胞内钙超载,终致心肌细胞舒张功能障碍。此外晚期糖基化终末产物及其受体(advanced glycation end product-receptor for advanced glycation endproducts,AGEs-RAGE)还可通过上调Beclin1、BNIP3等基因,诱导细胞自噬以激活心脏成纤维细胞,导致心肌纤维化,损害心功能[5]。
1.3 微血管炎症
Rush等[6]研究发现约91%的HFpEF患者合并冠状动脉疾病或冠状动脉微血管功能障碍。冠状动脉微血管功能障碍限制了心肌的最大供血和供氧量,使得心肌细胞坏死、纤维化,形成心肌瘢痕,导致心室舒张功能障碍。与射血分数降低性心力衰竭患者相比,微血管内皮功能障碍在HFpEF中更普遍,是HFpEF发生的早期标志,且心肌活检显示HFpEF患者一氧化氮生物利用度降低[7],影响NO-sGC-cGMP信号通路,环鸟苷酸浓度降低,影响了心脏氧利用以及心脏能量底物代谢的调节过程,导致了炎症反应及脂肪毒性的发生,进一步影响心肌细胞功能[8]。
1.4 心肌能量代谢障碍
相较于正常心肌组织,肥胖的HFpEF患者心脏脂肪酸摄取和脂肪酸氧化不平衡,由此产生的脂质过载导致甘油二酯、神经酰胺、甘油三酯和其他脂质物种在心肌内过度堆积,造成脂毒性,而葡萄糖攝取降低和葡萄糖氧化减少,进一步导致腺苷三磷酸产生缺陷[9]。长链脂肪酸会诱导DNA甲基转移酶的过度表达,促进巨噬细胞向M1方向极化,减少抗炎M2巨噬细胞的比例,相反,短链脂肪酸和酮能减少单核细胞产生肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)和核因子-κB(nuclear factor-κB,NF-κB),并调节核苷酸结合域样受体蛋白3(NOD-like receptor protein 3,NLRP3)炎症小体来发挥局部抗炎效应[10],对HFpEF的发生发展起到抑制作用。
1.5 其他机制
胰岛素抵抗是导致高血压及2型糖尿病发生发展的共同病理生理基础,与HFpEF患者心功能恶化密切相关[11],其使心肌消耗更多的游离脂肪酸,增加了心脏对缺血和压力负荷的敏感性,促进心脏重塑。在心房颤动发作后恢复期心肌成纤维细胞活性增加以及胶原蛋白、弹性蛋白纤维的沉积,促进心肌纤维化,导致舒张功能出现障碍[12],而被动充盈减少加之心房颤动发作期间丧失心房收缩又可导致相应的搏出量减少,加重HF症状。
2 HFpEF的预防和治疗
在药物治疗方面,近年来研究表明SGLT2i在治疗HFpEF患者上取得了良好效果,且具有降糖、改善肾功能及降低心房颤动诱发率的作用,是首个改善HFpEF患者预后的药物。研究[13]发现恩格列净通过减少线粒体钙超载和ROS的生成改善糖尿病和HFpEF患者的内皮功能。还可抑制NF-κB,减少白细胞介素-1β及NLRP3炎性小体的激活,降低线粒体膜通透性转换(mitochondrial membrane permeability transition,MMPT),减轻肾小球硬化[14]。在吡非尼酮的二期临床研究[15]中发现与安慰剂组相比,其可减少细胞外容积,改善心肌纤维化,对HFpEF患者起到一定治疗作用。