基于双金属高核钛氧簇基电极的高性能超级电容器的制备
罗 稳 靳 林*, Palanisamy Kannan 侯进乐 霍 鹏 姚金忠 王 鹏*,,5,6
(1周口师范学院河南省稀土功能材料重点实验室,周口 466001)
(2嘉兴学院生物与化学工程学院,嘉兴 314001)
(3聊城大学化学化工学院,聊城 252000)
(4复旦大学航空航天系,上海 200433)
(5嘉兴学院G60研究院,嘉兴 314001)
(6西安理工大学仪器科学与技术博士后流动站,西安 710048)
0 Introduction
Titanium-oxo-clusters (TOCs) have attracted significant attention in research due to their potential as precursors for soluble molecular titanium-oxo materials and nano-TiO2, which possess uniform scale and precise structure[1-3]. Among the numerous reported TOCs modified by metal doping and organic ligands, it can be found that the calculated electronic states of small clusters (nuclei number<10) vary significantly with the number of clusters′ nuclearity[4-6]. This highlights the significant potential for the synthesis and application research of high-nuclearity TOCs. Ti12[Ti12O16(OR)16](R=ethyl or isopropyl) has been extensively studied and widely applied in photocatalysis,photoelectric conversion,etc., likely due to its band gap that can be effectively regulated by doping transition metal ions[7-9].In recent decades, research on Ti12-based transitionmetal doped TOCs has primarily focused on functional ligands used to stabilize the structure of TOCs, such as pyrocatechol, benzoic acid, and 1, 10 - phenanthroline[10-12]. Therefore, developing novel functional ligandstable Ti12-based transition-metal doped TOCs and exploring their applications in emerging fields remain significant challenges.
Supercapacitors,with their ability to store and discharge energy quickly and efficiently,present a promising solution to the energy-storage crisis[13-14].At present,the main electrode materials that can be used as supercapacitors are carbon materials, MXenes, layered double hydroxides, black phosphorus, and perovskites[13-14].Recently,some metal-oxo-clusters (MOC)based energy storage devices have been reported,i.e.,AlO6and CuⅠ-MOFs containing[Mo8O26]4-clusters based supercapacitors[15-16]. However, their energy storage capacity and cycle performance are still lower than common energy storage materials in most studies, which were attributed to the introduction of low energy density metal elements and ligands with complex structures that are not resistant to high current environments[17-18]. Moreover,although the experimental and theoretical results of these MOC-based supercapacitors have revealed some details of energy storage, the diversity and systematisms of MOCs used in model studies are still incomplete, especially the TOC-based supercapacitor model has never been reported. This is mainly due to the low energy and easy breakage of Ti—O bonds, leading to the instability of the cluster structure and a sharp decline in electrical properties[4,19]. Therefore, it is necessary to search for a newly available ligand with a simple structure that can enhance the overall structural strength of the crystal cluster and keep the structure stable under an electrical environment. Meanwhile,another transition element needs to be introduced inside the lattice to further adjust the bond energy and increase the overall energy density of the system.
In this work,as a proof-of-concept study,the novel TOCs with electrical energy storage capabilities were successfully synthesized. TOCs with different transition metals(Zn and Cd)were obtained by a simple onestep solvothermal method, using thiophenol (SPh) as the chromophore and transition metals (Zn and Cd) as the second metal.The experimental results showed that the synthesized TOCs (Ti11clusters) had good energy storage capacity. Among them, the supercapacitor based on Zn-Ti11showed an excellent discharge capacity(175 F·g-1at 1 A·g-1)and provided the maximum power density and energy density of 9.5 W·kg-1and 463 Wh·kg-1,respectively.This work provides a reliable method for producing titanium-oxide-based energy storage devices with high energy density.
