Selected dietary plant-based proteins for growth and health response of Nile tilapia Oreochromis niloticus
Tzodoq Obrero Magbanua,Janice Alano Ragaza
Ateneo Aquatic and Fisheries Resources Laboratory,Department of Biology,School of Science and Engineering,Ateneo de Manila University,Katipunan Avenue,Loyola Heights,Quezon City,Metro Manila,1108,Philippines
Keywords: Nile tilapia Plant-based proteins Soybean proteins Copra proteins Pea proteins Corn proteins Palm kernel proteins Algal proteins
ABSTRACT Tilapia culture is one of the largest sectors of global aquaculture.Among the different species of tilapia,Nile tilapia (Oreochromis niloticus) is perhaps the top cultured species.The production of Nile tilapia has been continually increasing throughout the years resulting in genetic deterioration.Several tilapia strains with better growth performance and adaptive capability to survive in different culture conditions have been developed to alleviate the crisis.Increased demand for Nile tilapia implies higher farming cost.Plant-based proteins are utilized as partial or complete fishmeal replacements to reduce feed cost.However,these proteins can adversely affect and alter growth and feed performance,carcass composition and indices,and gut and hepatic health.This review discusses the use of seven plant-based proteins: namely,soybean,copra,pea,corn,palm kernel,microalgae,and seaweed as a Nile tilapia aquafeed.Different processing methods are employed to produce several types of plant-based proteins.Processed plant-protein types,when utilized as an aquafeed ingredient,vary in its effect on the performance,hemato-immunological parameters,and gut and hepatic health of Nile tilapia.Studies have shown that Nile tilapia can effectively maximize plant-based protein diets based on the preparation method,type of plant source,amino acid supplementation,and inclusion levels of the plant proteins.These readily available crops should be considered as primary protein sources for aquaculture.Hindrances to the use of plantbased proteins as a main dietary protein are limiting amino acids,presence of anti-nutritional factors,and the competition between its demand as human food and as animal feed.
1.Nile tilapia aquaculture
Tilapia is one of the most farmed fishes globally.This species is native to the Middle East and Africa before it was introduced worldwide in the 1900s (Fitzsimmons,2000).Tilapia culture is said to be profitable because it necessitates low-maintenance and considered to be disease-resistant fish.It can grow and breed in various conditions and reach marketable sizes of 600–900 g in just six months (Fitzsimmons,2000;Yue et al.,2016).Tilapia can thrive in substandard environmental conditions,including deficient dissolved oxygen and elevated ammonia levels,in which generally other fish species could not survive in (Fitzsimmons,2000).Several tilapia species are cultured worldwide.These include the blue tilapia (Oreochromis aureus),Mozambique tilapia(Oreochromis mossambicus),Zanzibar tilapia (Oreochromis hornorum),blackchin tilapia (Sarotherodon melanothron),and the most commercially farmed Nile tilapia (Oreochromis niloticus).
Capture fisheries are considered the sole source of fish products before fish farming was introduced.Nile tilapia farming has faced a lot of hurdles and setbacks since it started.Farmed tilapia are genetically inferior compared to their wild counterparts.Their decreased fecundity,growth rate,and survivability (Ordonez et al.,2014;Ansah et al.,2014)have caused lower production and profitability.To alleviate the crisis,breeding experiments were initiated to develop genetically improved Nile tilapia.
In the Philippines,tilapia strains with better growth performance and adaptive capability to survive in different culture conditions were developed (Romana-Eguia et al.,2019;Eguia &Romana-Eguia,2016).Some of the more notable genetically improved strains tilapia strains(Table 1) were: genetically improved farmed tilapia (GIFT);Freshwater Aquaculture Center selected tilapia (FaST);brackishwater enhanced selected tilapia (BEST);and genetically enhanced tilapia-excellent strain(GET-EXCEL) (Magbanua &Ragaza,2020).
