Antioxidant effects of the aqueous extract of turmeric against hydrogen peroxide-induced oxidative stress in spotted seabass (Lateolabrax maculatus)
Zhe Wng,Xiuqin Wng,Xueshn Li,Kngle Lu,Ling Wng,Xuekun M,Ki Song,**,Chunxio Zhng,*
a Xiamen Key Laboratory for Feed Quality Testing and Safety Evaluation,Fisheries College of Jimei University,Xiamen,361021,China
b Guangdong Yuehai Feed Group Co., Ltd., Guangdong,Zhanjiang,524000,China
Keywords: Turmeric Medicinal plant Hydrogen peroxide Oxidative stress Lateolabrax maculatus
ABSTRACT Intensive aquaculture-induced oxidative stress is detrimental to fish health and yield.Medicinal plants show promise as natural health boosters and antioxidants in the aquaculture industry.Therefore,this work investigated the effects of turmeric aqueous extract (TAE) on the growth performance,antioxidant status,and hydrogen peroxide (H2O2)-induced oxidative stress in spotted seabass (Lateolabrax maculatus).Fish were fed diets supplemented with 0 (Con),2 (TAE2),or 4 (TAE4) g/kg TAE for eight weeks,then were injected with H2O2.The results showed that dietary supplementation of TAE did not affect fish growth,feed utilization,or body composition.TAE treatment increased liver antioxidant enzyme activities and decreased liver malondialdehyde content and serum levels of glutamate oxalate transaminase,glutamate pyruvate transaminase,and lactate dehydrogenase.Furthermore,the increases in mortality,liver malondialdehyde content,and serum biomarkers of liver injury in the H2O2-treated fish were inhibited as a consequence of the TAE treatment.In addition,TAE treatment activated the Nrf2/Keap1 pathway in the liver,supported by the up-regulated expression of nrf2,ho-1,and gclc,and down-regulated keap1 expression.Overall,dietary incorporation of TAE protected against H2O2-induced oxidative stress in spotted seabass probably by enhancing antioxidant capacity through the Nrf2/Keap1 pathway.
1.Introduction
Aquaculture has become one of the fastest-growing sectors satisfying the increasing demand for marine products (Naylor et al.,2021).However,the rapidly expanding aquaculture industry promotes the production of free radicals and reactive oxygen species (ROS),which disturbs the metabolic and antioxidant balance in organisms,eventually leading to disease and massive mortalities (Ciji &Akhtar,2021).In recent decades,widely used synthetic antioxidants have raised increasing safety concerns in humans and cultured animals (Sandoval-Vargas et al.,2020).Therefore,the introduction of side effect-free antioxidants is essential for feasible and sustainable aquaculture.
Medicinal plants,especially traditional Chinese herbs (TCHs),have been widely used to treat human diseases due to their various pharmacological activities (Chen et al.,2018;Chu et al.,2018).Currently,growing researches interest in using plant ingredients and phytochemicals as functional additives in the aquaculture industry,as reviewed by Tadese et al.(2021).In contrast to chemical additives,plant ingredients have the unique advantages of being more widely distributed,lower cost,having fewer side effects,and producing less environmental pollution (Tadese et al.,2021).Increasing research has revealed that dietary administration of plant-derived antioxidants could aid in the stress tolerance of farmed animals (Dawood et al.,2020;Dawood et al.,2022;Pan et al.,2022).Turmeric (Curcuma longaL.),a medicinal herb belonging to the Zingiberaceae family,has shown growth-promoting,antioxidant,anti-inflammatory,and anti-viral properties in human and animal models (Bhowmilk et al.,2009;Mishra et al.,2011).Kim et al.(2019) reported that water was an efficient solvent to extract antioxidant components (e.g.,total phenolic compounds and flavonoids) from turmeric.This same study highlighted the radical scavenging activities of the water extract of turmeric.The application of turmeric and its active ingredient is becoming increasingly appealing in the aquaculture industry (Fagnon et al.,2020;Kim et al.,2021).However,the stress-relieving effects of turmeric have only started being investigated in aquatic animals.
Hydrogen peroxide (H2O2) has been widely used as an efficient oxidizing agent to induce oxidative stress in vivo and in vitro because of its easy metabolization into highly reactive hydroxyl radicals that attack organic molecules (Cheng,Ma,et al.,2021).Our recent study found that turmeric aqueous extract (TAE) alleviated the H2O2-induced toxicity in primary culture hepatocytes from spotted seabass (Lateolabrax maculatus) (Wang et al.,2021).The present study aimed to determine whether TAE could improve the antioxidant status and tolerance to H2O2-induced oxidative stress in spotted seabass,as well as unveil the molecular mechanisms.The results of this study contribute to further research and application of herbal additives in aquaculture.
