Growth performance,fatty acid profile,gut,and muscle histo-morphology of Malaysian mahseer,Tor tambroides post larvae fed short-term host associated probiotics
Mohammo Kamruzzaman Hossain ,Sairatul Dahlianis Ishak ,Shumpei Iehata ,Mat Noorin Nooriyana ,M Aul Kaer ,Amok Bolong Aol-Munafi ,*
a Department of Fisheries (DoF), Matshya Bhabon,Ramna,Dhaka,1000,Bangladesh
b Higher Institution Centre of Excellence (HICoE), Institute of Tropical Aquaculture and Fisheries (AKUATROP), Universiti Malaysia Terengganu,Kuala Nerus,21030,Malaysia
c Faculty of Fisheries and Food Sciences,Universiti Malaysia Terengganu,Kuala Nerus,21030,Malaysia
d Alternative Aquatech,Victoria,3029,Australia
Keywords: Enhance culture Enterococcus faecalis Aeromonas sp.Muscle hypertrophy Short-term feeding Tor tambroides
ABSTRACT Host associated probiotics (HAPs) provide health benefits to the host when administered as dietary supplement.However,a short-term probiotics application strategy has yet to be optimized.A 90-days study was conducted to evaluate the response of Malaysian mahseer,Tor tambroides post larvae fed with basal diet enriched HAPs for 30-days,and its response following another 60-days feeding with only basal diet.Three experimental diets(Enterococcus faecalis strain 2674 (T1),Aeromonas sp.strain A8-29 (T2) and E.faecalis strain FC11682 (T3)) were prepared by spray-coating each HAPs on a basal diet at 1 ×108 CFU/g feed.Differences in growth performances,whole-body proximate and fatty acid composition,muscle morphometry,and gut morphology were evaluated.Results showed that after 30 days,T3 fish produced highest growth.All HAP treatment groups showed better muscle distribution profile,improved fatty acid composition,and higher villus length,width and area,than control group.After 90 days,the growth of T3 fish was still the highest.Muscle distribution profile and villus growth were higher in HAP treatments,although only total n-6 PUFA,total MUFA,linoleic acids,and linolenic acids in HAP treated fish remained high after probiotics withdrawal.No difference in whole-body proximate composition was observed in both 30 and 90 days.Collective findings demonstrated that short-term application of HAPs at an early stage could be used to boost T.tambroides growth,with E.faecalis strain FC11682 showing the best efficacy.
1.Introduction
Aquaculture is rapidly expanding in many countries and the exponential growth trend for this industry will continue for the sake of global food security due to forecasted decrease in open water fisheries production (Cabello,2006;Froehlich et al.,2018).Large scale aquaculture face issues in health management like stress,disease,and environmental degradation which affect production and cause huge economic loses in aquaculture (Scarfe &Palić,2020;Subasinghe,2005).In this context,antibiotics and disinfectants have commonly been used to control disease outbreaks in fish farming (Feˇckaninová et al.,2017).Nonetheless,the indiscriminate use of antibiotics and anti-bacterial chemicals becomes damaging towards the environment as it develops antibiotic resistant bacteria that can be adverse to not only the fish gut microbiota but also its surrounding aquatic environment (Cabello,2006;Kovalakova et al.,2020;Sharifuzzaman &Austin,2009).Human health is also affected by the deposition of antibiotic residuals in fish and fishery products when consumed (Petersen et al.,2002;Reverter et al.,2020).Comparatively,vaccines,probiotics,and immunostimulants are regarded as better,successful aquaculture health management strategies (Aly et al.,2008a,2008b;Lazado &Caipang,2014).Probiotics are applied in aquaculture for nutritional benefit,and improvement of fish health through well documented pathogen inhibitory and nutritional enzymatic activity (Hai,2015;Lazado &Caipang,2014;Liu et al.,2020).Thus,probiotics have become popular alternative approaches to antibiotics in fish health management strategies.
Probiotics as functional feed additives have been used to enhance utilization of essential nutrients such as fatty acids,vitamins,and essential amino acids (Simón et al.,2021).Recent advances focus on its function in changing lipid metabolism in terrestrial animals,with the majority of these studies elucidating serum fatty acid conversions(Ashayerizadeh et al.,2009;Naseem et al.,2021;Shirani et al.,2019).Commercial probiotic preparations used in aquaculture,such as lactic acid bacteria (LAB) and bifidobacteria,are isolated from dairy and fermented food products (Fenster et al.,2019;Ramezani-Fard et al.,2014).Administration of the commercial LAB probiotic in the diet of the common carp (Cyprinus carpio) is shown to improve fillet quality by increasing polyunsaturated fatty acids (PUFA) content (Baesi et al.,2017).Other experimental non-host origin probiotics have also reported positive effect on the growth performances of various cyprinid fish such asCatla catla(Das et al.,2013),Cyprinus carpio(Baesi et al.,2017),Labeo rohoita(Ray et al.,2010),orCirrhinus mrigala(Ghosh et al.,2008).In comparison,host associated probiotics (HAPs),i.e;bacteria from the genusBacillus,Lactobacillus,Enterococcus,andCarnobacteriumisolated from the fish body,are considered to be part of the natural defense system with benefits include improved growth,feed value,and enzymatic digestion activity,and inhibition of pathogenic microorganisms(Lazado et al.,2015;Van Doan et al.,2020).Native HAP bacteria isolated from the digestive tract i.e;Lactobacillus plantarum,L.delbrueckiisp.,L.acidophilus,andL.bulgaricus,are mutually linked with habitat parameters (Prihanto et al.,2020),have better adaptive capacity in the intestinal mucus (Mohammadian et al.,2018),and in turn promote higher nutrient digestibility and improved immunity (Mohammadian et al.,2017,2019).HAPs such asPsychrobactersp.,could also reduce saponin toxicity in the host gut,thus counteracting the negative effects of plant-based ingredients like soybean meal (Wanka et al.,2018).
Studies with varied probiotic feeding duration,or short-term use of probiotic remain very limited (Aly,Abdel-Galil Ahmed,et al.,2008;Sharifuzzaman &Austin,2009).The feeding duration of probiotics diet in tilapia (Oreochromis niloticus) culture for short periods is shown to enhance fish growth performances as reported by Tachibana et al.(2020).Different feeding duration,such as pulse feeding has been examined as application of probiotic diet alternating between every 7 and 14 days for a total of 84 days.Short-term starvation or restricted use of feed are practiced by fish farmers as aquaculture management strategy to maintain water quality and to prevent mortality caused by disease outbreaks (Shoemaker et al.,2003).Apart from such strategies,probiotics in diets are also used to solve water quality problems,and for stress management due to handling (Caruso et al.,2011;Davis &Gaylord,2011).Short-term probiotic application could be very helpful strategy in optimization of probiotic cost,and overall farming sustainability,if improvements in growth performances or disease resistance could be demonstrated (Sahu et al.,2008).
The Malaysian mahseer fish,Tor tambroidesis a species from the large-bodied cyprinid family found throughout south-east Asia from Malaysia and Indonesia to southern China,regarded as of high market demand as a food,game,or ornamental fish (Lau et al.,2021).One of the bottlenecks inT.tambroidesfarming is the slow growth of the species in captive condition resulting in a long culture period (Chowdhury et al.,2016;Soon et al.,2014).Considering its high commercial value,scarcity,and increasing demand in the market,the species was selected for this present study in evaluation of short-term application of dietary supplementation with HAPs.A shorter application period of probiotic diet supplement to boost growth may positively affect the culture of this species in captive condition,with lower consumption of probiotics.Therefore,the present study aimed to evaluate the short-term application of probiotic dietary supplementation and its effects on slow growingT.tambroidespost larvae.The evaluation involved determining growth parameters (weight gain %,specific growth rate SGR%,feed conversion ratio (FCR),survival %),whole body proximate composition,fatty acid profile,and muscle growth,with study of gut morphology,after short-term (30 days) dietary probiotics application,and after the withdrawal of probiotics dietary supplementation.