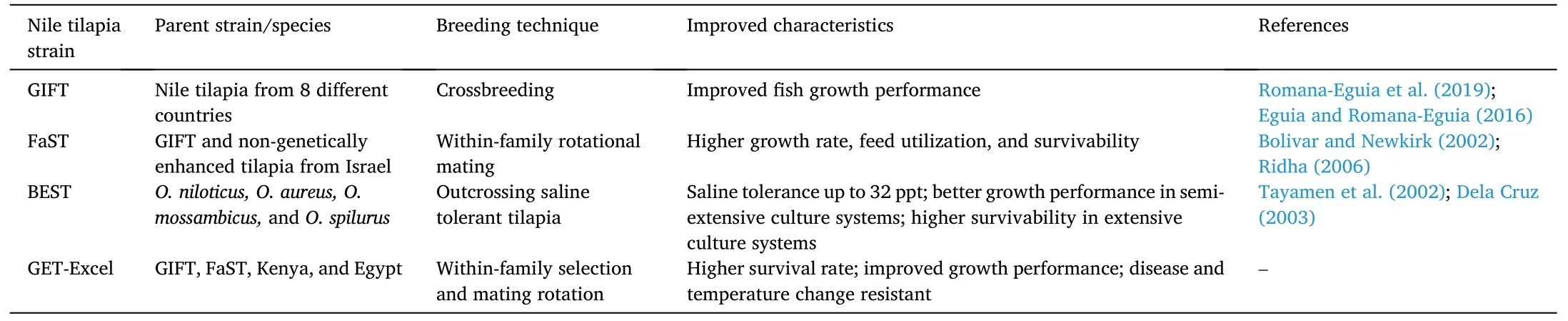
Table 1 Genetically enhanced and improved Nile tilapia O.niloticus developed in the Philippines.
The GIFT strain is the first genetically improved tilapia strain developed with better growth performance over wild and captured tilapia.Nile tilapia strains that came from the Philippines,Israel,Singapore,Thailand,Taiwan,Egypt,Ghana,Kenya,and Senegal,are usually selected for a series of crossbreeding (Magbanua &Ragaza,2020).
The FaST strain was named after the institution that developed its breeding techniques,the Freshwater Aquaculture Center of Central Luzon State University in the Philippines.The progeny developed—FaST strain showed superior qualities such as growth performance,survivability,and feed utilization over non-genetically improved tilapia(Ridha,2006) using within-family rotation mating of a GIFT strain together with an Israel-sourced Nile tilapia strain (Bolivar &Newkirk,2002).
The BEST strain was developed through outcrossing of saline tolerant tilapia,which could tolerate 0–32 ppt salinity (Tayamen et al.,2002).This development may limit the use of freshwater for tilapia farming.The BEST strain has been shown to have greater growth performance in semi-extensive culture systems and better survival in extensive culture systems (Labastida et al.,2015).
The GET-EXCEL strain was developed as a globally competitive tilapia strain.Since inbreeding caused genetic deterioration in the quality of tilapia reared in rural areas,the GET-EXCEL strain was developed with temperature and disease resistant qualities.Moreover,it had greater growth performance and survivability over its predecessors,the GIFT and FaST strains (Dela Cruz,2003).
Recent data show that tilapia ranked fourth biggest species group in 2018 with 6 million tonnes of production,accounting to more than 40%increase from 1990 (FAO,2020).Asia dominated the contribution of the tilapia production at 68.8% followed by Africa at 21.8% (Martínez-Cordero et al.,2021),wherein Nile tilapia was seen to be the most farmed tilapia species.In some countries like the Philippines and Mexico,tilapia ranked second most important farmed fish due to its market potential,production,demand,and sustainability (Martínez-Cordero et al.,2021;Dey &Gupta,2000).In the Philippines alone,tilapia covered 40% of total farmed fish in 2015 valued at USD 470 million in 2015 (BFAR,2016).
2.Dietary plant-based proteins for Nile tilapia
In the aquaculture industry,feeding accounts almost 50% of the total production costs (Mzengereza et al.,2014).The protein source of aquafeeds accounts as the most expensive feed component.Fishmeal is the gold standard protein source due to its balanced amino acid content,which is favorable for proper fish growth and development.
Fishmeal is an industrial product made from wild-caught fish and fish by-products.Overfishing is not a perceived issue fishmeal processing because the fishing industry is generally heavily monitored and managed.Fishmeal has a stable and steadfast production that ranges from 6 to 6.5 tons per year (Miles &Chapman,2006).
Global aquaculture heavily depends on fishmeal for aquafeed production.However,its price has continually inflated due to the increased demand for fish and fish products,which in turn,has immensely impacted the market price.Today,various plant-based proteins are used to partially or completely replace fishmeal to reduce feed costs.