1 Experimental
1.1 Materials and instruments
All the analytical pure reagents were purchased from Adams and used without further purification.[Me4N]2[M4(SPh)10] (M=Cd, Zn) was prepared by the reported methods[20-21].UV-Vis absorbance spectra were obtained on a Shimadzu UV-2600 spectrometer. FTIR spectra were recorded on a Nicolet 6700 spectrophotometer (KBr pellet). X-ray diffraction (XRD) measurements were carried out using a Rigaku CCD X-ray diffractometer with Ni-filtered CuKαradiation (λ=0.156 nm, 2θ=5°-30°, 40 kV, 40 mA). Transmission electron microscopy (TEM) images were obtained on a Tecnai G220 TEM with carbon film-coated grids. The photocurrent experiments were performed on a CHI650 electrochemistry workstation in a three-electrode system.The microcrystal samples were mixed with Nafion -D521 (Alfa) and coated directly on a cleaned ITO electrode (50 Ω·cm2), which was used as the working electrode. A Pt plate and a saturated calomel electrode(SCE) were used as the auxiliary electrode and the reference electrode, respectively. The working electrodes were positioned 20 cm from the light source with an effective irradiation area of 0.5 cm2. An aqueous solution of Na2SO4(0.1 mmol·L-1, 100 mL) in a quartz cell was used as the supporting electrolyte.
1.2 Syntheses of Zn-Ti11 and Cd-Ti11
1.2.1 [ZnTi11O14(SPh)(OiPr)17](Zn-Ti11)
Analytically pure titanium tetraisopropoxide(Ti(OiPr)4,0.15 mL,0.39 mmol)and[Me4N]2[Zn4(SPh)10](0.006 0 g,0.003 mmol)were mixed in 0.3 mL acetonitrile and anhydrous isopropanol (2∶1,V/V). The mixture was placed in a thick Pyrex tube (0.7 cm in diameter, 15 cm in length) and quickly degassed by argon.The sealed tube was heated under autogenous pressure at 60 ℃for 7 d and then cooled to room temperature to yield colorless block-shaped crystals (53% yield based on Ti). The crystals were rinsed with isopropanol and preserved in a sealed glass tube. The crystals were rinsed with isopropanol for crystal structure and XRD analysis. Selected FTIR data (KBr, cm-1): 3 060(b),2 966(b), 2 922(w), 2 866(w), 2 613(w), 1 462(s), 1 373(vs), 1 324(w), 1 137(w), 1 015(w), 856(m), 668(m),529(s), 464(w). [ZnTi11O14(SPh)(OiPr)17]: Calcd. for C57H124ZnO31STi11(%,Mw=1 929.88): C, 35.44; H, 6.43;S,1.66.Found(%):C,34.98;H,6.22;S,1.60.
1.2.2 [CdTi11O14(SPh)(OiPr)17](Cd-Ti11)
Analytically pure Ti(OiPr)4(0.15 mL, 0.39 mmol),[Me4N]2[Cd4(SPh)10] (0.006 7 g, 0.004 mmol) were mixed in 0.3 mLN,N-dimethyl formamide (DMF) and anhydrous isopropanol (2∶1,V/V). The mixture was placed in a thick Pyrex tube (0.7 cm in diameter, 15 cm in length) and quickly degassed by argon. The sealed tube was heated under autogenous pressure at 60 ℃for 5 d and then cooled to room temperature to yield colorless block-shaped crystals (55% yield based on Ti). The crystals were rinsed with isopropanol and preserved in a sealed glass tube. Selected FTIR data(KBr, cm-1): 3 065(b), 2 963(b), 2 925(w), 2 864(w),2 615(w),1 466(s),1 375(vs),1 328(w),1 139(w),1 013(w), 856(m), 666(m), 530(s), 465(w). [CdTi11O14(SPh)(OiPr)17]:Calcd.for C57H124CdO31STi11(%,Mw=1 976.91):C, 34.59; H, 6.27; S, 1.62. Found(%): C, 33.98; H,6.02;S,1.57.
1.3 X-ray crystallographic analysis
The single crystal measurements of Zn-Ti11and Cd-Ti11were carried out on a Bruker APEX-ⅡCCD diffractometer with graphite monochromated MoKα(λ=0.071 075 nm) radiation at room temperature. The structures were solved by direct methods using SHELXS-2016 and the refinements were performed againstF2using SHELXL-2016. All the non-hydrogen atoms were refined anisotropically. The hydrogen atoms were positioned with idealized geometry and refined with fixed isotropic displacement parameters.Relevant crystal data, collection parameters, and refinement results can be found in Table S1 (Supporting information).