2.Materials and methods
2.1.Preparation of herbal extract and experimental diets
Turmeric was purchased from a local pharmacy in Xiamen,China.After being oven-dried and grounded,the turmeric powder was immersed in distilled water for 1 h before being boiled for 30 min.The turmeric extract was collected using a 0.22 μm filter.The residues were re-boiled and filtered again.Finally,the aqueous extract was blended and evaporated to achieve a concentration of 1 g/mL.The solution was stored at 4◦C before being mixed into the fish feed.
Three iso-nitrogenous (45% crude protein) and iso-lipidic (12%crude lipids) diets were formulated (Table 1),including a basal diet(Con),2 and 4 g/kg TAE diet (TAE2 and TAE4).The diets were produced following our established procedure (Zhang et al.,2018).All feed ingredients were grounded and passed through a 250 μm sieve.The oil,herbal solution,and distilled water were separately added to the blended feeds and mixed thoroughly.The pellets with two sizes (diameter: 1.5 mm and 2.5 mm) were produced by a two-screw extrusion machine (F-76,Guangzhou Huagong Optical Mechanical and Electrical Technology Co.,Ltd.,China) and dried at 55◦C overnight.The dried feeds were packed and stored at -20◦C until use.
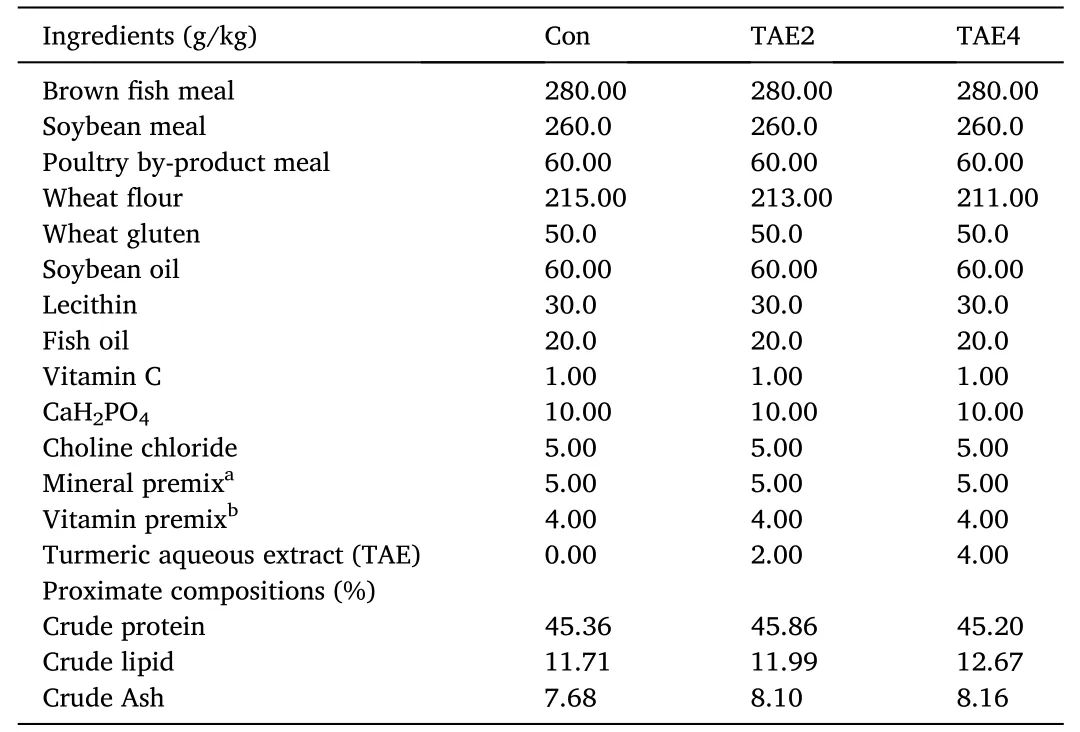
Table 1 Formulation and proximate composition of the experimental diets.
2.2.Fish and feeding trial
Spotted seabass juveniles were obtained from a marine hatchery(Zhaoan,China) and transported to the fisheries laboratory of Jimei University.After being acclimated to the laboratory conditions for two weeks,a total of 180 fish (mean initial weight: 15.31 ± 0.17 g) wereequally randomized to nine tanks (400 L).Fish were randomly allocated to triplicate per treatment and hand-fed to apparent satiation twice daily(8:00 and 17:00) for 56 days.Approximately 40% of water in each tank was renewed after each feeding.During the feeding period,the water temperature was 29—31◦C,pH was 5.9—6.8,and dissolved oxygen >6 mg/L.
2.3.Sample collection
At the end of the feeding trial,fish were anesthetized with eugenol(1:10000) after fasting for 24 h.The fish number and body weights were recorded individually.Three fish per tank were randomly selected for body composition analyses.Six fish per tank were sampled to determine somatic indices based on the weights of whole bodies,livers,viscera,and intraperitoneal fat.Blood samples were collected from the caudal vein using sterile syringes and stored overnight at 4◦C.Then,serum samples were collected by centrifugation (2500×g,10 min) and stored at-80◦C for biochemical analyses.Liver tissues were collected and stored in liquid nitrogen for RNA extraction.All analyses were performed in triplicates.