Plant-based protein diets for aquafeeds have long been recognized to be inferior to fishmeal (Hardy,2006) because of insufficient amino acid profile,lower palatability,and presence of anti-nutritional factors(ANFs) that affect the overall cultured fish performance (Gatlin et al.,2007).However,several studies have shown that plant-based protein diets such as corn gluten feed (Wu et al.,1995) and cottonseed meal(El-Sayed,1990) are superior to fishmeal in terms of cost-benefit analysis.For instance,plant-based protein mix consisting of soybean meal,sunflower meal,cottonseed meal,and linseed meal were fed to juvenile Nile tilapia for 16 weeks.The complete replacement of fishmeal with the plant protein mix did not cause staggering differences in the growth performance and carcass proximate composition of Nile tilapia.Although it has lower apparent protein digestibility,the cost-benefit analysis showed that it surpassed fishmeal (El-Saidy &Gaber,2002).
Aside from being a cost-effective and a sustainable replacement for fishmeal,plant-based proteins can support appropriate fish health and growth performance.Recently,Helmiati and Isnansetyo (2021) evaluated the immune response of red tilapia fed fermentedMoringa oleiferaleaves at levels up to 30% fishmeal replacement.Replacing the fishmeal up to 20% fermentedM.oleiferaleaves improved fish immunity by markedly increasing the plasma protein,hematocrit,and leukocrit levels.Moreover,the phagocytic index and activity were notably stimulated (Helmiati &Isnansetyo,2021).
In another study,Nile tilapia were fed dephytinized canola meal as fishmeal replacement between 12.5 and 50%.At 25% fishmeal replacement,growth performance remained unaltered (Mohammadi et al.,2020).Moreover,digestive enzymes (i.e.,lipase,amylase,and alkaline protease),and mucosal innate immunity (i.e.,immunoglobulin content,mucosal lysozyme,alkaline phosphatase,and alkaline protease activity) were not affected by the inclusion of the dietary canola meal.Although the liver showed normal levels of catalase,superoxide dismutase,and glutathione peroxidase values,gene expression of the liver enzymes was upregulated upon inclusion of processed canola meal.Furthermore,intestinal pathological changes were observed,such as,villus detachment and lamina propria expansion in fish fed 30% inclusion level (Mohammadi et al.,2020).
The primary goal of utilizing plant-based proteins is to provide a more economical alternative to fishmeal.The economic efficiency of plant-based proteins is based on increased profits,reduced production costs,and avoided losses.However,finding a cheaper alternative is not the sole criterion.These plant-based proteins must also be sustainably produced without compromising fish growth and health.Hematological and histopathological analysis are conventional tools used to identify overall fish health and response to different proteins and diets.
Some of the plant-based protein sources discussed in this review paper are soybean meal,copra meal,pea meal,and corn meal.These plant sources are readily and widely available globally at lower prices than fishmeal.These plant-based protein sources are also sufficiently proteinaceous to cover and meet dietary protein requirements of many fish species.Although plant-based proteins generally lack amino acids like lysine and methionine (Gatlin III et al.,2007),this is mitigated byminute inclusions of crystalline amino acids.The review paper also covers the different plant protein processes,inclusion levels and effects thereof on Nile tilapia growth,feed,and overall health performance.
3.Soybean-based proteins
Soybean (Glycine maxL.) is one of the most proteinaceous (40%–51%protein content) and low-cost fishmeal replacements (Montoya-Camacho et al.,2019).Having a huge production worldwide,it is very abundant and readily available in the market.Some of the largest contributors of soybean production worldwide are Brazil,the United States,China,and Argentina.Since 1961,the soybean industry has shown increasing production for several decades (Masuda &Goldsmith,2009).Almost 40% or 150.1 million tonnes of soybean products have been exported in 2017 at USD 58 billion (Organisation for Economic Co-operation and Development &Food and Agriculture Organization of the United Nations,2019).Recently,soybean has been recognized as the most traded agricultural commodity because of its three-fold increase in the past 20 years (Montanía,Fernández-Núñez,&Márquez,2021).
Although soybean contains the major essential amino acids for fish growth and development,it can cause growth retardation due to naturally occurring anti-nutritional factors (ANFs).Several processes have been adopted to mitigate the negative effects of anti-nutritional factors on fish performance.Currently,various soybean-based proteins primarily differing in their processing methods are used in aquafeeds.Soybean-based proteins include soybean meal,soybean protein concentrate,soybean protein isolates,soybean peptides,and fermented soybean proteins.
3.1.Soybean meal
Soybean meal (SBM) is widely used as a feed component for fish,pigs,and poultry (Dersjant-Li,2021).In the world trade perspective,SBM accounts to 62.5% of total oilseed-based products at a value of 243 million tons in 2014 (Heuzé et al.,2020).The worldwide production was dominated by China followed by the USA,Argentina,Brazil,and European Union-28.SBM prices remain constant at USD 400 per short ton(USDA,2021).