心肌能量代谢也逐渐引起学者们的重视,被认为是HFpEF的潜在治疗靶点。一项动物实验[16]显示增加β-羟丁酸水平可减少NLPR3炎性小体的形成,并拮抗促炎细胞因子引发的线粒体功能障碍和纤维化,从而缓解HFpEF小鼠的心功能障碍。也有研究[17]发现补充丁酸盐可抑制组蛋白去乙酰化,升高蛋白磷酸酶2Cm及其编码基因ppm1k的蛋白水平,增加支链氨基酸氧化分解,防治肥胖诱发的HFpEF。此外中药提取物如白藜芦醇被证明可以通过激活Sirt1、降低Smad3乙酰化和转录活性,从而对HFpEF诱导的不良心脏重塑起到保护作用[18],虽尚无大型临床试验证明改善能量代谢及服用中药提取物在HFpEF中的治疗作用,但并不妨碍其为HFpEF的治疗带来希望。Adropin是一种由肝脏分泌的肽激素,可以降低血脂、血糖、血压、改善胰岛素抵抗和保护血管内皮,被证明通过作用心肌细胞膜G蛋白偶联受体19激活细胞内MAPK-PDK4信号通路,从而发挥改善HFpEF的作用[19]。在手术治疗方面,房室间隔分流器为HF,尤其是HFpEF治疗的一项里程碑式技术,其可通过降低患者左心系统压力治疗HFpEF。但REDUCE LAP-HF Ⅱ结果发现在左室射血分数≥40% 的HF患者总体人群中放置房室间隔分流器并未降低HF事件的总发生率或改善患者健康状况[20]。
3 HFpEF合并症的预防和治疗
3.1 HFpEF合并高血压的预防和治疗
Schnelle等[21]研究评估了386例HFpEF患者使用螺内酯或安慰剂治疗一年后血浆样本中的92种生物标志物,发现螺内酯可升高MMPT等促进心脏重塑的指标,但也可以升高半乳凝素-9等抗动脉粥样硬化标志物的水平,故其在HFpEF中的作用机制及治疗效果尚不明晰。Jackson等[22]认为与缬沙坦相比,沙库巴曲缬沙坦使收缩压的下降幅度更大,16周后,沙庫巴曲缬沙坦组抵抗性高血压患者的收缩压控制比例为47.9%,缬沙坦组为34.3%(OR=1.78,95% CI 1.30~3.43)。Ledwidge等[23]对250例高血压合并HFpEF前期的患者进行观察,结果显示患者充盈压指标降低的情况下,沙库巴曲缬沙坦可以增加患者的左心房容积指数。然而,还需要做更多的工作来了解沙库巴曲缬沙坦对HFpEF前期患者的长期获益和风险状况。Patel等[24]在OPTIMIZE-HF选取1 620例HFpEF患者,分析发现钙通道阻滞剂与HFpEF患者的全因死亡率、HF住院率和全因住院率并不相关。而Wang[25]等纳入3 440例HFpEF患者,平均随访3.4±1.7年后发现,服用钙通道阻滞剂的患者全因死亡率、心血管和非心血管死亡风险明显低于未服用钙通道阻滞剂的患者。
3.2 HFpEF合并糖尿病的临床治疗方针
尽管多数降血糖药在单药或联合治疗中能够提供良好的控制血糖和抑制氧化应激的作用,但它们未能显示出降低心血管死亡率的可能。噻唑烷二酮类药物被发现能够通过诱导合成CYP450s、激活趋化因子及炎症反应等对小鼠心肌细胞产生毒性作用[26]。在2023年ACC HFpEF共识[2]中也指出禁用噻唑烷二酮类药物治疗合并糖尿病的HFpEF患者。亦有研究[27]显示与使用口服降血糖药相比,接受胰岛素治疗糖尿病合并HF的患者与死亡率、住院和再入院风险增加息息相关。