2.4.H2O2 induced oxidative stress
A pilot experiment was conducted to determine the appropriate dose of H2O2for inducing oxidative stress in spotted seabass.Four groups of fish were injected intraperitoneally with H2O2of 0,300,600,and 1200 mM,respectively.After the injection,blood samples were sampled for biochemical analysis at 24 and 48 h.The results showed that H2O2induced a dose-and time-depended hepatotoxicity,with significant increases in serum glutamate oxalate transaminase (GOT) and glutamate pyruvate transaminase (GPT) (Fig.1).Fish treated with 1200 mM H2O2showed a higher mortality;hence,the H2O2dose used for the formal stress induction was set as 600 mM for 48 h.
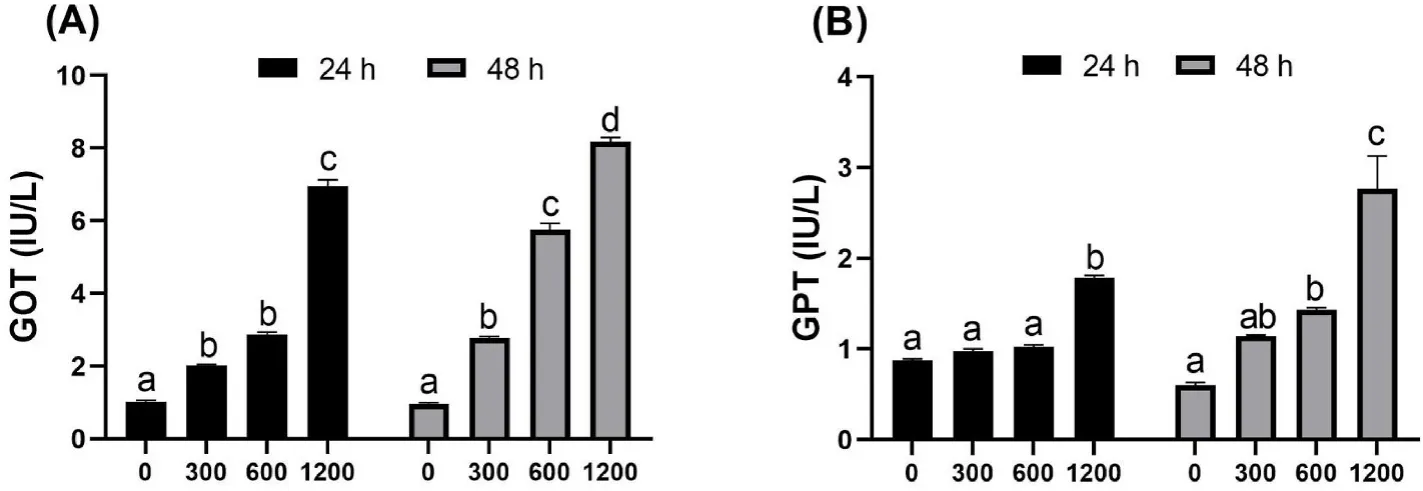
Fig.1.Serum GOT (A) and GPT (B) levels of spotted seabass after injection with H2O2 at concentrations of 0,300,600,1200 mM.Different letters placed above columns indicated significant differences (P <0.05).
Forty-eight hours after the 56-day feeding trial,eight fish were randomly selected from each tank and injected intraperitoneally with 600 mM H2O2.Fish fed the basal diet and treated with the same aliquot of sterile water were allocated as the positive control (PC).Forty-eight hours after the injection,blood and liver samples were sampled for further analysis.
2.5.Proximate analysis
Proximate compositions of fish bodies and experimental diets were determined following the guidelines of AOAC (2002).The moisture content was determined by drying samples at 105◦C until reaching a constant weight.Crude protein was analyzed according to the Dumas method.Crude lipid and crude ash were determined according to the methods of ether extraction and combustion (550◦C for 6 h),respectively.
2.6.Biochemical analysis
Liver samples were homogenized in phosphate-buffered saline (PBS)and centrifuged at 4000×gfor 10 min.Then,the homogenized solution was collected and stored at -80◦C for biochemical analysis.Total antioxidant capacity (T-AOC),superoxide dismutase (SOD),catalase(CAT),glutathione peroxidase (GPx),malondialdehyde (MDA),lactate dehydrogenase (LDH),GOT and GPT were separately determined by using standard assay kits (Nanjing Jiancheng Bioengineering Institute,Nanjing,China).