SBM is commonly prepared by either extracting oils or through mechanical means.In oil extraction,soybeans are firstly subjected to heat treatment at 60◦C.This process does not severely affect the protein content of soybean.It can denaturize some ANFs (Dunford,2012).Hence,it prevents growth retardation when SBM is used as aquafeed.However,overprocessing and overheating may cause reduction of some thermolabile essential amino acids like methionine and lysine (Lokuruka,2011),which are commonly found to be limiting in soybean meal(Gatlin III et al.,2007).After cooking,the seeds are subjected to crushing and dehulling to promote easier solvent extraction.The usual solvents used for soybean oil extraction are hexane and ethanol (Dunford,2012).The whole process removes ANFs and results in a product with 48% crude protein (Heuzé et al.,2020).
On the other hand,a mechanical method for soybean meal preparation includes pressing and extruding processes.Soybeans are dehulled and cracked before passing through a screw press to produce soybean flakes.The flakes are then subjected to a dry extruder.The extruded flakes are fed to a screw press to remove oils (Heuzé et al.,2020).
3.2.Soybean protein concentrate
Soybean protein concentrate (SPC) is another low-cost,yet proteinrich soy-based functional and nutritional feed raw material.In 2010,around 500,000 metric tons of SPC were produced,70% of which were used for human consumption (Hardy,2010).SPC come from defatted soybean meal where carbohydrates and some flavors are removed (Guo,2009),including ANFs like trypsin inhibitors (Lenehan et al.,2007).
The general basic processes involved in SPC preparation are: 60%–80% aqueous ethanol extraction;acid leaching;and moist heat water leaching (Guo,2009).These processes allow the separation of insoluble proteins with carbohydrates via centrifugation producing SPC upon spray-drying.There has been an increasing interest in using SPC as an alternative protein source for aquafeeds due to its notable characteristics and beneficial effects on fish.
SPC contains 65%–67% crude protein.It was found to exhibit high protein and energy digestibilities that are comparable to fishmeal and superior to soybean meal when fed to trout and sea bass (US Soybean Export Council (USSEC),2008).Similarly,SPC can effectively replace fishmeal in Atlantic salmon (Storebakken et al.,2000) and golden pompano (Ren et al.,2021;Wu et al.,2015).Interestingly,sea bream positively responded to SPC inclusion up to 70% inclusion level,as there were no detrimental effects on growth and feed utilization (Biswas et al.,2019).
Perera and Yúfera (2016) have examined the nutritional effects of SBM and SPC as an early diet for juvenile zebrafish.The expression of genes responsible for inflammation,intestinal nutrient absorption,luminal digestion,and extracellular matrix reduction (i.e.,il1b,tnfa,il10,mmp9,mmp13,try,alpi,slc15a1,fabp2,andfabp6) were measured.After 3 days of feeding,all the genes were expressed in zebrafish fed SPC except foril10andfabp6,which act as anti-inflammatory cytokine and bile acid recycler,respectively.On the other hand,SBM-fed zebrafish showed lower expression offabp6,cytokine-related genes (i.e.,tnfa,il10,il1b),and extracellular matrix remodeling genes (i.e.,mmp9,mmp13).An inflammatory response in juvenile zebrafish was observed as the fish’s immediate reaction to dietary SPC (Perera &Yúfera,2016).
3.3.Soybean protein isolate
Like SPC,soybean protein isolate (SPI) commonly starts from defatted soybean meal via aqueous-alkaline extraction (with a pH 7–10).The extraction process collects the soy proteins and soluble carbohydrates.The residue from the extraction usually contains insoluble carbohydrates which are segregated via centrifugation (Guo,2009;Wang et al.,2004).The proteins precipitate upon extraction at isoelectric point at pH 4.5.The last step involves mechanical processing of proteins via decantation and washing before spray-drying (Guo,2009).The whole process allows SPI to contain 85%–90% proteins in dry matter basis(Tang,2019).
SPI has been reported to have high nutrient digestibilities.In a study,Atlantic cod fed SPI showed high protein and energy apparent digestibility coefficients (Tibbets et al.,2006).Moreover,a study showed no significant difference in protein,energy,dry matter,and amino acid apparent digestibility coefficients in juvenile white shrimp (Litopeneaus vannamei) fed SPI-based diets (Cruz-Suárez et al.,2009).