二甲双胍和二肽基肽酶4(dipeptidyl peptidase-4,DPP-4)抑制剂对HF的影响尚有争议。有研究[28]显示,二甲双胍可通过使心肌腺苷酸活化的蛋白激酶活性正常化,改善脂肪酸氧化和减轻氧化应激而发挥防止左心室肥厚,改善心功能的作用。而Dia等[29]的实验却发现二甲双胍并不能阻止心肌细胞向肥厚和舒张功能障碍发展的进程。DPP-4抑制剂通过抑制心脏代偿机制,加重了心肌损伤[30]。Mu[31]等分析认为与安慰剂或其他降血糖药相比,DPP-4对2型糖尿病患者的脑钠肽(brain natriuretic peptide,BNP)或N末端脑钠肽前体(N-terminal pro-brain natriuretic peptide,NT-proBNP)水平并没有显著的调节作用。但Zakaria[32]等却发现DPP-4抑制剂可减少线粒体应激和细胞死亡,并通过降低心脏血管紧张素Ⅱ、增加血管紧张素1-7来改善心脏血流灌注,具有良好的心脏保护潜力。
当前的降血糖药治疗HFpEF的研究热点主要集中于SGLT2i和胰高糖素样肽-1受体激动剂(glucagon-like peptide-1 receptor agonist,GLP-1 RA)。Withaar等[33]通过研究发现,利拉鲁肽可以改善HFpEF小鼠心脏代谢紊乱的状态,减轻心肌肥厚和心肌纤维化症状,降低心房重量及钠尿肽水平。近期临床研究[34]结果显示,无论是否伴有2型糖尿病,恩格列净等SGLT2i均可改善HFpEF患者心功能,降低心血管不良事件的发生风险。故在糖尿病合并HFpEF的治疗中,应率先考虑SGLT2i及GLP-1 RA的使用。
3.3 HFpEF合并冠心病的临床治疗原则
在血脂管理方面,黄鑫涛等[35]研究发现高剂量瑞舒伐他汀可能通过抗炎、抗心肌纤维化等机制改善HFpEF患者的心室重塑、心室舒张功能及心功能,降低主要不良心血管事件发生率,且不增加不良反应。对于C反应蛋白水平升高的EF≥40%的HF患者,使用他汀类药物的患者的生存趋势更优,能够降低缺血性心脏病患者的死亡风险[36]。故他汀类药物或许可以通过降脂、抗炎等作用对心血管疾病起到一定的预防和治疗作用。
3.4 心房颤动伴HFpEF的临床治疗
SGLT2i及沙库巴曲缬沙坦对心房颤动也有一定治疗作用。有研究[37]表明达格列净能将房颤事件的风险降低约19%。Li等[38]一项实验研究发现沙库巴曲缬沙坦可通过抑制胶原蛋白Ⅰ和Ⅲ、NT-proBNP、Ca2+浓度等的增加及ICaL密度的下降来治疗房颤。而对于手术治疗,Zhu[39]等一项meta分析显示,与药物治疗相比,射频消融在改善心房颤动合并HF患者左室射血分数、心功能和运动能力方面效果更佳。
4 总结与展望
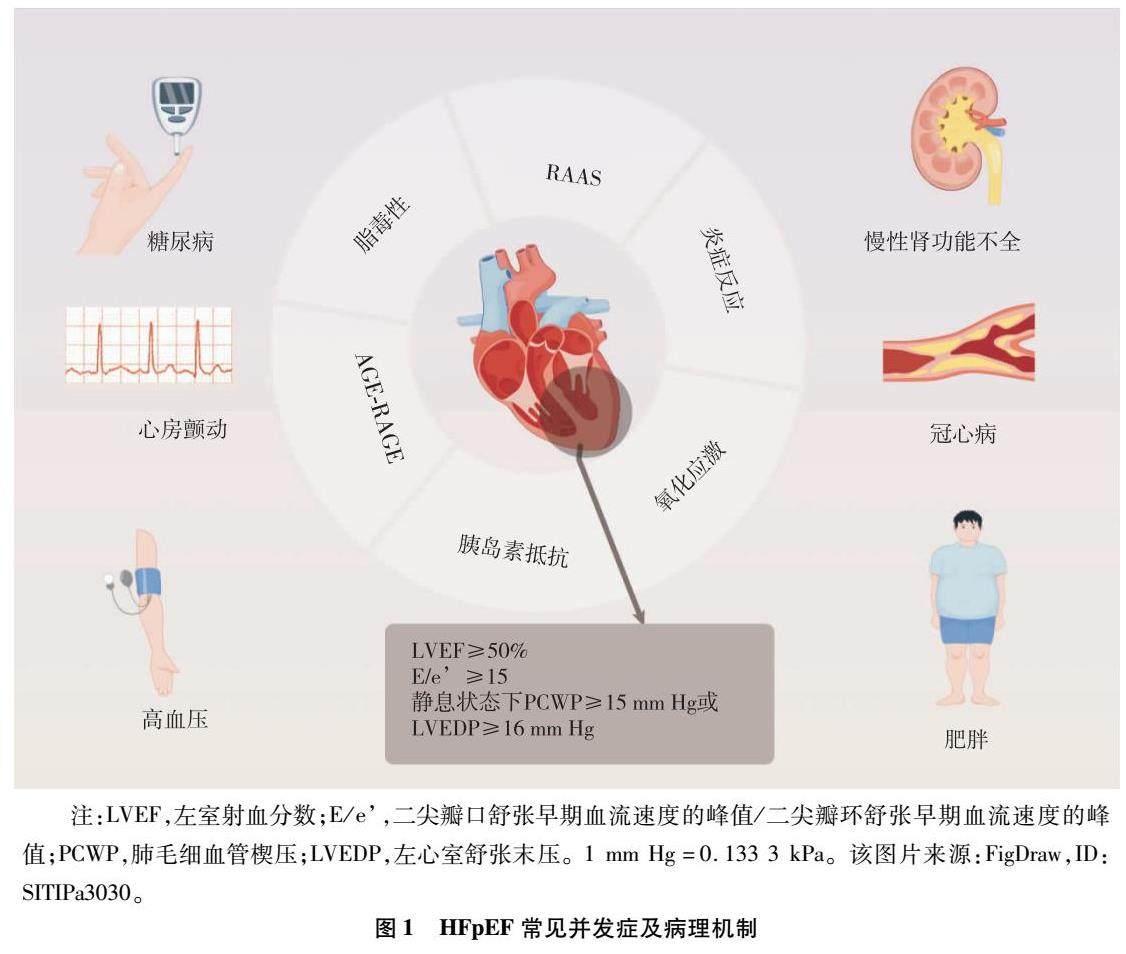
当前研究主要认为HFpEF的发生与RAAS系统、AGEs-RAGE、冠状动脉微血管炎症等介导的细胞自噬、氧化应激、炎症反应及心脏能量代谢障碍相关。血糖升高导致AGEs-RAGE的表达增强,生成过量活性氧,激活NF-κB并释放白细胞介素-6、TNF-α等,诱导细胞凋亡,引起线粒体功能障碍,还可引起脂质代谢紊乱,并导致肾系膜细胞损伤、血管内皮细胞破坏及心肌细胞的功能障碍,促进肥胖、肾功能不全、冠心病、房颤、HFpEF等的发生[40-43]。HFpEF机制复杂,合并症之间相互影响,共同促进HFpEF的发生发展,见图1。