2.Materials and method
2.1.Preparation of probiotics and experimental diets
Experimental diets,with three different HAPs supplemented diets,were prepared according to methods described by Asaduzzaman,Iehata,et al.(2018),and Iehata et al.(2015),with some modification.Probiotics used in this studyE.faecalisstrain 2674,E.faecalisstrain FC11682.andAeromonassp.A8-29 were previously isolated from the gut ofT.tambroides(Hossain et al.,2022).Briefly,selected bacterial strains were used to mass culture,and density CFUml-1were then determined for further inclusion in basal diet.Selected strains were separately grown.Aeromonassp.A8-29 were grown in 50 ml TSB(Tryptone Soya broth,TSB;Merck KGaA Germany) for 24 h,whileE.faecalisstrains (strain 2674 and FC11682) were grown in 50 ml MRS broth (De man,Rogosa and Sharpe agar,MRS;Merck KGaA Germany)for 48 h at room temperature incubator shaker at 100 rpm.The cultures were subsequently centrifuged at 3000 rpm for 10 min,pellets harvested and washed thrice in phosphate buffer solution (PBS,PH 7.4),and then resuspended in the same amount of PBS solution.
Commercial sinking crumble (dry feed) from Gold Coin Specialities Sdn.Bhd.were selected as basal diet throughout the feeding trial,following the recommended nutritional requirements forT.tambroidesspecies stated by Kamarudin et al.(2012),in which the minimum requirements for protein and lipid are 40% and 5%,respectively.The proximate composition of the basal diet was pre-determined (Table 1)according to AOAC method (AOAC 1997),and fatty acid composition was determined (Table 2) by following one step method (Abdulkadir &Tsuchiya,2008).Dilution of the probiotics in PBS solution (1 × 108CFU/mL) was added into basal diets by pump sprayer which was sterilized by chlorine and 70% ethanol before use (Kuebutornye et al.,2020).Prior to the actual preparation,several trials were conducted to optimize the incorporation success of the probiotic bacteria,identifying the targeted 1 ×108CFU/g concentration,which was determined both in plate count,and by optical density (OD600=0.1) at web length of 600 nm measured by spectrophotometer.The control diet was prepared by spraying only PBS (without any probiotic) into the basal diet,which was subsequently dried in an aseptic condition under laminar flow until initial weight of feed was achieved.Then the experimental diets were stored at 4◦C for further use in the feeding trial (Amstrong et al.,2016).Fatty acid content of experimental diets was then determined with results shown in Table 2.The viability of bacteria in dry feed,and water immersed feed were predetermined by following method described by Abarike et al.(2018).Colonies were counted and converted to CFU/gor CFU/mL.
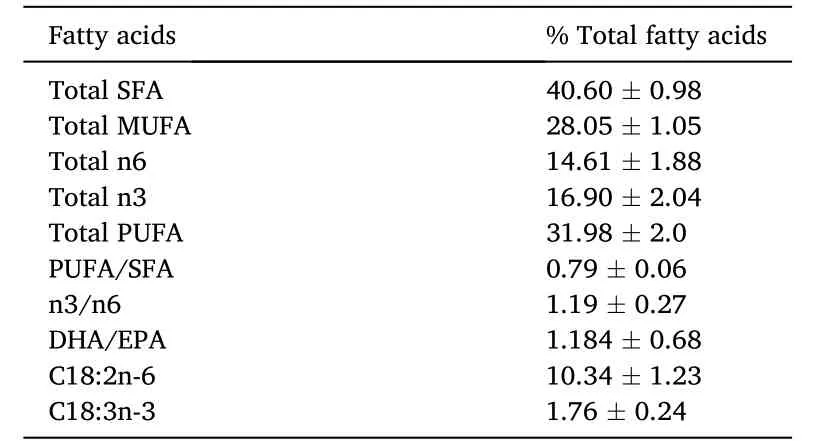
Table 2 Fatty acid composition of commercial diet used to prepare the basal diet and experimental probiotic diets for T.tambroides post larvae.
2.2.Experimental design and feeding
A 4 ×3 experimental design was done for this study.There were four group treatments,namely T1 (E.faecalisstrain 2674),T2 (Aeromonassp.A8-29),T3 (E.faecalisstrain FC11682),and Control (no probiotic),witheach experimental treatment tested in triplicates.Three hundred healthy fish (initial average weight;0.24 ± 0.01g) were randomly distributed into twelve tanks (120 L volume each) consisting of 25 individuals per tank,arranged in a completely randomized design.Probiotics diet were provided twice daily (9:00 a.m.and 4:30 p.m.) at 3% body weight(Asaduzzaman,Iehata,et al.,2018),and feed amount adjusted weekly based on growth (Abarike et al.,2018).Continuous aeration was provided by air stone diffusion connected to a central air compressor.The tanks were cleaned weekly to reduce the risk of bacterial growth,and water quality was measured daily using YSI probe (YSI ProDSS multiparameter-Model number: 550A,USA) and with commercial test kits (API Brand,Malaysia),while maintaining acceptable level of dissolved oxygen (DO) >6 ppm,pH 7.5–8.20,temperature 26.00 ±4.0◦C,alkalinity 73–107 ppm,and NH30.0 ppm.Uneaten feed,and faeces were removed as required.Data collection for growth parameters was done every 15 days for monitoring purposes and only data collected on the final day of each experiment was subjected to statistical analysis(Andani et al.,2012;Asaduzzaman et al.,2018).The experiment was divided into two parts,where the first part was short-term (30 days)probiotic treatment,and the second part started after withdrawal of probiotic diet,and shift to normal feeding for an additional 60 days.All experiments were conducted in line with Universiti Malaysia Terengganu and European laboratory animal welfare legislation,as well as the guiding principles for the use and care of laboratory animals.
2.2.1.Experiment part 1:short-term use of probiotic supplement on T.tambroides post larvae
In experiment part 1,a 30-day feeding experiment was conducted in 120 L tanks with triplicate for each treatment.The first (T1),second (T2)and third (T3) groups were fed diets supplemented with 1 × 108CFU/mg cells of HAPE.faecalisstrain 2674,Aeromonassp.strain A8-29,andE.faecalisstrain FC11682 respectively for 30 days.The control group was fed with basal diet.The fish were fed two times daily at the rate of 3% of the body weight for 30 days.After 30 days of application of probiotic diets,samples were collected to measure growth,biochemical and conduct histological analysis.Remaining fish were carried over for the second part of the experiment.
2.2.2.Experiment part 2:effects on growth,after withdrawal of probiotic and shift to normal feeding
At the end of experiment part 1,remaining fish was carried over for experiment part 2 in the same tanks and set up.Fish from replicates of the same treatments were pooled and graded before being randomly distributed as new replicates,stocking the fish as close to average body weight as much as possible.Then,the fish in experiment part 2 were shifted to normal feed for an additional 60 days,without probiotic,to investigate and compare effects after withdrawal of probiotic.Final sampling was executed at day 90,following overnight starving.From each tank,fish were individually weighed,measured,data recorded,and sample fish collected and processed,for analysis of growth,fatty acid,gut morphology,and hypertrophic muscle growth.
2.3.Sample collection
Prior to final sampling,all fish were fasted for 24 h at the end of both feeding trials and fish were collected from each tank,counted,and weighed by digital balance.Data were recorded from which total weight,SGR (%),and food conversion ratio (FCR) were calculated.For the final whole body proximate and fatty acid analysis,five fish were randomly selected from each replication tank and maintained at -80◦C until freeze drying.At the same time three fish samples close to the mean weight from each probiotic treatment tank,and control tank,were collected for histological analysis.Briefly,the fish were anaesthetized by using clove oil (50 mg/L),and then were dissected aseptically for collection of gut samples.Immediately after anaesthetization,muscle tissue samples were cut transversely near the dorsal fin region,mid guts removed,and samples dipped in Bouin’s solution for 24 h (ratio of fixative and sample,10:1).Later fixed sample was transferred to 70%ethanol for further histological study following methods described by Chen et al.(2020).