2.7.Real-time quantitative PCR (RT-qPCR)
Total RNA extraction,cDNA synthesis,and RT-qPCR were performed following our recent publication (Cheng,Li,et al.,2021).Briefly,the total RNA of live samples was extracted using RNAiso kits (VazymeBiotech Co.,Ltd.China).The concentration and purity of RNA was determined with a NanoDrop 2000 spectrophotometer (Thermo Fisher,USA).Agarose gel electrophoresis was used for checking the integrity of RNA.And then,the total RNA was reversely transcribed to cDNA.RT-qRCR was performed on ABI stepOne plus (Thermal Cycler,USA).The reaction system (20 μL) included the following: 2 μL of cDNA,0.4 μL of each primer,10 μL of PerfectStartTMGreen qPCR SuperMix (TransGen Biotech Co.,Ltd.China),and 7.2 μL of ddH2O.The reaction conditions were as follows: 95◦C for 1 min,followed by 45 cycles of 95◦C for 5 s and 60◦C for 15 s.The primers for amplification were designed based on the nucleotide sequences of spotted seabass (Table 2).The relative gene expression was calculated following the 2-△△Ctmethod (Livak &Schmittgen,2001).
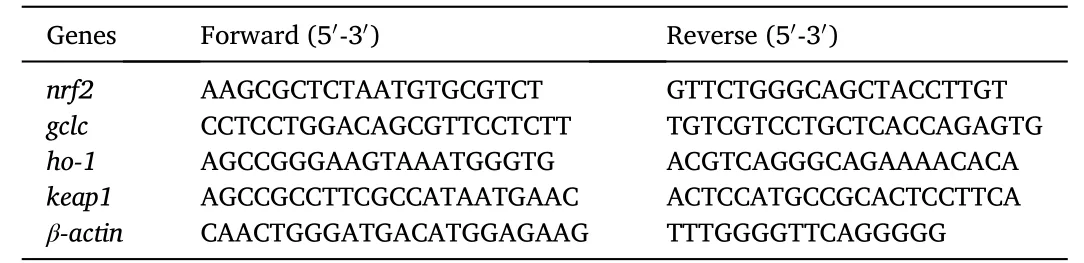
Table 2 Sequences of the PCR primers used in this study.
2.8.Calculation and statistical analysis
WhereW1andW0were the mean final and initial body weights,respectively.The Shapiro-Wilk test was used for assessing normal distribution of data.Then,data were analyzed by one-way ANOVA followed by Tukey’s test (SPSS 20.0).Statistics withP<0.05 were regarded to be significant,and data were presented as mean ±S.E.M.of triplicate groups.
3.Results
3.1.Growth performance
After the 56-day feeding trial,the WG was not significantly affected by dietary TAE supplementation (Table 3),while it showed an increasing trend with increasing dietary TAE levels (P>0.05).Furthermore,survival,FR,FE,CF,and somatic indices (HSI,VSI,and IFR) showed no significant difference among all groups (P>0.05).No difference in the contents of whole-body moisture,crude protein,crude lipid,and ash was detected among all groups (P>0.05,Table 4).These results indicated that dietary supplementation of TAE did not influence the growth performance,feed utilization,or body composition of spotted seabass.

Table 3 Growth performance,feed utilization and somatic indices of spotted seabass.
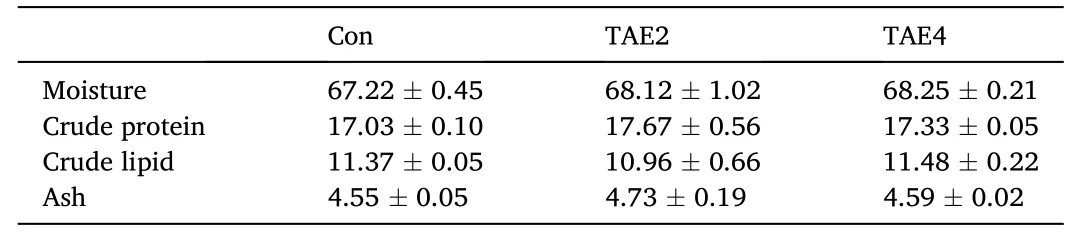
Table 4 Body composition analysis (%,wet weight) of spotted seabass.
3.2.Hepatic antioxidant enzymes and serum biomarkers
To explore the effects of TAE on the antioxidant status of spotted seabass,we detected the hepatic antioxidant enzymes and serum biomarkers for liver injury (Fig.2).Compared with the control,dietary TAE significantly increased the T-AOC level and activities of SOD,CAT,and GPx in the liver in a dose-dependent manner,while a reverse trend was observed in the activities of GPT,GOT,and LDH in the serum (P<0.05).These results indicated that dietary supplementation of TAE enhanced hepatic antioxidant capacity in spotted seabass.