CCDC:2217954,Zn-Ti11;2219848,Cd-Ti11.
1.4 Electrochemical measurements
The electrochemical properties of the as-prepared materials were studied on a CHI 660E electrochemical workstation.The experiments were performed in a threeelectrode configuration comprising the 1 cm2samples,the activated carbon (AC),and the Hg/HgO in 3.0 mol·L-1KOH as a working electrode, a counter electrode,and a reference electrode, respectively. To prepare the working electrode, porous carbon, acetylene black,and polytetra fluoroethylene (mass ratio of 8∶1∶1)were ultrasonically mixed in a solution containing distilled water and ethanol and then dried. The obtained sticky mixture was roll-pressed into a thin sheet, cut into small pieces, and fi nally pressed onto the nickel (Ni)foam current collector. The loading mass of the active material was approximately 2.0 mg·cm-2. Cyclic voltammetry (CV) measurements were carried out in a three-electrode setup in 3 mol·L-1KOH. Galvanostatic charge-discharge (GCD) and cycling tests were conducted in a two-electrode configuration in 3 mol·L-1KOH using a LAND CT2001A battery measurement system. The energy density (E) and the power density(P)were calculated from the following equations[22]:
whereI,t,m, and ΔVrepresent the discharge current(mA), the discharge time (s), the total mass of active materials (g), and the potential window of the electrode(V)in that order.
The asymmetric supercapacitor was fabricated by combining M-Ti11(M=Cd,Zn)and AC as a cathode and an anode in a 3 mol·L-1KOH electrolyte. The mass loadings of the cathode and anode were calculated by using Eq.3:
WhereC+,C-andm+,m-are the capacitance and mass of the positive and negative electrodes. In this study,the mass ratio of the cathode and the anode was set at 2.9∶1.
2 Results and discussion
2.1 Structural characterization
Colorless crystals of clusters Zn-Ti11and Cd-Ti11were obtained directly by one step in situ solvothermal synthesis at 60 ℃. All samples were carefully isolated by handpicking under a microscope.The identity of the collected cluster samples with the crystals for structure analysis was confirmed by comparing the experimental XRD patterns with the calculated patterns from the crystal data of the Zn-Ti11and Cd-Ti11clusters (Fig.S1 in the ESI).
The FTIR spectra of the clusters (Fig.S2) showed asymmetric and symmetric stretching vibrations of the SPh ligand. The isopropoxy groups were identified based on theνC—Hvibrations ranging from 2 860 to 2 975 cm-1and theνO—Cvibration around 1 010 cm-1.The bands around 760 cm-1are attributed to the Ti—O vibrations. The solid-state UV-Vis diffuse-reflection spectra of Zn-Ti11and Cd-Ti11are shown in Fig.S3a.The absorption data were calculated from the reflectance, which indicated no absorption above 400 nm(3.1 eV), consistent with their colorless nature (Fig.S3b).
As revealed by single-crystal X-ray diffraction analysis, compounds Zn-Ti11and Cd-Ti11are isostructural in the formula [MTi11O14(SPh)(OiPr)17] (M=Zn,Cd).Two clusters crystallize in the space groupCcand two clusters are included in a unit cell (Table S1). Fig.1a,1b,and S4 show the structures of Zn-Ti11and Cd-Ti11in ball-stick presentation. The structure of Zn-Ti11is discussed here in detail. Fig.1c and 1d give polyhedron views of Zn-Ti11. The Ti11cluster core is constructed by two Ti3O13subunits and one Ti5O13subunit. Six Ti (Ⅳ)atoms in two Ti3O13subunits adopt distorted octahedral geometry (green in Fig.1d) while the other five Ti (Ⅳ)adopt a trigonal bipyramid geometry (yellow in Fig.1d).The Zn atom takes a five-coordinated geometry with an SPh and fourμ3-oxo groups together to be the[ZnTi11O14(SPh)] subunit and 17 OiPr groups further stabilize the structure of the cluster, which is similar to the reported isostructural of [(CoⅠ)Ti11O14(OiPr)17][14]and [LnTi11O14(NO3)2(OiPr)17]·3H2O (Ln=Sm (1), Eu (2), and Gd (3))[8]clusters, in which the Co+, two [Eu (NO3)2]+, are replaced by a Zn(SPh) moiety. A unique characteristic of Zn-Ti11and Cd-Ti11, different from the CoTi11and LnTi11clusters,was coordinated with SPh and with better photoelectric performance. Fig.1e shows the cluster packing along theb-axis.