2.4.Growth performances analyses
Growth performances ofT.tambroidespost larvae was estimated in terms of mean final weight (g),weight gain (g),specific growth rate(SGR %),survival (%),and feed conversion ratio (FCR),which were analysed both at the end of short-term probiotic applications,and at the conclusion of the final period of feeding trial.Growth related parameters were calculated with the following five formulae according to Bullerwell et al.(2016):
2.5.Analysis of proximate,and fatty acid composition of whole-body T.tambroides
Proximate composition of both fish and experimental diet were analysed using standard AOAC methods (AOAC 1997).Dry matter and percent of moisture content were calculated weighing fish sample before and after freeze drying by following formula: Moisture=[(wet weight(g) -dry weight (g))/wet weight (g)] x 100 (Bullerwell et al.,2016).
Experimental diet and freeze-dried fish sample were ground to approximately less than 1.00 mm size using a grinder (Panasonic,model MX801S,Japan).The percent of moisture in both feed and fish were determined by drying sample at 105◦C for 12 h until a constant weight obtained,and ash content was determined by burning sample at 550◦C for 12 h in Muffle furnace.Quantification of crude protein was by Kjeldahl method,and crude fat content was measured by using ether extract by Soxhlet method (FOSS Labtech model Sweden).
Freeze dried samples in triplicates of whole body ofT.tambroides,and of the experimental diets,weighing 200–300 mg each,were taken for one-step method of fatty acid analysis,which combined the extraction and esterification process using a single tube following method described by Abdulkadir and Tsuchiya (2008).The fatty acid methyl esters (FAMEs) were used in determination of fatty acid composition,with fatty acids separated,and quantified by gas chromatography with Mass spectrometer (GC MS-QP2010 Ultra).The peak area of each fatty acid was compared to the overall peak area of all fatty acids in the sample to obtain individual fatty acid percentages (Mian et al.,2017).
2.6.Histological analysis
After 30 days of probiotic treatment,and at the conclusion of the final period of feeding trial,histological analysis were studied following method described by Karahmet et al.(2014),with some modification.The samples previously collected and stored in Blouin’s solution were fixed for 24 h,then the samples were transferred and re-transferred three times to 70% ethanol,and finally preserved in 70% ethanol until tissue processing.Fixed tissues were processed following standard histological technique,by Leica automatic tissue processor.
2.6.1.Muscle morphology
To study the muscle morphometry (cell hypertrophy and hyperplasia),the number of muscle fibres and their diameters were measured,within the cross-sectional area of transverse section compartmental area of each section (Kuebutornye et al.,2020).Epaxial white muscle samples below the dorsal fin were collected for morphological and morphometric analyses (n=6).Fixed skeletal muscle samples were processed with a series graded level ethanol,using Leica automatic tissue processor.Processed samples were embedded in paraffin block on a cold plate,and tissue cut at 5–7 μm in Leica Microtome machine and placed on previously marked glass slide.Thereafter,glass slides were dried in hot plate for 24 h and stained with Hematoxyline-Eosine (H&E),then dried and covered with DPX.Then the slides were observed under compound microscope,Nikon Eclipse 80i,and individual fibre area measured by using computerized image analyse system/software NIS elements illustrator D5 10.00,(64 bit).Six fish per treatment group with 600 (6 of 100) fibres was measured per treatment.The fibres were distributed into classes according to diameter (d,μm) and classified into 3 groups;class 10: d ≤10,class 10–30: 10 ≥d ≥30 class 30–50: 30 ≤d≤50,class 50–80: 50 ≤d ≤50 (Asaduzzaman et al.,2017a,2019;Nebo et al.,2013),and the muscle fibre frequency was determined by the number of fibres in each diameter class compared to the total number of fibres.
2.6.2.Gut morphology analysis
Previously fixed gut samples were processed in a Leica™ automatic tissue processor,and embedded in paraffin wax on a cold plate.Then,the embedded samples were cut at 5–8 μm thickness and placed on glass slides.Slides were placed at a hot plate overnight for drying before staining.Dried slides were used in H&E staining method,for analysing gut morphology parameters such as villus height,villus width,villus area,and crypt depth in probiotic treated fish.Stained samples were dried at room temperature for 30 min before mounting with DPX.Minimum 5,and maximum 10 villi were used to measure evenly.Villi located in the mid-gut were chosen for measurement.A total 6 fish samples were used in gut morphology analysis,and the maximum possible number of villi were selected to measure the parameters.Measurements were performed using the image-analysis application software Sigmascan Pro 5 (Systat software Inc,San Jose,CA,USA).
2.7.Statistical analysis
Assumptions of normal distribution and homogeneity of variances were checked before running the statistical analysis.The frequency distribution of fast skeletal muscle fibres in distinct classes was represented as a percentage,and transformed using an arcsine square root transformation.Data from each treatment was submitted to a one-way analysis of variance (ANOVA) using SPSS (version 20;IBM,Chicago,IL,USA).This was followed by a Tukey multiple range test to evaluate the differences across treatments,when significant differences (P<0.05) were found.To describe hyperplasia and hypertrophy,the muscle fibres of various diameter were divided into size classes.The data were expressed as a percentage and underwent an arcsine square root transformation before statistical analysis.The presence of lowest size class10 fibres was a predictor of hyperplasia,while fibres larger than class 30 were considered to indicate hypertrophy.
3.Results
3.1.Feeding trials
3.1.1.Experiment part 1:short-term use of probiotic supplement on T.tambroides post larvae
Growth performances ofT.tambroidespost larvae for all treatments both after receiving short-term (30 days) HAPs diet supplements has been represented in Table 3.After short-term dietary supplementation with HAPs,T.tambroidespost larvae showed significantly (P<0.05)better growth in all probiotic treatment groups as shown in Table 3.Group T3 (E.faecalis-2674)showed the highest final weight (g),weight gain (g),weight gain (%),SGR (%),FCR and survival (%) followed by T2(Aeromonassp.) group and lowest was observed in control group.All water quality parameters observed for 30-day probiotic treatments were within the acceptable limits for finfish aquaculture and observed to have no significant difference among the treatments during application of dietary supplementation with host probiotics or non-probiotics feed.
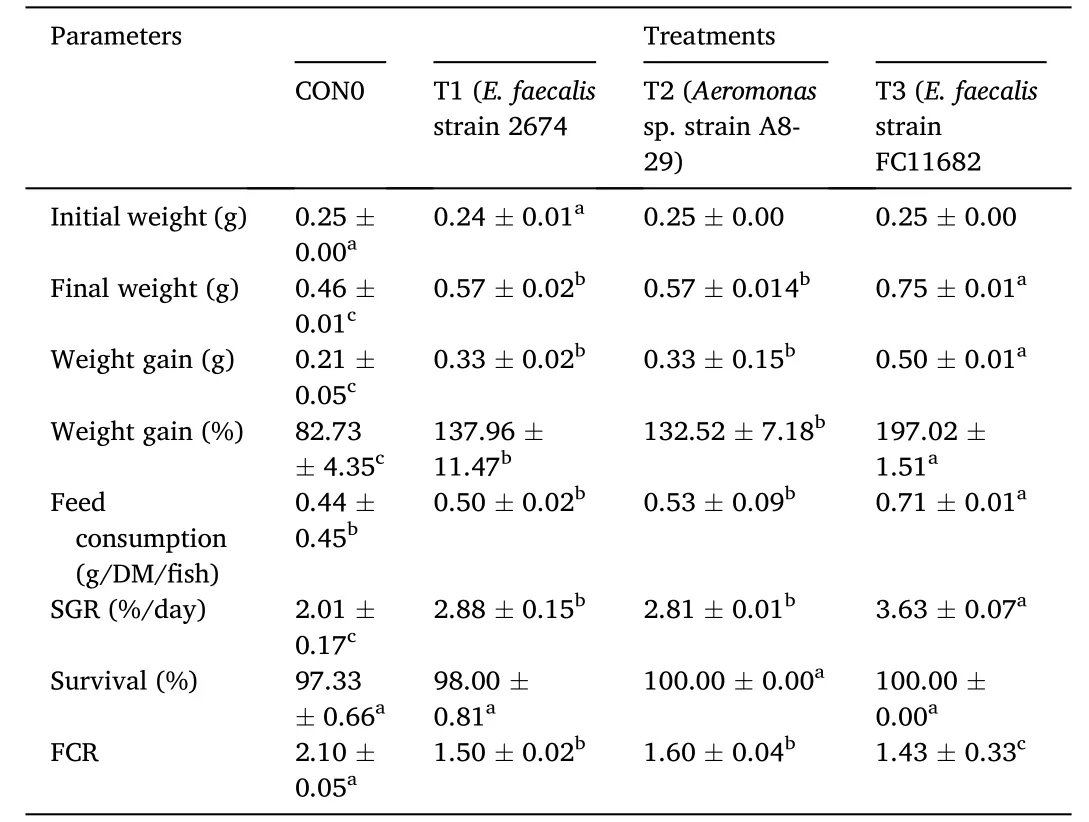
Table 3 Growth parameters of fish after receiving short-term (30 days) of probiotics diet supplement.