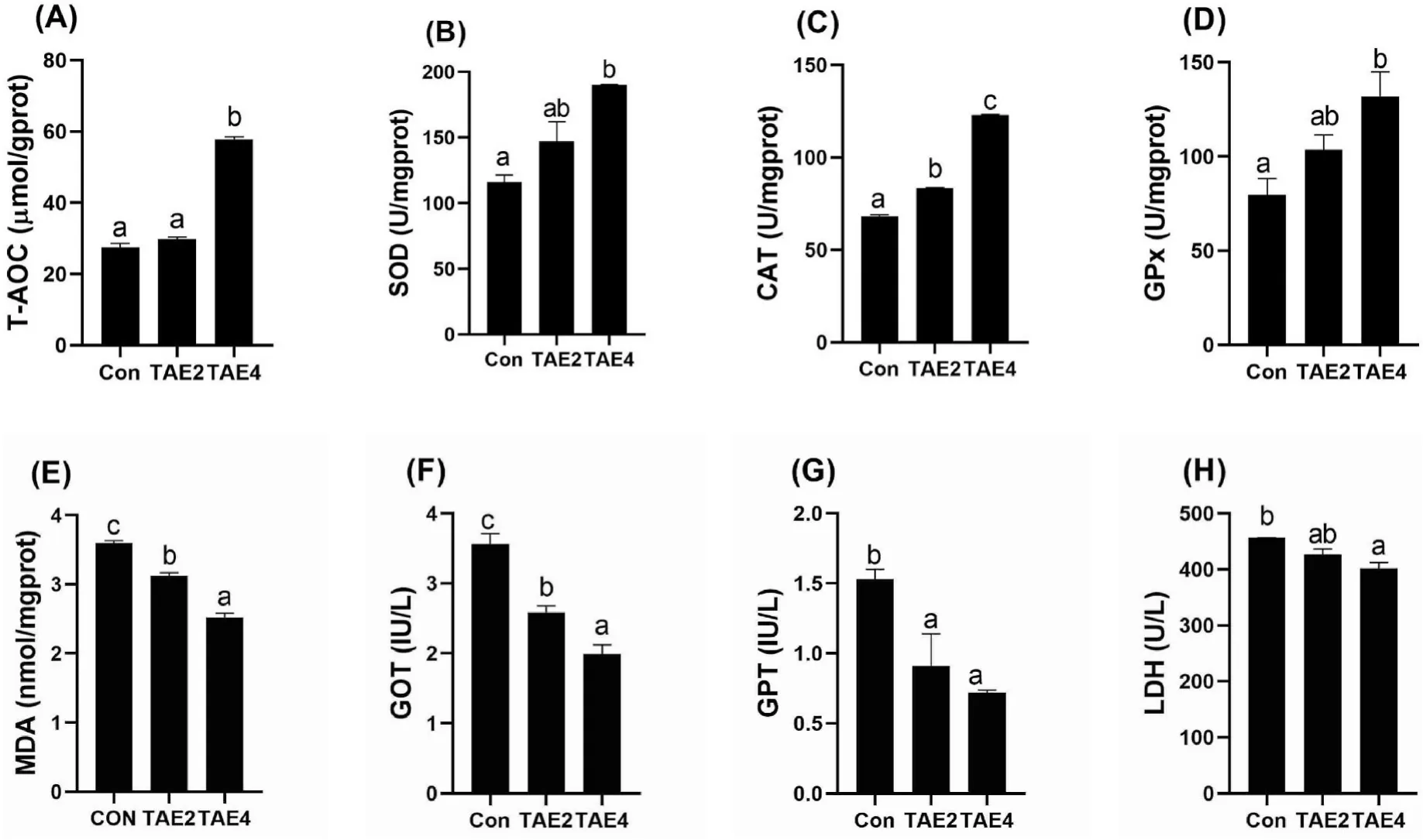
Fig.2.Liver antioxidant enzyme activities (A—E) and serum biomarkers (F—H) of spotted seabass.Different letters placed above columns indicated significant differences (P <0.05).Fish were fed diets supplemented with 0 (Con),2 (TAE2),or 4 (TAE4) g/kg TAE for eight weeks.
3.3.Nrf2 signaling in the liver
To probe the mechanism of the antioxidant activity of TAE,we proceeded to study the Nrf2 signaling (Fig.3).The results showed that both TAE2 and TAE4 significantly increased the expression ofnrf2,ho-1,andgclcin the liver (P<0.05),while thekeap1expression showed no difference among all groups (P>0.05).These results indicated that theactivation of the Nrf/Keap1 pathway was one possible mechanism through which TAE improved the antioxidant capacity of spotted seabass.