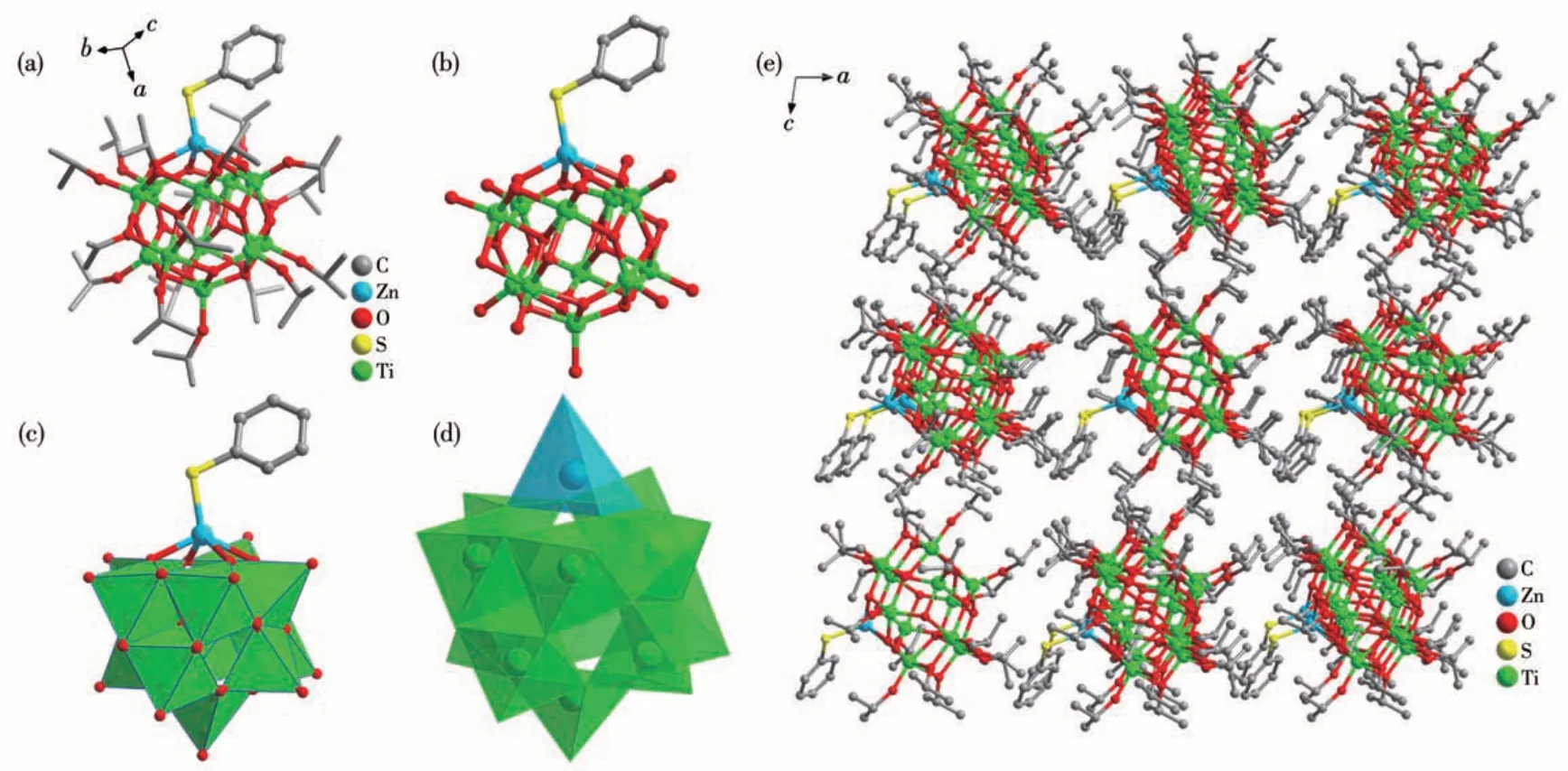
Fig.1 Ball-stick(a,b)and polyhedron(c,d)views of cluster Zn-Ti11 in one direction and the cluster packing viewed from the b direction(e)
2.2 Electrochemical performance
The electrochemical performance of Zn-Ti11and Cd-Ti11as supercapacitor working electrodes was tested in a three-electrode system with 3 mol·L-1KOH as the electrolyte. All samples in this paper were attached and measured on Ni foam substrates. According to the research of Xu et al.[23-24], only a very slight current was recorded on the bare Ni foam electrode, so it is reasonable to ignore the capacitive contribution of Ni foam.