3.1.2.Experiment part 2:effects on growth,after withdrawal of probiotic and shift to normal feeding
Similar growth performances were also evident at the end of the 90 days feeding trial which was 60 days after withdrawal of probiotic diets as shown in Table 4.Growth parameters after application of nonprobiotic basal diets on fish for 60 days,including final weight (g),weight gain (g),SGR (%),and FCR,were significantly (P<0.05) higher in T3 group,followed by T1 and T2,with the lowest values recorded for the control.Survival was highest in T3 group but there were no significant differences (P<0.05) among the groups.All water quality parameters observed for the subsequent treatment 60 days after withdrawal of probiotic diets were within the acceptable limits for finfish aquaculture and observed to have no significant difference between treatments.
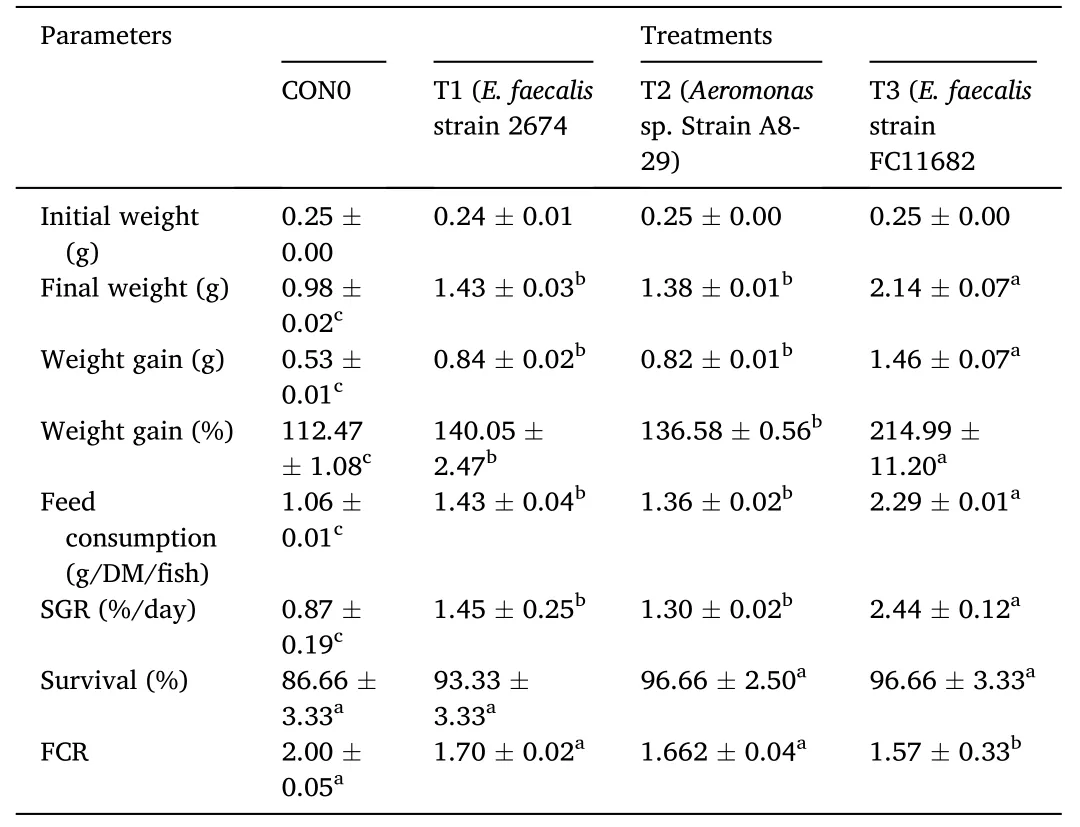
Table 4 Final growth parameters of fish at day 90 after 60 days withdrawal of probiotic diets.
3.2.Effects of HAPs on whole-body proximate,and fatty acid composition of T.tambroides post larvae
The whole-body proximate and percentage of total fatty acids compositions of whole body ofT.tambroidespost larvae fed different HAPs diets after 30 days and at the end of feeding trial after withdrawal of probiotics,were shown in Table 5 and Table 6,respectively.The proximate compositions such as,moisture (%),crude protein (%),crude lipids (%) were neither significantly affected by the short-term probiotic diets,nor after withdrawal of probiotic dietary supplementation.Regarding whole body fatty acid profiles,significantly (P<0.05) higher percentages values were recorded both for fish fed with HAPs compared to control group and for fish following withdrawal (60 days) of probiotic diets.For total MUFA,linoleic acid (C18:2n-6),linolenic acid (C18:3n-3),and total n6 fatty acids,the higher values were recorded for shortterm probiotic treatment groups.The highest total MUFA values were recorded for T1(29.36%),followed by T2 (25.91%) and T3 (24.83%).For n6 PUFA,the highest value was recorded for T3 (17.27%),followed by T1(16.20%) and T2 (15.24%),with the lowest recorded for control(12.86%).The ratios of n3/n6 values were lower in the treatments group compared to control.In the second part of the study,after withdrawal of probiotic treatments,the fatty acid profiles of whole body were significantly higher for total MUFA,linoleic acid,linolenic acid,total n6 fatty acids,in the treatments group compared to control.
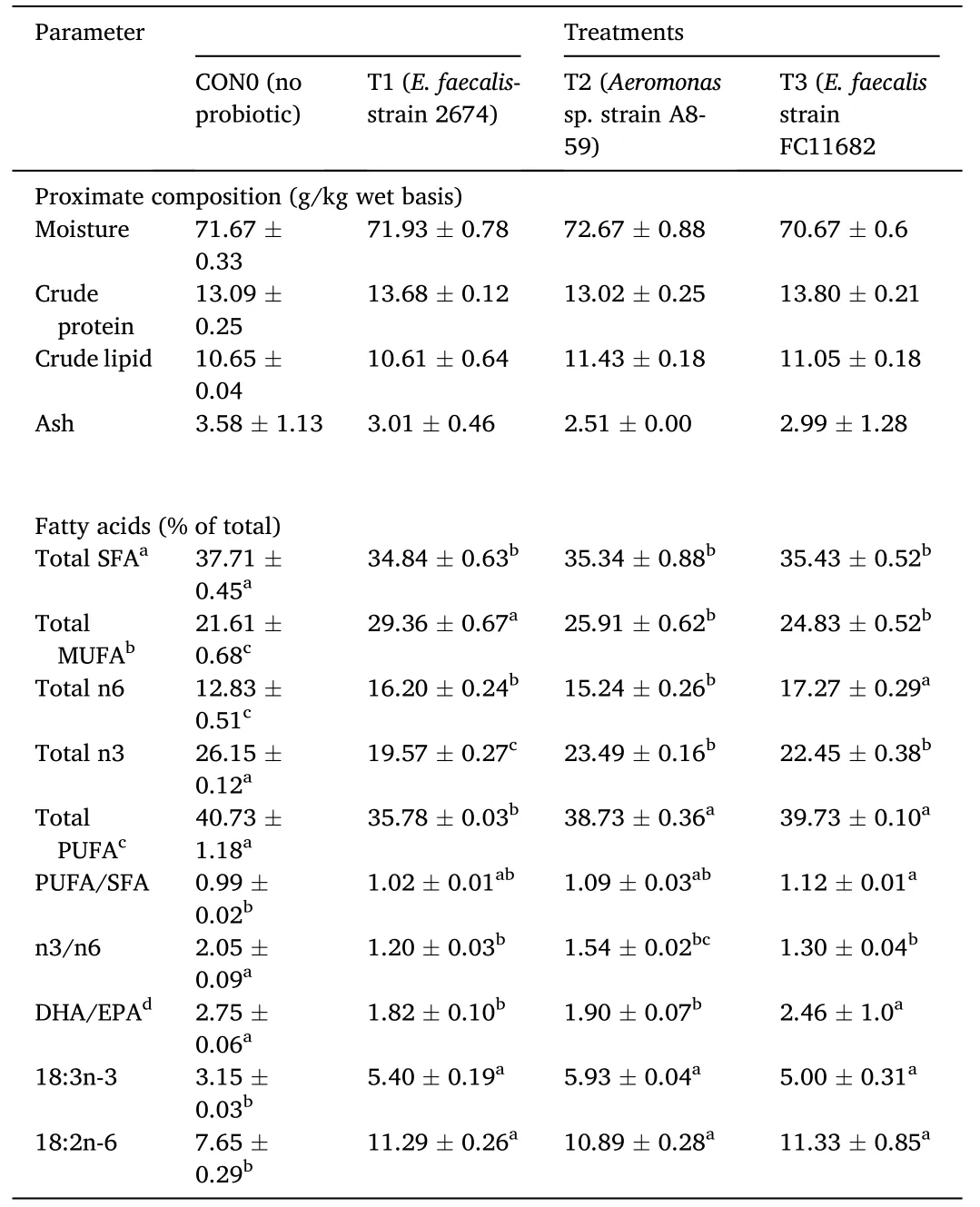
Table 5 Proximate and fatty acid composition of whole body of T.tambroides post larvae after receiving short-term (30 days) of probiotics diet supplement.