Although SBM,SPC,and SPI can support normal fish growth,deleterious effects were still observed in fish when fed more than the optimum inclusion levels.Deng et al.(2010) compared the effects of dietary cholesterol in Japanese flounder (Paralichthys olivaceus) fed fishmeal-,fish protein concentrate-(FPC),SPC-and SPI-based diets.SPI-based diets increased specific growth rate and feed intake when compared to FPC-based diets.Moreover,dietary cholesterol supplementation increased hepatic lipids except fish fed fishmeal-based diets.Hence,the use of soybean-based products may change the fish response to cholesterol supplementation,causing fatty liver and arteriosclerotic lesion in the fish (Deng et al.,2010).
3.4.Soybean peptides
Soybean peptides are mainly extracted through protein fractionization and protease hydrolysis during fermentation.The isolated peptides usually weigh below 1000 Da (Puchalska et al.,2014).Because they are rich in proline,histidine,and lysine (Chen et al.,1995),soybean peptides contain antioxidant properties like hydroxyl radicals and single oxygen quenching (Peña-Ramos &Xiong,2001;Chen et al.,1998),anticarcinogenic,hypocholesterolemic,and immune-stimulating activities (Guo,2009).
Mamauag et al.(2011) studied the growth and immune response of Japanese flounder fed soy peptide hydrolysate (SPH) up to 40% inclusion as an alternative protein source.Improved feed intake,growth rate,and feed efficiency were evident in flounder fed 20% SPH.However,significant growth reduction was observed when the flounder was fed more than 20% inclusion level.The carcass proximate values,liver and digestive proteolytic enzyme activities,and blood chemical profile (i.e.,urea,total bilirubin,aspartate aminotransferase,and alanine aminotransferase) remained unaltered.However,the total cholesterol levels were lower in flounders fed 10% and 20% SPH.
3.5.Fermented soybean-based proteins
Several studies have tried to limit the effects of ANFs from soybean in the overall fish growth and development.For many years,fermentation of soybeans is used as a method to alleviate and mitigate the problem of naturally occurring ANFs in soybean proteins.Soybeans are first dehulled,soaked at room temperature for 2–3 h,steamed for another 2 h,fermented,and finally incubated (Shrestha,Dahal,&Ndungutse,2010).The most common fermenting agent used for soybean processing isBacillus subtilis(Yang et al.,2007).Its use in soybean processing clearly showed reduction in ANFs (Mukherjee,Chakraborty,&Dutta,2016).
In a study,fermented soybean meal (FSBM) viaBacillus pumillusSE5(up to 80% inclusion level) replaced 35% fishmeal in diets for spotted sea bass (Lateolabrax maculatus) (Rahimnejad et al.,2021).Although it was suggested that FSBM can substitute fishmeal at levels between 26.9% and 37.1%,growth retardation and lower nutrient digestibilities were evident in sea bass fed higher FSBM.Similarly,the fish’s gut structure and enzymatic activities (i.e.,superoxide dismutase and lysozyme activities) were found to be adversely affected.
In white shrimp (Litopenaeus vannamei),however,FSBM sufficiently promoted growth and immune response (Lin &Chen,2022).Although the inclusion levels of FSBM were high (i.e.,25%–100%),there were no differences in growth,feed efficiency,survivability,and hepatopancreas morphology.The shrimps fed 75% FSBM diets exhibited higher hemocyte count,phenoloxidase activity,and apparent digestibility coefficient of dry matter,lipid,and protein,further suggesting that high inclusion levels (i.e.,75% FSBM) do not adversely affect in white shrimp growth and innate immune responses (Lin &Chen,2022).Interestingly,similar results on growth performance,total hemocyte count,and phenoloxidase activity were observed in a separate study by Cherdkeattipol et al.(2021),whenBacillus-fermented FSBM was fed to white shrimp.Moreover,the additional challenge test againstVibrio parahaemolyticusshowed white shrimps exhibited higher survival rate and lesser hepatopancreas bacterial infections when fed 25% FSBM,suggesting improved immune response.
Proper and sufficient fermentation affects the fish response to FSBM(Yamamoto et al.,2010).In a study,rainbow trout fed FSBM fermented viaBacillusspp.for 10 h with 30% water did not cause any detrimental effects on fish intestinal morphology compared to other fermentation conditions.And although lower growth rate was observed,the fermentation of soybean meal increased feed digestibility.