The CV curves of Zn-Ti11and Cd-Ti11are shown in Fig.2. The presence of multiple pairs of redox peaks at Zn-Ti11and Cd-Ti11electrodes indicates the nature of typical pseudocapacitive behavior[25], which can be attributed to the redox reactions of cations in the Ti11electrode,as shown in Eq.4 and 5[26].Moreover,the oxidation peak(Ox2,ca.0.54 V)and reduction peak(Re1,ca. 0.34 V) for Cd-Ti11,which is closely consistent with the values for Zn-Ti11can be attributed to the existing of thiophenol[27]. However, Cd-Ti11exhibited two redox peaks (Ox1/Re1,ca. 0.409 V and Ox2/Re2,ca. 0.479 V) slightly shift compared to that of Zn-Ti11(ca. 0.412 and 0.484 V) due to the coordination of different metal atoms (i. e.Zn and Cd) and thiophenol within the lattice, which indicates that there may be differences between the electrochemical properties of Cd-Ti11and Zn-Ti11electrodes.
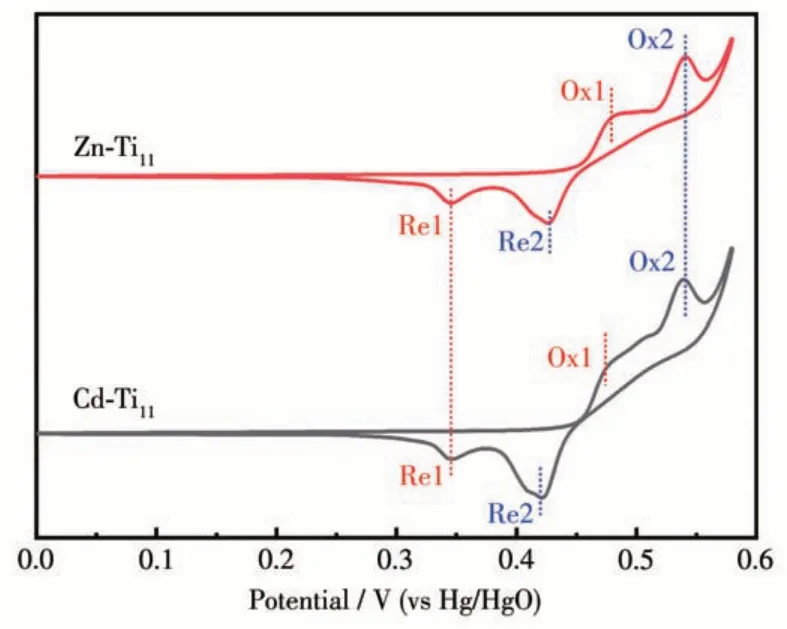
Fig.2 CV curves of Zn-Ti11 and Cd-Ti11 at the scan rate of 2 mV·s-1
The Zn-Ti11and Cd-Ti11electrodes showed the same symmetrical redox peaks at different scan rates(ranging from 1 to 30 mV·s-1),indicating that these Ti11clusters had excellent rate capability and relatively low resistance (Fig. 3a and 3c). The linear relationship between the square root of the scan rate (v1/2) and the peak current density (ip) provides