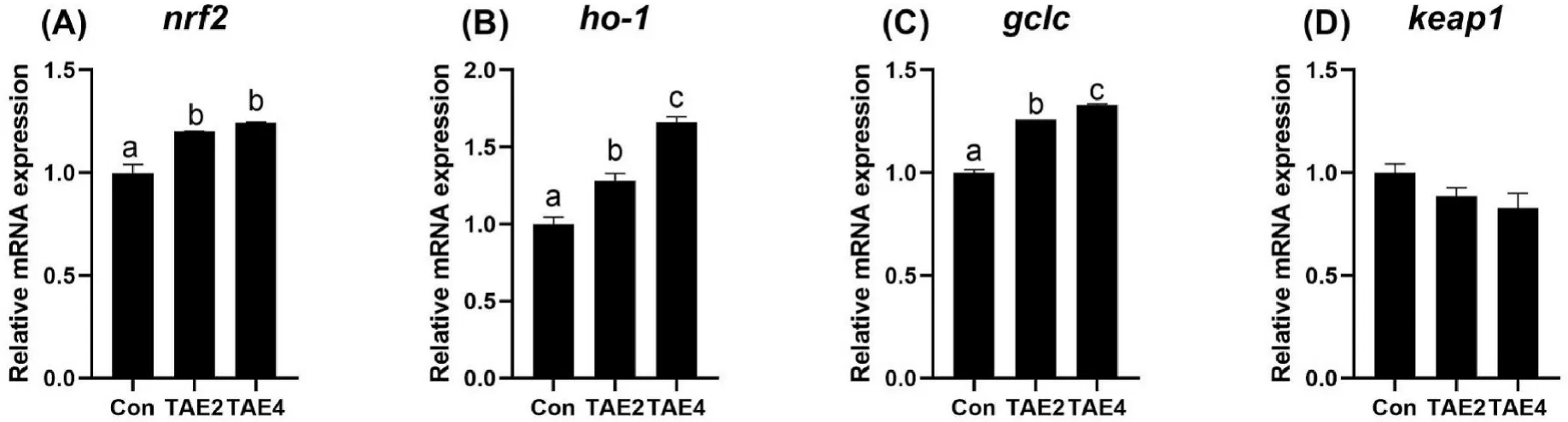
Fig.3.Relative mRNA expression of nrf2,ho-1,gclc,and keap1 in the liver of spotted seabass.Different letters placed above columns indicated significant differences(P <0.05).Fish were fed diets supplemented with 0 (Con),2 (TAE2),or 4 (TAE4) g/kg TAE for eight weeks.
3.4.Tolerance to H2O2 stress
3.4.1.Survival and serum biomarkers
After the 56-day feeding trial,fish were injected with H2O2for further analyses (Fig.4).Fish fed the basal diet displayed significantly decreased survival in the present of H2O2(P<0.05).TAE treatment at 2 and 4 g/kg significantly increased fish survival compared with the control (P<0.05).Furthermore,we examined the liver damage-related biomarkers of GOT,GPT,and LDH.The results showed that H2O2significantly increased the activities of GOT,GPT and LDH in the serum(P<0.05),all of which was inhibited and even restored to the level of the PC group by TAE treatment.These results indicated that the TAE exerted a protective effect against H2O2-induced oxidative stress in spotted seabass.
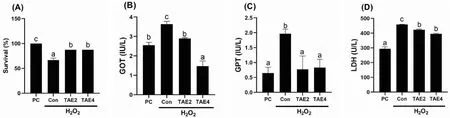
Fig.4.Survival (A) and serum biomarkers including GOT,GPT and LDH (B—D) of spotted seabass after H2O2 injection.Different letters placed above columns indicated significant differences (P <0.05).Fish were fed diets supplemented with 0 (Con),2 (TAE2),or 4 (TAE4) g/kg TAE for eight weeks;PC,positive group.
3.4.2.Hepatic antioxidant response
To identify the relationship between liver damage and antioxidant effects,we detected the antioxidant enzyme activities (Fig.5).The results showed that H2O2suppressed T-AOC levels and activities of SOD and CAT in the liver (P<0.05),which were abolished by dietary TAE (P<0.05).Furthermore,TAE treatment significantly enhanced liver GPx activity compared with the control (P<0.05).Moreover,TAE treatment significantly reduced the H2O2-induced elevation in liver MDA content(P<0.05).
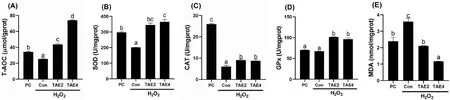
Fig.5.Liver T-AOC level (A),antioxidant enzymes activities (B—D),and MDA content (E) of spotted seabass after H2O2 injection.Different letters placed above columns indicated significant differences (P <0.05).Fish were fed diets supplemented with 0 (Con),2 (TAE2),or 4 (TAE4) g/kg TAE for eight weeks;PC,positive group.
We proceed to determine the expression of genes involved in the Nrf/Keap1 pathway (Fig.6).H2O2stress induced significantly higher expression ofnrf2,ho-1,andgclcand lowerkeap1expression in the liver(P<0.05).Furthermore,the TAE4 treatment significantly increased the expression ofnrf2,ho-1,andgclcamong the groups (P<0.05).TAE significantly decreased liverkeap1expression in a dose-dependent manner compared with the control (P<0.05).These results indicated that dietary TAE supplementation protected against H2O2-induced oxidative stress in spotted seabass,which was partly due to the activation of antioxidant defense through the Nrf2/Keap1 pathway.