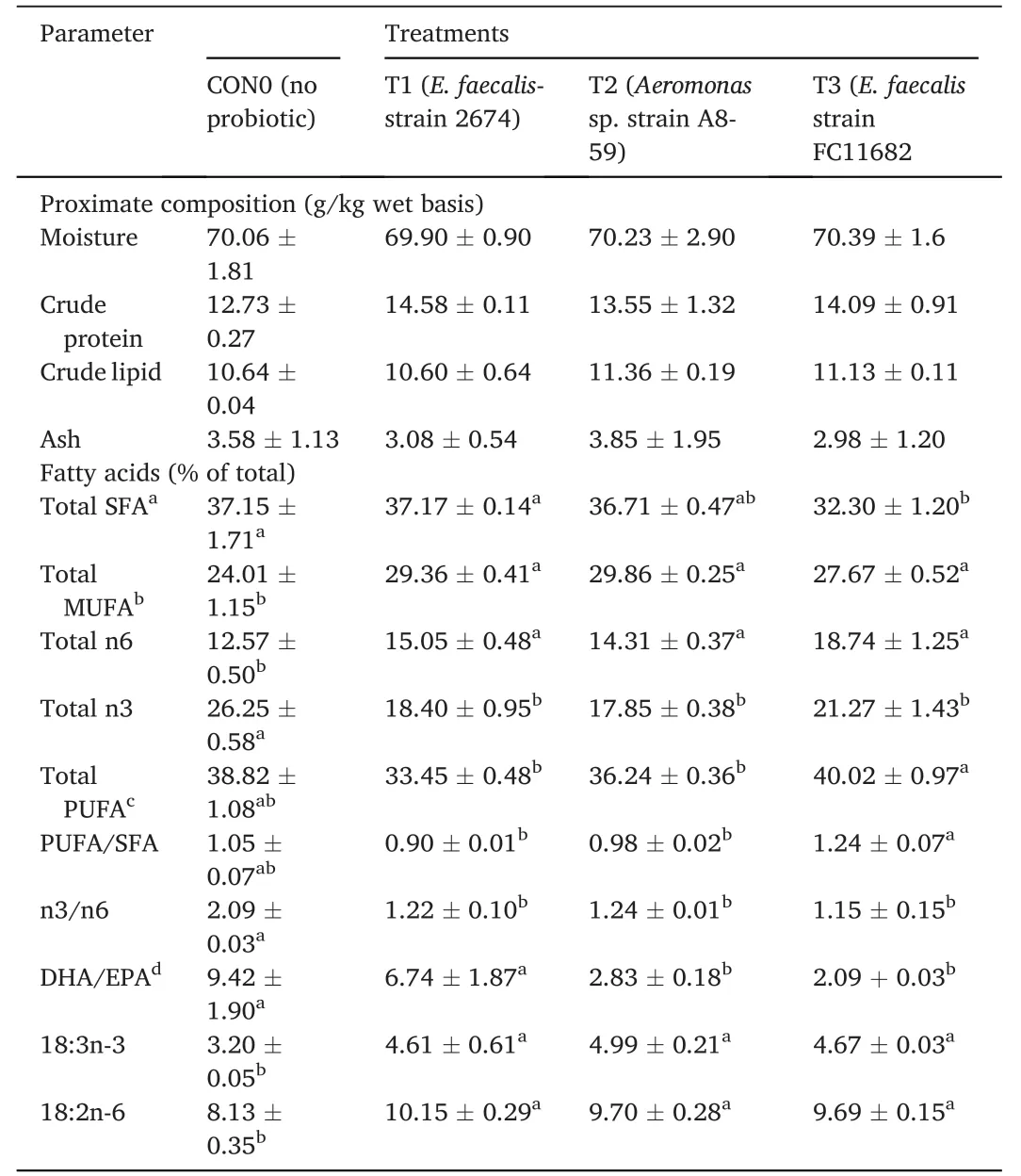
Table 6 Proximate and fatty acid composition of whole-body T.tambroides post larvae at the final period (90 days) fed control diets after withdrawal of short-term probiotic diets application.
3.3.Effects of HAPs on muscle morphology
Muscle samples of transverse sections were collected both at the final period of the short-term (30 days) use of probiotic diet,and the end of experiments (after withdrawal of probiotics),for T1(E.faecalis 2674),T2(Aeromonassp.strain A8-59),T3 (E.faecalisFC11682),and control(CON0).In this muscle histology analysis,class 10 fibres were considered as newly recruited hyperplastic fibres,class 10–30 were considered as growing intermediated fibres,and class 30–50 and class 50–80 were considered as hypertrophic fibres.Microscopic analysis showed that round-shaped muscle fibres were separated,and connective tissues were dispersed in mosaic pattern with different types of diameters.Fig.1 shows the frequency of distribution of muscle fibre after short-term probiotic treatment,and Fig.2 shows the frequency of distributionafter withdrawal of probiotic diets at the final period (day 90).Fig.3 displays microscopic pictures showing different muscle fibres.After short-term (30 days) application of probiotic in T1,T2,and T3,the frequency diameter for all classes were similar in all group (Asaduzzaman et al.,2019;Nebo et al.,2013)
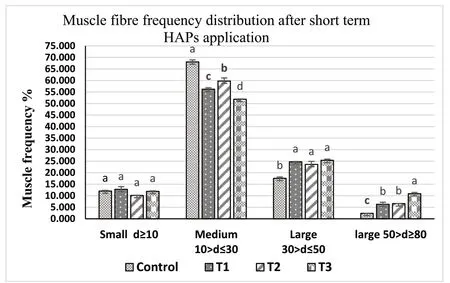
Fig.1.Muscle fibre frequency of skeletal muscle of T.tambroides post larvae fed short-term (30 days) probiotics diets and control diets.Values=Mean ±Standard error of triplicate measurements (n-3).The mean values indicating by the different superscript letter in each parameter expresses significant differences at P ≤0.05.ANOVA was done followed by Tukey test*T1: fish fed probiotic bacteria,E.faecalis strain 2674;*T2: Aeromonas sp A8-29;and *T3: E.faecalis strain FC11682 and Control: no probiotic.