further evidence that a Faraday redox reaction occurs on the electrode surface(Fig.3c and 3d).Moreover,CV curves can also provide greater insight into the charge storage kinetics of Ti11electrodes. Generally, the following equation proposed by Lindström et al.[28-29]can be used to depict the relationship between the scan rates (v) and peak current density(i):
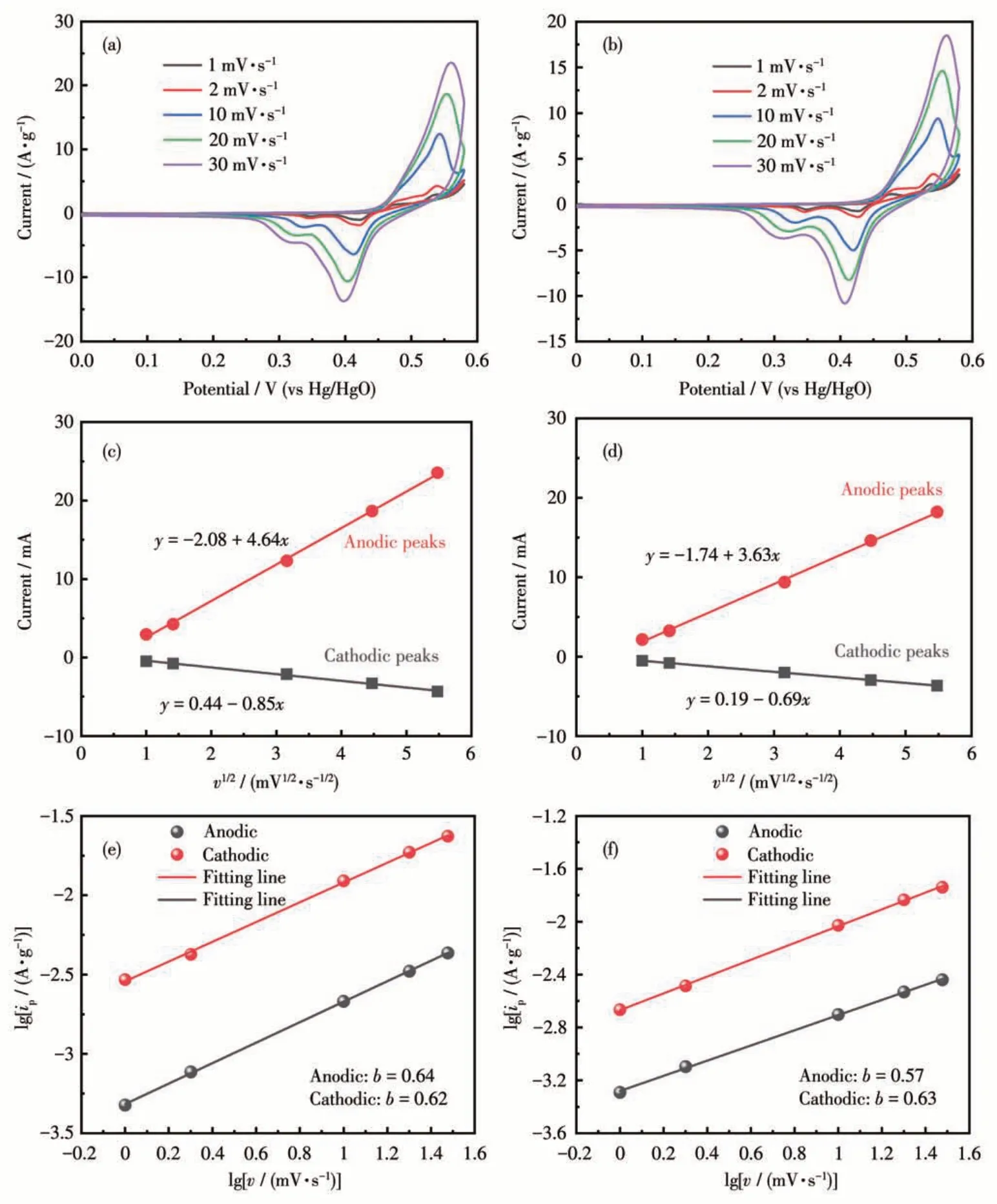
Fig.3 CV curves of(a)Cd-Ti11 and(b)Zn-Ti11 at various scan rates;Corresponding relationships between v1/2 and ip of(c)Cd-Ti11 and(d)Zn-Ti11 in voltage range of 0-0.6 V(vs Hg/HgO);Determination of the b-value of(e)Cd-Ti11 and(f)Zn-Ti11 using the relationship between lg ip and v
whereaandbare adjustable parameters. Specifically,kinetic limitations can be estimated from the limiting cases of theb-value;b=0.5, which suggests a diffusioncontrolled (or battery-like) behavior, caused by slowly semi-infinite linear ion diffusion;b=1.0, which indicates a capacitive behavior related to a fast-Faraday redox reaction on the surface. Calculated from CV curves with scan rates ranging from 1 to 30 mV·s-1, all of theb-values of the anodic and cathodic peaks for both Zn-Ti11and Cd-Ti11electrodes were between 0.5 and 1, which suggests that this electrochemical reaction is both surface-controlled and diffusion-controlled(Fig.3e and 3f).