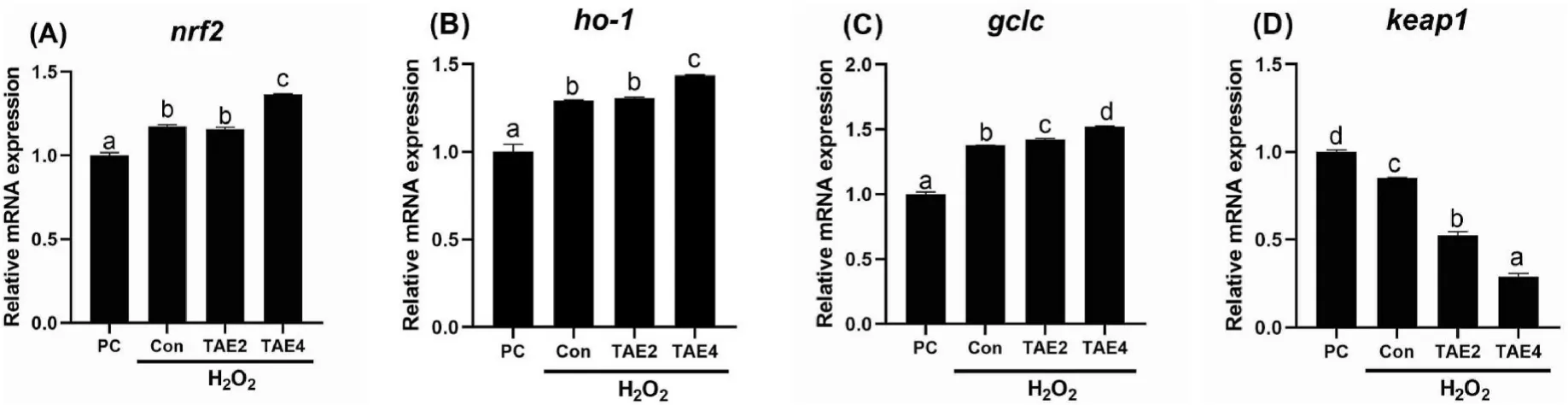
Fig.6.Relative mRNA expression of nrf2,ho-1,gclc,and keap1 genes in the liver of spotted seabass after H2O2 injection.Different letters placed above columns indicated significant differences (P <0.05).Fish were fed diets supplemented with 0 (Con),2 (TAE2),or 4 (TAE4) g/kg TAE for eight weeks;PC,positive group.
4.Discussion
Medicinal herbs are conducive to animal health for their abundant content of amino acids,proteins,lipids,vitamins,and active components modulating biological functions (Tadese et al.,2021).Turmeric,a common Chinese herbal medicine,contains compositions with antioxidant,anti-inflammatory,and anti-viral pharmacological properties(Mahmoud et al.,2014;Rajabiesterabadi et al.,2020).The growth-promoting effect of turmeric on aquatic animals remains controversial.Yusuf et al.(2017) noted that dietary incorporation of 0.2% turmeric increased the WG of juvenile tilapia (Oreochromisniloticus),but higher turmeric levels (0.4 and 0.8%) resulted in reduced growth.In addition,a significant dose-dependent (1—5 g/kg) enhancement of the specific growth rate was found in common carp (Cyprinus carpioL.) that were fed turmeric-supplemented diets for 10 weeks(Abdel-Tawwab &Abbass,2017).In the present study,the WG of spotted seabass showed an increasing trend with increased dietary TAE levels (2—4 g/kg),indicating that a wider dose range should be investigated in future studies.In addition,dietary TAE had little effect on feed utilization and body composition of fish,suggesting that this treatment is safe.
As a major ROS,H2O2was proven to cause cellular oxidative stress and induce MDA generation,an endogenous genotoxic product of lipid peroxidation (Jia,Gu,et al.,2019).In addition,cellular oxidative damage is often accompanied by the secretion of cytosolic enzymes,such as GPT,GOT,and LDH (Peng et al.,2022).Studies have revealed the hepatoprotective effect of plant ingredients as functional additives for farmed fish (de Freitas Souza et al.,2019;Jia,Gu,et al.,2019;Zhou et al.,2015).In the present study,the significant increases in mortality,liver MDA content,and serum GPT,GOT,and LDH levels in the H2O2-treated fish were inhibited as a consequence of TAE treatment,indicating that the TAE exerted a protective effect against H2O2-induced oxidative stress in spotted seabass.The antioxidant effects of TAE might be due to various bioactive components.For instances,flavonoids,the main components occurring ubiquitously in turmeric,are believed to be powerful free radical scavengers (Chu et al.,2000;Priyadarsini et al.,2003).A recent study analyzed the flavonoids in the water extract of turmeric,including diosmetin,quercitrin,and rutin,all of which showed antioxidant potentials in vivo and in vitro studies (Kim et al.,2021).