Fermented soy pulp (FSP) was also observed as a feasible fishmeal replacement in African catfish diets.Results showed improvement in the specific growth rate,weight gain,and condition factor in African catfish fed 50% FSP.Moreover,blood parameters such as lymphocytosis and red blood cell count,and gut bacterial load were elevated in the same treatment.On the other hand,the total protein,globulin,and albumin values in the control group were lower than those in the FSP-fed fish groups.Intestinal histology of fish fed 50% FSP showed normal villus structure,goblet cell arrangement,tunica muscularis,and epithelial barriers when compared to the other treatments (Kari et al.,2021).In another study,the same FSP inclusion level was also observed to elicit favorable effects on the digestive enzyme activities,immune-related gene expressions,and muscle amino acid profile (Kari et al.,2022).Increased protein digestibility,lipase and amylase activities,and muscle amino acid profiles (isoleucine,arginine,leucine,and histidine) were reported in African catfish fed 50% FSP.Moreover,50% inclusion of FSP resulted in upregulation of immune-related genes such as transforming growth factor-β1,nuclear factor-kβ,lactose synthase B protein (lyzg),and heat shock proteins (hsp90a),further suggesting that FSP inclusion improves and enhances health-related indices (Kari et al.,2022).
3.6.Growth response of Nile tilapia when fed soybean-based proteins
3.6.1.Soybean meal
SBM lacks sulfur-containing amino acids,such as,cysteine,methionine,and lysine (El-Sayed,1999),which are needed by tilapia.The lacking amino acids can be supplemented with crystalline amino acids(CAA).Nile tilapia fed soybean meal-based diet supplemented with CAA was found to exhibit increased productive value,feed utilization,and fat and energy retentions (Goda et al.,2007).Interestingly,the addition of methionine to SBM diets significantly enhanced feed retention ratio,specific growth rate,protein gain,and retention efficiency in hybrid tilapia (O.niloticus×O.mossambicus) (Figueiredo-Silva et al.,2015).Moreover,the supplementation of 0.9% dietary methionine in corn-soy meal significantly increased growth performance and whole-body protein content of Nile tilapia (Prabu et al.,2019).