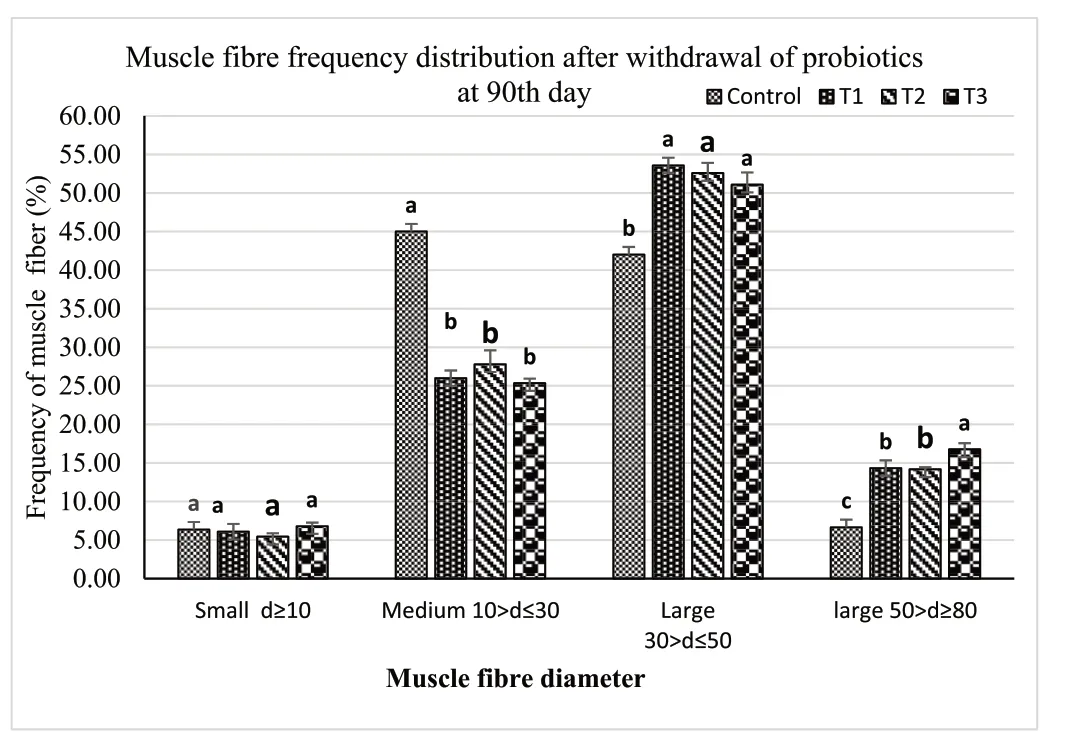
Fig.2.Muscle fibre frequency of skeletal muscle of T.tambroides post larvae at final period (after 90 day) after short-term administration of HAPs T1:E.faecalis strain 2674.T2: Aeromonas sp.A8-29 and T3: E.faecalis strain FC 11682 Values=Mean ± Standard error of triplicate measurements (n-3).The mean values indicated by the different superscript letter in each parameter express significant differences at P ≤0.05.
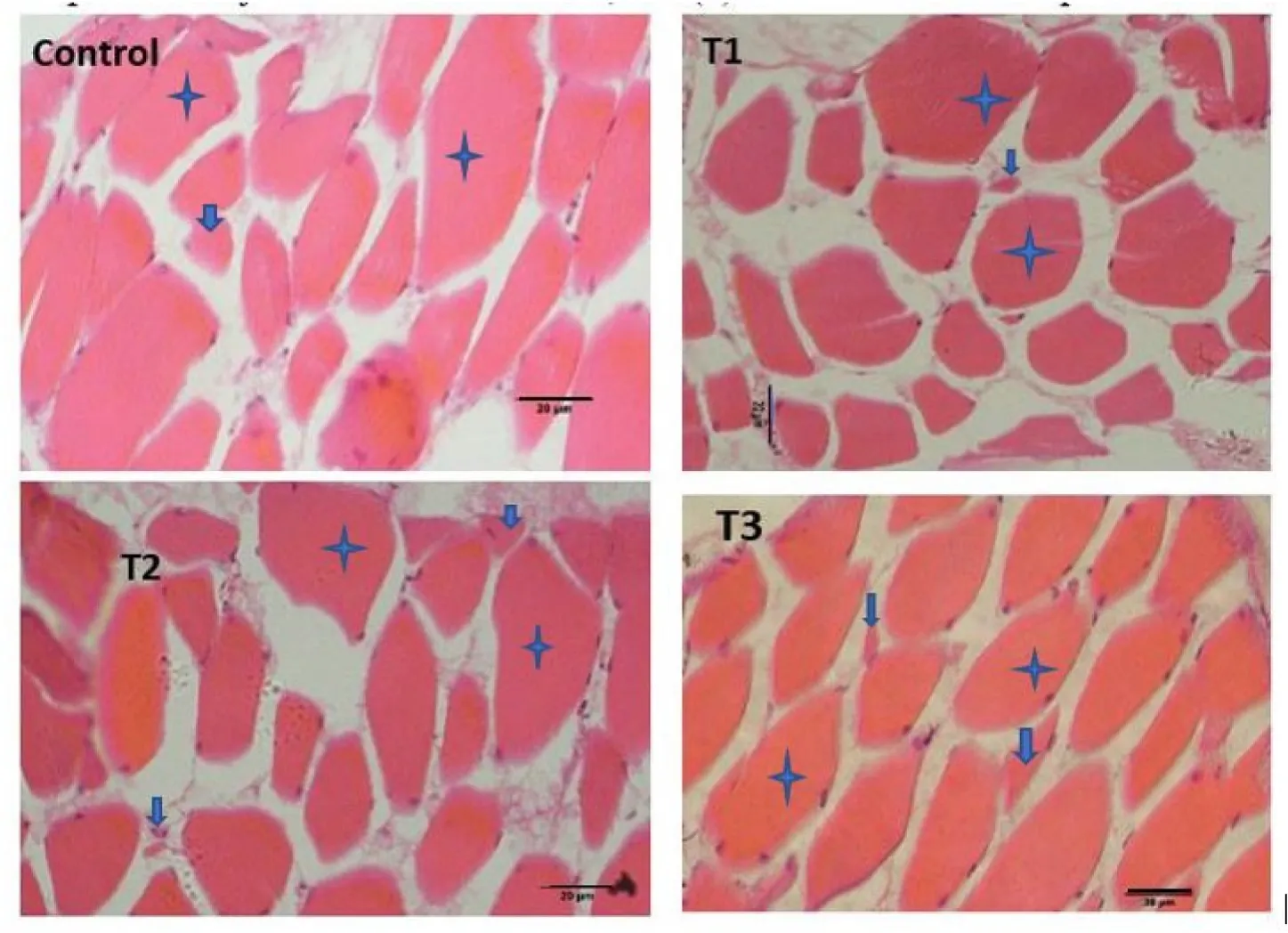
Fig.3.Skeletal muscle of T.tambroides post larvae fed different HAP after 30 days,(a) T1: treatment with E.faecalis strain 2674;(b) T2: fed with probiotic Aeromonas sp;(c) T3: fed with probiotic E.faecalis strain FC11682;and (d) C: fed Control diet (star marks indicate hypertrophic muscle fibre and arrow marks indicate hyperplastic fibres,scale: 20 μm.
A morphometric analysis of muscle indicated that both dietary supplementation with different HAPs for 30 days,and at the end of feeding trial at day 90 displayed no effect on hyperplastic small diameter fibre Class 10.Hypertrophic large diameter fibres such as,Class 30–50 and Class 50–80 reported significantly higher values for fish fed short-term HAPs diet in T1 (E.faecalisstrain 2674),T2 (Aeromonassp.A8-29),and T3 (E.faecalisFC11682),where highest values for both classes were reported for group T3,followed by T2 and T1,and lowest value for control group.However,Class 10–30,considered for intermediate growing fibres,recorded significantly lower value for T1,T2,T3 groups,with the highest value recorded for control group.Muscle morphometric data at the final period (90 day) (Figs.2 and 3) after withdrawal of probiotics,demonstrated that large diameters fibre classes were significantly more prevalent in T1,T2,and T3 groups,with the highest prevalence in T3 followed by T1 and T2.The lowest value was recorded for control group.