Among the fish antioxidant system,antioxidant enzymes,such as SOD,CAT,and GPx act as a first defense counterbalancing ROS stress by promoting its conversion into inert species.Specifically,SOD catalyzes the dismutation of O2-into O2and H2O2,CAT stimulates the conversion of H2O2into O2and H2O,and GPx,mainly from mitochondria,decomposes peroxides into H2O or alcohol (Sandoval-Vargas et al.,2020).In addition,the enhanced activity of antioxidant enzymes contributes to organism’s defense against various external stressors,such as water quality deterioration,overcrowding,and high ambient temperature (Ciji&Akhtar,2021).The present study demonstrated that dietary TAE supplementation enhanced the activities of SOD,CAT,and GPx in the presence and absence of H2O2,indicating an improvement in antioxidant capacity of spotted seabass.Similar results have been observed in studies on zebrafish (Danio rerio) (Kim et al.,2021),common carp(Cyprinus carpio) (Rajabiesterabadi et al.,2020),and perch (Anabas testudineus) (Manju et al.,2012).These results indicated that dietary TAE improved antioxidant status to help preventing H2O2-induced oxidative stress in spotted seabass.
The transcription factor Nrf2 is a common regulator of antioxidant systems in organisms (Ma,2013).Keap1,a cytoplasmic actin-binding protein,inhibits Nrf2 activity by sequestering it in the cytoplasm.The Nrf2/Keap1 pathway ameliorates oxidative stress by mediating phase II detoxifying/antioxidant enzymes,such as HO-1 and GCLC (Jang et al.,2009).Generally,Nrf2 enters the nucleus and promotes the biosynthesis of antioxidant enzymes by binding to antioxidant response elements(AREs) during mild to moderate oxidative stress (Jia,Du,et al.,2019).In the present study,the transcription level ofnrf2,as well as the downstreamho1andgclclevels in the liver,were upregulated by H2O2injection,while the activity of antioxidant enzymes SOD and CAT was decreased in the circumstances.We hypothesized that the activity of Nrf2,followed by the antioxidant enzymes in the liver,was suppressed at the early stage of H2O2-induced stress,and Nrf2 expression was restored as a compensatory mechanism during oxidative stress in its latter stage.In addition,TAE4 treatment significantly increased the expression ofnrf2,ho1,andgclc,and reducedkeap1gene expression in H2O2-treated fish.Liver SOD,CAT,and GPx activities displayed positive correlations withnrf2expression,thus,the antioxidant enzyme activity most likely increased as a result of Nrf2 activation by TAE.Similar observations have also been reported in punti (Puntius sophore) (Mahanty et al.,2017),golden pompano (Trachinotus ovatus) (Liu et al.,2021),and Grass Carp (Ctenopharyngodon idella) (Wu et al.,2022).These results indicated that TAE exerted antioxidant effects in spotted seabass,which was mainly due to the modulation of Nrf2/Keap1 pathway.
In conclusion,dietary supplementation of 2—4 g/kg TAE protected against H2O2-induced oxidative stress in spotted seabass,characterized by the increased fish survival and antioxidant enzyme activity,as well as the suppressed secretion of cytosolic enzymes in the liver.The antioxidant effect of TAE was partly associated with the activation of the Nrf2/Keap1 pathway (Fig.7).Further studies are needed to identify the specific active component responsible for the beneficial activity of TAE.

Fig.7. Dietary TAE mitigates H2O2-induced oxidative stress in spotted seabass through the Nrf2/Keap1 signaling pathway.
Ethics statement
All animal protocols were approved by the Animal Ethical Committee of Jimei University (Xiamen,China).
CRediT authorship contribution statement
Zhe Wang:Data curation,Writing — review &editing.Xiuqin Wang:Formal analysis,and,Validation.Xueshan Li:Visualization,and,Validation.Kangle Lu:Resources,and,Investigation.Ling Wang:Investigation,and,Validation.Xuekun Ma:Resources,and,Investigation.Kai Song:Resources,Methodology,and,Supervision.Chunxiao Zhang:Conceptualization,Funding acquisition,and,Supervision.
Declaration of competing interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this study.
Acknowledgements
This work was supported by China Agriculture Research System(CARS-47) and the Natural Science Foundation of Fujian Province of China (grant number: 2019J01060380).
 Aquaculture and Fisheries2024年1期
Aquaculture and Fisheries2024年1期
- Aquaculture and Fisheries的其它文章
- Selected dietary plant-based proteins for growth and health response of Nile tilapia Oreochromis niloticus
- The roles of polysaccharides in tilapia farming: A review
- The effects of mixed prebiotics in aquaculture: A review
- Growth performance,fatty acid profile,gut,and muscle histo-morphology of Malaysian mahseer,Tor tambroides post larvae fed short-term host associated probiotics
- Evaluation of growth performance,feed efficiency and nutrient digestibility of red hybrid tilapia fed dietary inclusion of black soldier fly larvae(Hermetia illucens)
- Effects of dietary astaxanthin enrichment on enhancing the colour and growth of red tilapia,Oreochromis sp.