The acceptable and optimum SBM inclusion levels in Nile tilapia diets vary.Generally,as shown in Table 2,normal Nile tilapia growth response was reported in SBM inclusion levels between 25% and 80%(Zhao et al.,2011;Llanes &Toledo,2011;El-Saidy &Gaber,2002;Soltan,Ibrahim,Fatma,&FathElbab,2001).Without CAA supplementation and in a long-term feeding duration (i.e.,6 months),SBM can replace up to 25% fishmeal in Nile tilapia diets without affecting growthperformance (Pervin,Halima,et al.,2020).Higher body weight,body length,and specific growth rate were observed in tilapia fed 0 and 25%soybean meal-based diets.In another study,up to 50% SBM in diet showed no differences in growth and feed performance and carcass proximate composition (Ahmad et al.,2020).Growth retardation was observed in tilapia fed more than 50% SBM.
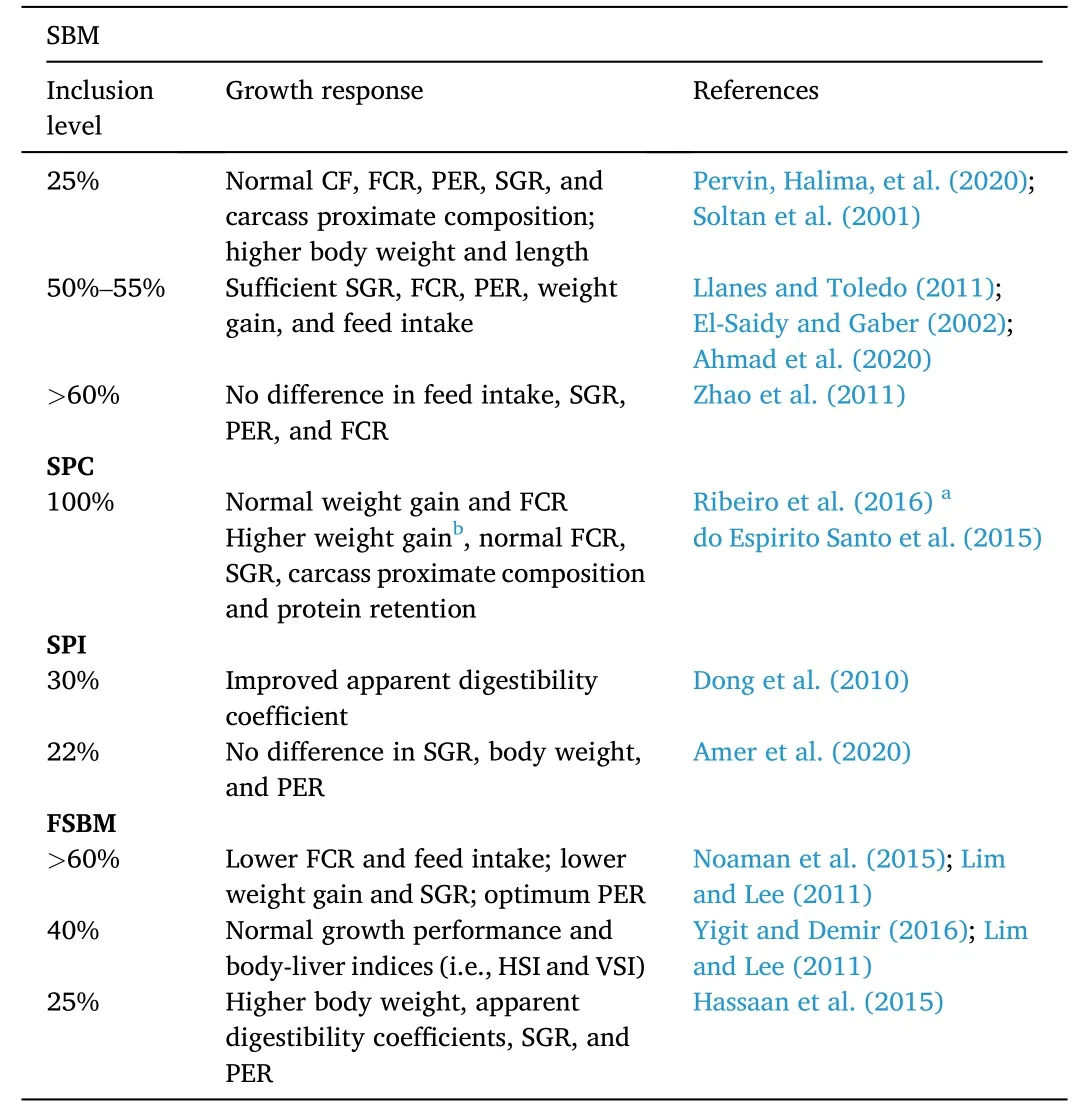
Table 2 Effects of soybean-based proteins on growth response of Nile tilapia O.niloticus.
3.6.2.Soybean protein concentrate
There are only a few studies that explored the use of SPC,as sole or in combination with other protein sources,fishmeal replacement for Nile tilapia diets.When SPC was combined with meat and bone meal and completely replaced fishmeal in the diet,no significant differences in weight gain and feed conversion ratio were observed (Ribeiro et al.,2016).In a two-part study,SPC was tested with and without CAA (i.e.,methionine and threonine) supplementation in Nile tilapia diets.In the first trial,growth and feed performances and carcass and liver compositions were not directly affected by high dietary SPC inclusion (i.e.,33%–100%) even without CAA supplementation (do Espirito Santo et al.,2015).However,in the second trial,SPC with CAA supplementation had increased weight gain without affecting other growth parameters.Interestingly,apparent digestibility coefficients of dry matter and crude protein were higher in tilapia fed SPC without CAA supplementation (do Espirito Santo et al.,2015).
Although studies on the use of dietary SPC in Nile tilapia diets are limited,more recently,however,dietary SPC has been evaluated positively as a partial fishmeal replacement for other fish species such as rainbow trout (Collins et al.,2012),hybrid grouper (Epinephelus fuscoguttatus×E.lanceolatus) (Mohd Faudzi et al.,2018),juvenile tench(Carral et al.,2021),Coho salmon (Yoo et al.,2021),common sole(Saleh et al.,2021),blackhead seabream (Kalhoro et al.,2018),Japanese flounder (Deng et al.,2006) and juvenile tambaqui (Martins et al.,2020).