注:LVEF,左室射血分数;E/e,二尖瓣口血流速度的峰值/二尖瓣环运动速度的峰值;PCWP,肺毛细血管楔压;LVEDP,左心室舒张末期压力;1 mm Hg=0.133 3 kPa。
图1 HFpEF常见并发症及病理机制
HFpEF患者常合并多种疾病,由多种病理生理紊乱导致HFpEF的发生和发展,因此可能需要联合使用多种药物才能有效减缓疾病的发展。除SGLT-2i外,吡非尼酮和支链氨基酸氧化激动剂、短链脂肪酸等改善心肌能量代谢的药物也給HFpEF的治疗带来希望。临床需要尽早识别高血压、糖尿病、冠心病、心房颤动等HFpEF合并症并及时进行评估,判断其与HFpEF转归预后的相关性,进行合理预防诊断及遵循相关指南进行治疗。
利益冲突 所有作者均声明不存在利益冲突
参考文献
[1] Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction[J]. Nat Rev Cardiol,2020,17(9):559-573.
[2] Kittleson MM,Panjrath GS,Amancherla K,et al. 2023 ACC Expert Consensus decision pathway on management of heart failure with preserved ejection fraction:a report of the American College of Cardiology Solution Set Oversight Committee[J]. J Am Coll Cardiol,2023,81(18):1835-1878.
[3] Teuber JP,Essandoh K,Hummel SL,et al. NADPH oxidases in diastolic dysfunction and heart failure with preserved ejection fraction[J]. Antioxidants (Basel),2022,11(9):1822.
[4] Budde H,Hassoun R,Mugge A,et al. Current understanding of molecular pathophysiology of heart failure with preserved ejection fraction[J]. Front Physiol,2022,13:928232.
[5] Liang BR,Zhou Z,Yang ZQ,et al. AGEs-RAGE axis mediates myocardial fibrosis via activation of cardiac fibroblasts induced by autophagy in heart failure[J]. Exp Physiol,2022,107(8):879-891.