The electrochemical properties of Cd-Ti11and Zn-Ti11electrodes were further investigated. Fig.4a and 4b shows GCD curves of Cd-Ti11electrodes at the range of 0.1 to 1 A·g-1. Although these electrodes had long discharge times at low current densities (452 F·g-1at 0.1 A·g-1for Cd-Ti11and 709 F·g-1at 0.1 A·g-1for Zn-Ti11)(Fig.4c),their Coulombic efficiencies appeared unsatisfactory (only 69% for Cd-Ti11and 59% for Zn-Ti11).With the increasing current density,the Coulombic efficiency increased significantly.When the current density was up to 1 A·g-1, the Coulombic efficiencies of Cd-Ti11and Zn-Ti11electrodes were 96% and 93%,respectively (Fig.4d). Although Cd-Ti11electrode had a larger voltage window (0-0.92 V) compared that of Zn-Ti11electrode (0-0.60 V) (Fig.S5), the discharge specific capacitance of Zn-Ti11electrode wasca. 175 F·g-1at 1 A·g-1, significantly better than that of the Cd-Ti11electrode (ca. 76 F·g-1at 1 A·g-1) and many related MOCbased electrodes (Table 1). Moreover, the specific capacitance of both Cd-Ti11and Zn-Ti11electrodes decreased almost linearly with increasing current density,which may be attributed to the electrode polarization.

Table 1 Summary of MOC-based composite electrodes of three-electrode supercapacitors
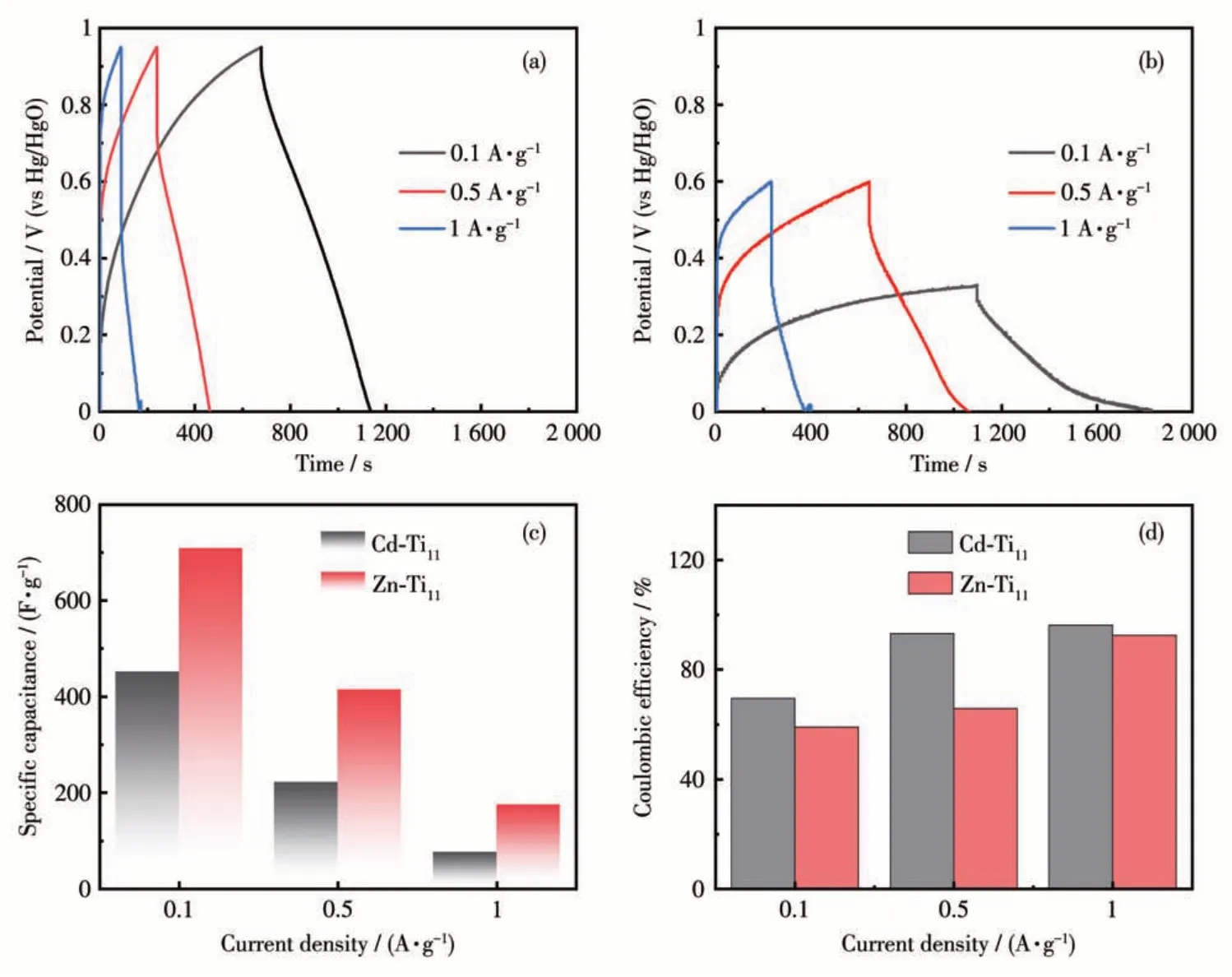
Fig.4 GCD curves of(a)Cd-Ti11 and(b)Zn-Ti11 scanned at various current densities;(c)Specific capacitances and(d)Coulombic efficiencies of Cd-Ti11 and Zn-Ti11 after IR-drop correction