Muscle fibre morphometric data after short-term probiotic application revealed that the higher growth in probiotic treated fish was due to hypertrophic muscle growth.
3.4.Effects of HAPs on gut morphology of T.tambroides
Histological representation ofT.tambroidesmidgut for all treatments is shown in Fig.4 and the measurements of mid gut both after short-term(30 days) probiotic application,and at the final period after withdrawal of probiotic treatment,are displayed in Table 7.Dietary supplementation ofE.faecalisandAeromonassp.treatment showed higher villus height,villus width,and villus area (P<0.05),compared to control group.Intestinal wall thickness and crypt depth did not significantly differ (P<0.05) among the treatments (see Fig.5).
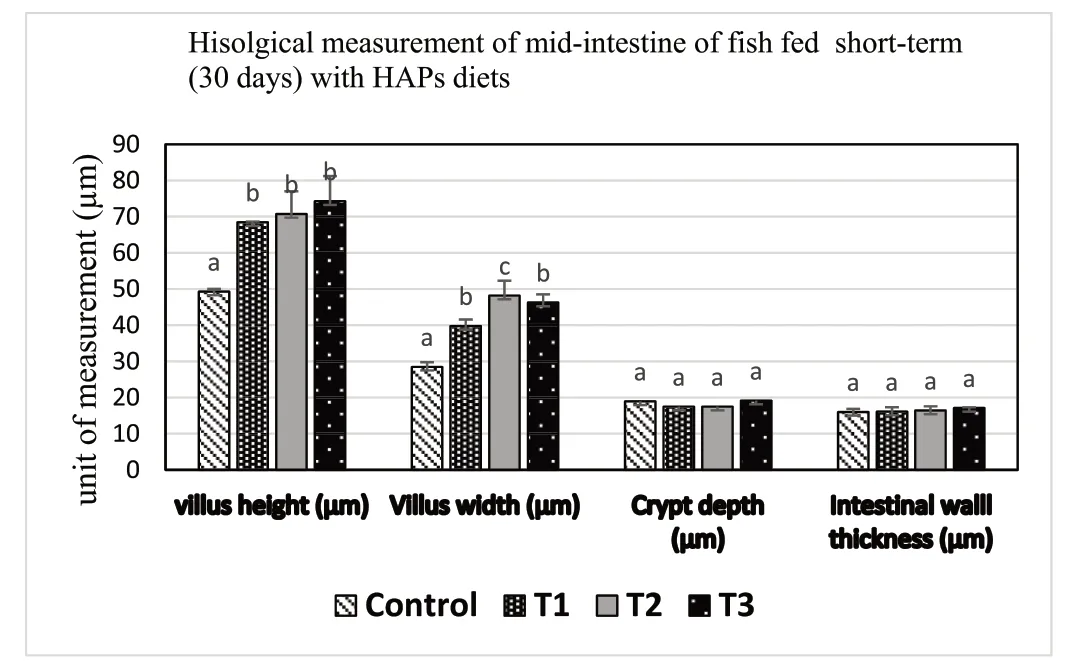
Fig.5.Morphological parameters of the mid-gut specimen of T.tambroides post larvae fed for short period (30 days) with different HAPs supplemented diets.
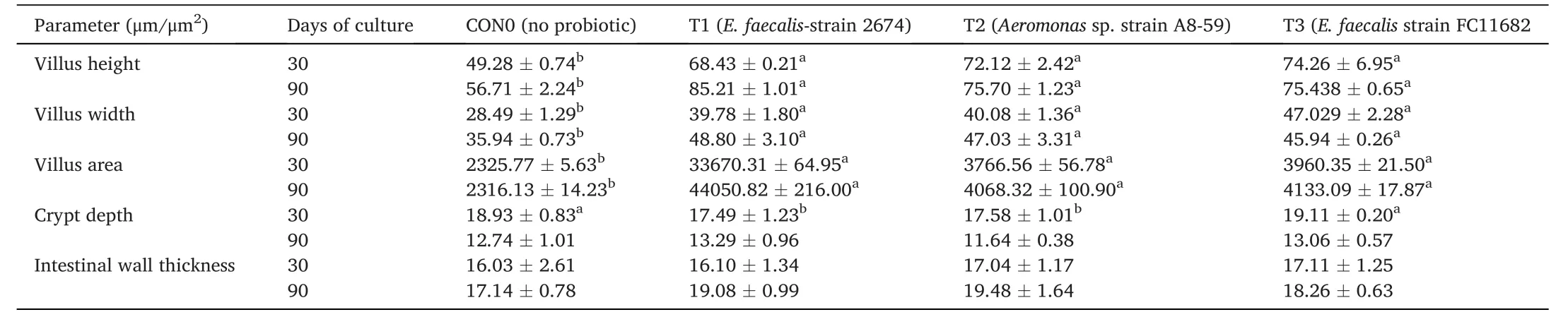
Table 7 Histo-morphological parameters of mid-gut samples of T.tambroides post larvae fed short-term dietary supplementation with host probiotics,and final period after withdrawal of probiotics.
4.Discussion
The beneficial effects of probiotic bacteria as starters,dietary supplementation,or as preventive culture have been reported before(Hamid et al.,2021;Mohammadian et al.,2019;Yang et al.,2014).Studies with application of probiotic bacteria have focused on the management of different feeding duration,such as 1–8 weeks feeding regime which led to enhanced growth and protection of fish against pathogen (Gildberg et al.,1997;Raida et al.,2003;Sharifuzzaman &Austin,2009).The current study investigated the effects of short-term(30 days) dietary supplementation with intestinal HAPs probiotics through dry basal feed on growth,carcass composition,fatty acid composition and histological changes in muscle morphometry and gut morphology ofT.tambroidespost larvae.Asaduzzaman et al.(2018a,b)reported higher growth forT.tambroidesfed a probiotic diet supplement withBacillussp.,Alcaligenessp.In this present study,fish fed for a short period withE.faecalis(strain 2674 and FC11682) andAeromonassp.showed significantly higher growth than those that received control diets,with no significant difference reported for survival.T.tambroideshas been described as a slow-growing fish species that takes a long time to reach bigger size than other fish (Chowdhury et al.,2016).In the early stage of life,they have shown higher SGR (%) which later decreased.Thus,the low SGR (%) has been accepted as reasonable for this particular species (Chowdhury et al.,2016;Ismail et al.,2013;Kamarudin et al.,2012).The application of probiotic dietary supplementation for a short period such as 28 days has provoked different growth responses in fish (Welker et al.,2012),and it has been shown to be important to formulate diet related to dose-time effect in studies with active additives(Hamid et al.,2021;Merrifield,Bradley,et al.,2010;Torrecillas et al.,2018).The findings of this study matched with some other previous studies regarding to weight gain (g),SGR (%),and FCR,where improved growth were observed in several kinds of fishes such as,rainbow trout,Oncorhynchus mykiss,T.tambroides,and hybrid catfish,(Clarias gariepinus×C.macrocephalus)(Asaduzzaman,Iehata,et al.,2018;Hamid et al.,2021;Sharifuzzaman &Austin,2009).Probiotics may influence higher growth because of their ability to regulate production of digestive enzymes,which in turn enhances feed utilization (El-Haroun et al.,2006;Mohammadi et al.,2022).