[6] Rush CJ,Berry C,Oldroyd KG,et al. Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction[J]. JAMA Cardiol,2021,6(10):1130-1143.
[7] Simmonds SJ,Cuijpers I,Heymans S,et al. Cellular and molecular differences between HFpEF and HFrEF:a step ahead in an improved pathological understanding[J]. Cells,2020,9(1):242.
[8] Schiattarella GG,Rodolico D,Hill JA. Metabolic inflammation in heart failure with preserved ejection fraction[J]. Cardiovasc Res,2021,117(2):423-434.
[9] Capone F,Sotomayor-Flores C,Bode D,et al. Cardiac metabolism in HFpEF:from fuel to signalling[J]. Cardiovasc Res,2023,118(18):3556-3575.
[10] Challa AA,Lewandowski ED. Short-chain carbon sources:exploiting pleiotropic effects for heart failure therapy[J]. JACC Basic Transl Sci,2022,7(7):730-742.
[11] Son TK,Toan NH,Thang N,et al. Prediabetes and insulin resistance in a population of patients with heart failure and reduced or preserved ejection fraction but without diabetes,overweight or hypertension[J]. Cardiovasc Diabetol,2022,21(1):75.
[12] Verhaert DVM,Brunner-La Rocca HP,van Veldhuisen DJ,et al. The bidirectional interaction between atrial fibrillation and heart failure:consequences for the management of both diseases[J]. Europace,2021,23(23 suppl 2):ii40-ii45.
[13] Mone P,Lombardi A,Kansakar U,et al. Empagliflozin improves the microRNA signature of endothelial dysfunction in patients with HFpEF and diabetes[J]. J Pharmacol Exp Ther,2023,384(1):116-122.
[14] Defronzo RA,Reeves WB,Awad AS. Pathophysiology of diabetic kidney disease:impact of SGLT2 inhibitors[J]. Nat Rev Nephrol,2021,17(5):319-334.
[15] Lewis GA,Dodd S,Clayton D,et al. Pirfenidone in heart failure with preserved ejection fraction:a randomized phase 2 trial[J]. Nat Med,2021,27(8):1477-1482.
[16] Deng Y,Xie M,Li Q,et al. Targeting mitochondria-inflammation circuit by beta-hydroxybutyrate mitigates HFpEF[J]. Circ Res,2021,128(2):232-245.
[17] Hatahet J,Cook TM,Bonomo RR,et al. Fecal microbiome transplantation and tributyrin improves early cardiac dysfunction and modifies the BCAA metabolic pathway in a diet induced pre-HFpEF mouse model[J]. Front Cardiovasc Med,2023,10:1105581.
[18] Zhang L,Chen J,Yan L,et al. Resveratrol ameliorates cardiac remodeling in a murine model of heart failure with preserved ejection fraction[J]. Front Pharmacol,2021,12:646240.
[19]李兵達. Adropin在射血分数保留型心衰中的作用及机制[D].江西:南昌大学,2022.
[20] Shah SJ,Borlaug BA,Chung ES,et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF Ⅱ):a randomised,multicentre,blinded,sham-controlled trial[J]. Lancet,2022,399(10330):1130-1140.
[21] Schnelle M,Leha A,Eidizadeh A,et al. Plasma biomarker profiling in heart failure patients with preserved ejection fraction before and after spironolactone treatment:results from the Aldo-DHF trial[J]. Cells,2021,10(10):2796.
[22] Jackson AM,Jhund PS,Anand IS,et al. Sacubitril-valsartan as a treatment for apparent resistant hypertension in patients with heart failure and preserved ejection fraction[J]. Eur Heart J,2021,42(36):3741-3752.
[23] Ledwidge M,Dodd JD,Ryan F,et al. Effect of sacubitril/valsartan vs valsartan on left atrial volume in patients with pre-heart failure with preserved ejection fraction:the PARABLE randomized clinical trial[J]. JAMA Cardiol,2023,8(4):366-375.
[24] Patel K,Fonarow GC,Ahmed M,et al. Calcium channel blockers and outcomes in older patients with heart failure and preserved ejection fraction[J]. Circ Heart Fail,2014,7(6):945-952.
[25] Wang X,Ju J,Chen Z,et al. Associations between calcium channel blocker therapy and mortality in heart failure with preserved ejection fraction[J]. Eur J Prev Cardiol,2022,29(9):1343-1351.