As shown in Fig.S6, the Zn-Ti11electrode exhibited a higher initial capacitance (114 F·g-1) at 1 A·g-1,compared to Cd-Ti11(74 F·g-1). Moreover, the Cd-Ti11-based cell failed inca.200 cycles,accompanied by violent fluctuations in specific capacity. However, the Zn-Ti11cell can be stably cycled for more than 500 cycles,with a capacitance retention rate of 84.7% and a decay rate of only 0.003% per cycle. The above results showed that the Zn-Ti11-based supercapacitor had a stronger structure and could operate stably for a long time at a higher current density.Furthermore,after calculation,Cd-Ti11and Zn-Ti11electrodes can provide the maximum power density and energy density of 9.5 W·kg-1and 463 Wh·kg-1, 8.7 W·kg-1and 324 Wh·kg-1,respectively, which are significantly better than many related reports in the literature[29-31], implying the great potential of Ti11clusters in the energy storage area.
3 Conclusions
In this study, two novel Ti11clusters (Zn-Ti11and Cd-Ti11)with fascinating structures were synthesized by introducing thiophenol and a second metal.Their applications in supercapacitors were investigated for the first time.The experimental results showed that Zn-Ti11-based supercapacitors showed higher specific capacitance than that of Cd-Ti11, and were better than most reported MOC-based electrode materials. Therefore,this work provides an effective strategy for designing and synthesizing novel TOC-based electrode materials with excellent specific capacitance and may open a new avenue for high-performance supercapacitors.
Acknowledgments:We are grateful for the financial support from the National Natural Science Foundation of China (Grant No.62204272), the Natural Science Foundation of Shandong Province(Grant No.ZR2021QB077),youth fund of Zhoukou Normal University (Grant No. ZKNUC2020044), Baiqing project(Grant No.CD70621019), and Jiaxing University SRT project(Grant No.8517221253).
Supporting information is available at http://www.wjhxxb.cn