It has been reported that feeding regimes or environmental factors can affect the muscle growth in fish (Chauvigné et al.,2003;Johnston,2001).Many studies reported a positive role of different types of probiotics in influencing growth parameters of different important culturable fish (Asaduzzaman,Iehata,et al.,2018;Shakur Aha et al.,2020;Nebo et al.,2013;Silva et al.,2021),and crustacean species (Nimrat et al.,2021;Toledo et al.,2019;Zuo et al.,2019).Nonetheless,only few researchers have evaluated the cellular system of improved growth due to effects of short-term dietary application of HAPs.Newly recruited hyperplastic cells are mainly smaller in size,and they increase the cross sectional area by increasing size of the cell due to the hypertrophic growth of cell (Asaduzzaman et al.,2018,2019).The different size of muscle fibres could be used as a tools to evaluate the growth of the fish exerted by the effects of dietary supplementation of host associated probiotics (Shakur Aha et al.,2020;Silva et al.,2021).In this present study,fibres less than 10 μm in diameter indicated hyperplasia,while higher than 10 μm represented the consequent growth of hypertrophic fibres (Asaduzzaman et al.,2017b;Nebo et al.,2013;Wanka et al.,2018).The presence of fibres smaller than 10 μm in diameter indicated the formation of newly recruited fibres and the variation in the percentages of these fibres resulted the growth rate of existing fibres.Previous studies reported similar results by applying various live and formulated diets forT.tambroideslarvae (Asaduzzaman et al.,2017a).Muscle morphometry analysis in the present study reported that a short-term application of different HAP diets increased the hypertrophic muscle growth ofT.tambroidespost larvae.HAP dietary supplementation produces bigger fish as HAP may assists in higher nutrient digestion and absorption (Mohammadian et al.,2017),which subsequently stimulate white muscle hypertrophic growth (Silva et al.,2021).
Proximate composition analysis results of fish fed different HAPs for the short-term demonstrated no significant variation among the treatments.The result is supported by some previous studies (Sharifuzzaman&Austin,2009;Tachibana et al.,2020).Fatty acid profile ofT.tambroidespost larvae was affected by the short-term dietary application of HAPs diets.Significantly higher levels of total MUFA,n6 fatty acids,linolenic acid (C18:3n-3),and linoleic acid (C18:2n-6),were recorded in probiotic groups compared to control group.Amounts of saturated fatty acid (SFA) were not improved by HAPs probiotics.The ratio of n3/n6 was higher in the control group.Previous study showed that percentage levels of MUFA detected in treatment group were higher than the control group,while the MUFA component found the most abundant in fish was oleic acid (C18:1) (Baesi et al.,2017).In probiotic,Shewanella baltica,dietary supplemented sole fish,positive correlation of both linolenic and linoleic acid was detected,as well as a significant rise in n-6 fatty acid (Solea senegalensis)(García de La Banda et al.,2010;Tapia-Paniagua et al.,2014).The ratio of n3/n6 values has exhibited similar values across probiotic treatments (Baesi et al.,2017;García de La Banda et al.,2010).The ratio of DHA/EPA in probiotic treatments was significantly higher in carp fish fed with commercialLactobacillussupplemented diet (Baesi et al.,2017).Gut bacteria or probiotics may generate enzymes such as cellulase,amylase,lipase,protease,and chitinase (Bairagi et al.,2002),which can also modulate the synthesis of important nutrients for fish,such as amino acids,fatty acids,and vitamins (Mohammadi et al.,2021;Nayak,2010;Semova et al.,2012).Microbes may improve fatty acid bioavailability by altering the synthesis of bile salt (Swann et al.,2011),by increasing absorption by lipolytic activity (Osman et al.,2021;Ringø et al.,1995),and by influence on physiological responses to absorption in the intestinal epithelium (Van Doan et al.,2020).
Probiotics may lower the rate of fatty acids oxidation.In this present study,probiotics increased both the linolenic acid (C18:3n-3) and linoleic acid (18:2n-6 fatty acids) in whole bodyT.tambroidespost larvae.Linolenic acid serves as precursor for eicosapentaenoic acid(C20:5n-3) and docosahexaenoic acid (C22:6n-3);and linoleic acid for arachidonic acid (C20:4n6) which subsequently acts as a precursor for eicosanoids,which are integral for an array of cellular activities including membrane integrity,immune response,gene regulation,renal and neural functions (Agaba et al.,2005;Leaver et al.,2008;Tocher,2003).This shows that probiotics is linked to improved growth by increasing lipid catabolism through β-oxidation and promotes intestinal absorption of key fatty acids metabolites (Nguyen et al.,2018;Semova et al.,2012).
The gastrointestinal (GI) tract is where the gut microbiota resides and a healthy bacteria diversity will competitively prevent pathogens from colonizing the gut.The gut-associated lymphoid tissue (GALT)mucosa lining the GI tract of the fish plays a vital role in fish immunity as the GALT mechanism will differentiate between pathogenic bacteria with autochthonous bacteria (Gatlin &Yamamoto,2022).Several studies have reported that various HAPs could modulate gut morphology by producing metabolites i.e;short-chain fatty acids(SCFAs) for energy,which result in greater intestinal epithelium growth that increases adsorptive surface for nutrient adsorption (Hoseinifar et al.,2019;Lazado et al.,2015;Merrifield,Bradley,et al.,2010).This intestinal growth can be indicated by villus height,villus width,ratio of villus width and height,intestinal wall thickness,villus area,goblet cell and density of microvilli (Merrifield,Dimitroglou,et al.,2010;Pirarat et al.,2011;Ramos et al.,2017;Standen et al.,2015;Zaineldin et al.,2021).In this study,the short-term application of HAPs influencedT.tambroidesgut morphology by increasing villus height,width and area.Similarly,previous study reported diets with different HAPs,such asAlcaligenessp.AFG22,Bacillussp.AHG22,that influenced intestinal histology ofT.tambroidesjuveniles (Asaduzzaman,Iehata,et al.,2018),and showed higher villus height in tilapia fish (Han et al.,2015;Pirarat et al.,2011;Xia et al.,2020).
5.Conclusion
Present study proved that administration of short-term (30 days continuous administration) probiotic in post larval stage ofT.tambroides,significantly influences growth performance,fatty acid profile,and gut and muscle histo-morphometry ofT.tambroidespost larvae.Shifting to normal feeding after withdrawal of short-term application of HAPs dietary supplementation after 30 days showed positive growth performance that continued up to day 90.It is concluded that this effect can optimize the expenditure and effort of probiotic application without hampering growth or chemical composition of the host fish.It can be concluded that short-term (such as 30 day) application of probiotic diet supplement inT.tambroidesfish can significantly improve growth parameters,hypertrophic muscle growth,and gut morphology compared to control.Moreover,some fatty acid related parameters,such as n-6 fatty acid,total MUFA,n3/n6 ratio,and concentration of linoleic and linolenic acid,were significantly improved after short-term application of probiotic dietary supplements,which may ultimately improve the nutritional quality of produced fish.The results have indicated the scale of opportunity available to further study,inclusive of study of duration and pulse feeding of probiotic diet supplementation impacts on growth performance,disease resistance,and immune parameters,for various cycle periods of culture and different sizes and age groups of fish,to achieve more complete findings on effects of the probiotics feeding duration on fish.
CRediT authorship contribution statement
Mohammod Kamruzzaman Hossain:Investigation,Formal analysis,Writing– original draft.Sairatul Dahlianis Ishak:Formal analysis,Writing– review &editing.Shumpei Iehata:Supervision,Methodology.NoorDiyana Mat Noordin:Writing– review &editing.Md Abdul Kader:Writing– review &editing.
Ambok Bolong Abol-Munafi:
Conceptualization,Supervision,Project administration,Writing– review &editing,Visualization,Resources,Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The study was funded by Universiti Malaysia Terengganu under the Research Intensified Grant Scheme (RIGS VOT NO 55192/5).
 Aquaculture and Fisheries2024年1期
Aquaculture and Fisheries2024年1期
- Aquaculture and Fisheries的其它文章
- Editorial special issue: Friendly nutritional strategies for sustainable aquaculture
- Hematobiochemical and histopathological alterations in Nile Tilapia(Oreochromis niloticus) exposed to ethidium bromide: The protective role of Spirulina platensis
- Effects of Moringa oleifera aqueous extract on the growth performance,blood characteristics,and histological features of gills and livers in Nile tilapia
- Evaluation of the nutritional value of Artemia nauplii for European seabass(Dicentrarchus labrax L.) larvae
- Antioxidant effects of the aqueous extract of turmeric against hydrogen peroxide-induced oxidative stress in spotted seabass (Lateolabrax maculatus)
- Marine yeast Rhodotorula paludigena VA 242 a pigment enhancing feed additive for the Ornamental Fish Koi Carp