[26] Jarrar YB,Jarrar Q,Abaalkhail SJ,et al. Molecular toxicological alterations in the mouse hearts induced by sub-chronic thiazolidinedione drugs administration[J]. Fundam Clin Pharmacol,2022,36(1):143-149.
[27] Liu J,Hu X. Impact of insulin therapy on outcomes of diabetic patients with heart failure:a systematic review and meta-analysis[J]. Diab Vasc Dis Res,2022,19(3):14791641221093175.
[28] Li J,Minczuk K,Massey JC,et al. Metformin improves cardiac metabolism and function,and prevents left ventricular hypertrophy in spontaneously hypertensive rats[J]. J Am Heart Assoc,2020,9(7):e015154.
[29] Dia M,Leon C,Chanon S,et al. Effect of metformin on T2D-induced MAM Ca2+ uncoupling and contractile dysfunction in an early mouse model of diabetic HFpEF[J]. Int J Mol Sci,2022,23(7):3569.
[30] Voros I,Onodi ZS,Toth VE,et al. Investigation of cardiotoxicity by dipeptidyl-peptidase-4 inhibitors in a human cardiomyocyte cell line as well as in samples from chronic heart failure patients[J]. Cardiovasc Res,2022,118(suppl 1):1.
[31] Mu L,Wang Z,Ren J,et al. Impact of DPP-4 inhibitors on plasma levels of BNP and NT-pro-BNP in type 2 diabetes mellitus[J]. Diabetol Metab Syndr,2022,14(1):30.
[32] Zakaria EM,Tawfeek WM,Hassanin MH,et al. Cardiovascular protection by DPP-4 inhibitors in preclinical studies:an updated review of molecular mechanisms[J]. Naunyn Schmiedebergs Arch Pharmacol,2022,395(11):1357-1372.
[33] Withaar C,Meems LMG,Markousis-Mavrogenis G,et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction[J]. Cardiovasc Res,2021,117(9):2108-2124.
[34] Filippatos G,Butler J,Farmakis D,et al. Empagliflozin for heart failure with preserved left ventricular ejection fraction with and without diabetes[J]. Circulation,2022,146(9):676-686.
[35]黃鑫涛,白保强,李之恒,等. 不同剂量瑞舒伐他汀对老年高血压并射血分数保留型慢性心力衰竭患者心室重构的影响[J]. 重庆医科大学学报,2022,47(1):66-73.
[36] Park JJ,Yoon M,Cho HW,et al. C-reactive protein and statins in heart failure with reduced and preserved ejection fraction[J]. Front Cardiovasc Med,2022,9:1064967.
[37] Zelniker TA,Bonaca MP,Furtado RHM,et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus:insights from the DECLARE-TIMI 58 trial[J]. Circulation,2020,141(15):1227-1234.
[38] Li LY,Lou Q,Liu GZ,et al. Sacubitril/valsartan attenuates atrial electrical and structural remodelling in a rabbit model of atrial fibrillation[J]. Eur J Pharmacol,2020,881:173120.
[39] Zhu X,Wu Y,Ning Z. Meta-analysis of catheter ablation versus medical therapy for heart failure complicated with atrial fibrillation[J]. Cardiol Res Pract,2021,2021:7245390.
[40] Gu MJ,Hyon JY,Lee HW,et al. Glycolaldehyde,an advanced glycation end products precursor,induces apoptosis via ROS-mediated mitochondrial dysfunction in renal mesangial cells[J]. Antioxidants (Basel),2022,11(5):934.
[41] Senatus L,Maclean M,Arivazhagan L,et al. Inflammation meets metabolism:roles for the receptor for advanced glycation end products axis in cardiovascular disease[J]. Immunometabolism,2021,3(3):e210024.
[42] Sun J,Xu J,Yang Q. Expression and predictive value of NLRP3 in patients with atrial fibrillation and stroke[J]. Am J Transl Res,2022,14(5):3104-3112.
[43] Wang S,Zhang J,Wang Y,et al. NLRP3 inflammasome as a novel therapeutic target for heart failure[J]. Anatol J Cardiol,2022,26(1):15-22.
收稿日期:2023-07-07