3.6.3.Soybean protein isolate
Only a few studies have shown that SPI can replace up to 30% of fishmeal in Nile tilapia diets.Dong et al.(2010) compared the apparent digestibility coefficients of different plant-based protein (i.e.,corn gluten meal,soybean meal,corn germ meal,soy protein concentrate,fermented soybean meal,malt sprouts,and soy protein isolate) diets for hybrid tilapia (O.niloticus×O.aureus).Like hybrid tilapia fed fishmeal-based diets,high dry matter,gross energy,and crude protein were evident in fish fed SPI.Amer et al.(2020) also observed that protein efficiency ratio,specific growth rate,and weight gain increased when methylated SPI replaced 22% fishmeal in Nile tilapia diets.In other fish species like rohu (Fawole et al.,2016) and juvenile starry flounder (Song et al.,2014),however,SPI-based protein diets were only found to partially replace fishmeal.
3.6.4.Fermented soybean meal
When soybean meal was subjected to solid state fermentation usingSaccharomyces ceverisiae,the fermentation process resulted in increased hydrolyzed amino acids,total protein content,and reduction of ANFs such as trypsin inhibitor and phytic acid (Hassaan et al.,2015).Nile tilapia fed 25% solid state fermented soybean had higher final body weight,protein efficiency ratio,specific growth rate,and apparent digestibility coefficients.Moreover,lowest gross energy and dry matter were observed in the absence of the dietary solid state fermented soybean (Hassaan et al.,2015).In another study,however,no significant differences in Nile tilapia growth performance when fed fermented soybean diet at high replacement values between 75% and 100% Noaman et al.(2015).In the same study,lower FCR and feed intake were observed.
In combination with other proteins,fermented soybean meal was found to partially replace fishmeal up to 50% for Nile tilapia diets.A combination of fermented soybean meal and whey induced highest growth rate and feed efficiency at 30% inclusion level.Moreover,the hepatosomatic index,viscerasomatic index,feed conversion ratio,and protein efficiency ratio remained unaltered.At more than 50% inclusion level,lower growth and feed efficiency (Yigit &Demir,2016;Lim &Lee,2011) were observed.
3.7.How do soybean-based proteins affect the body indices of Nile tilapia?
3.7.1.Soybean meal
Replacement of fishmeal with SBM up to 75% sufficiently induced proper growth of juvenile hybrid tilapiaO niloticus×O.aureus.Aside from normal growth response,blood serum chemistry showed normal hepatic and intestinal amylase activities based on the serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase values,which were also similarly reported in another study (Abdelghany,2003).Moreover,the immune response of tilapia decreased when fishmeal was replaced by soybean meal at levels higher than 50%.Beyond this level,lysozyme and superoxide dismutase activities were reduced (Lin &Luo,2011).Changes in the enzymatic activities in fish are due to several factors including fish feed utilization,feeding behavior,and presence of ANFs (Lin &Luo,2011).
In a recent study,normal liver health was evident upon fishmeal replacement with soybean as IGF-1 expression was upregulated (Ismail et al.,2020).The apparent improvement of growth upon soybean supplementation increasesigf-1expression as it corresponds to teleost somatic growth (Hack et al.,2018).
GIFT Nile tilapia strains were also tested to determine the optimum protein level of SBM in the practical diet.Dietary protein levels were prepared at 25%–45% of each iso-caloric diet.Dietary protein levels tended to be directly proportional to the final weight,lipid content,hepatosomatic index,and condition factor of the GIFT strains.Hematological and immunity-related parameters (i.e.,red blood cells and white blood cells,lysozyme and superoxide dismutase activities,and immunoglobulin M) were not directly affected by varying protein levels.However,41.6% dietary protein was required for proper growth of the Nile tilapia GIFT strains (Kpundeh et al.,2015).
Although SBM inclusion in Nile tilapia diets is promising (Table 3),other studies have shown that it can induce enteritis in several fish species (Booman et al.,2018;Krogdahl et al.,2015;Zhang et al.,2021).This is partly due to the different ANFs that are naturally occurring in soybeans (Sahlmann et al.,2013).Enteritis can be described through evaluation of the histological changes in the intestinal tissues,including thickening of submucosa and lamina propria,increase in goblet cells and immune-related cells,and reduction of mucosal folds (Urán,2008).In a study,plant protein-based diets including soybean meal,sunflower meal,hydrolyzed feather meal,rice bran,rapeseed meal,and dried distillers’ grains with solubles were fed to Nile tilapia to evaluate the changes in the intestinal morphology.Although the highest apparent digestibility coefficients were exhibited by SBM-based diets,only the SBM-based diet fed tilapia exhibited abnormal intestinal tissue changes(i.e.,increase in goblet cells and thickening in both submucosa and lamina propria) (Tran-Ngoc et al.,2019).
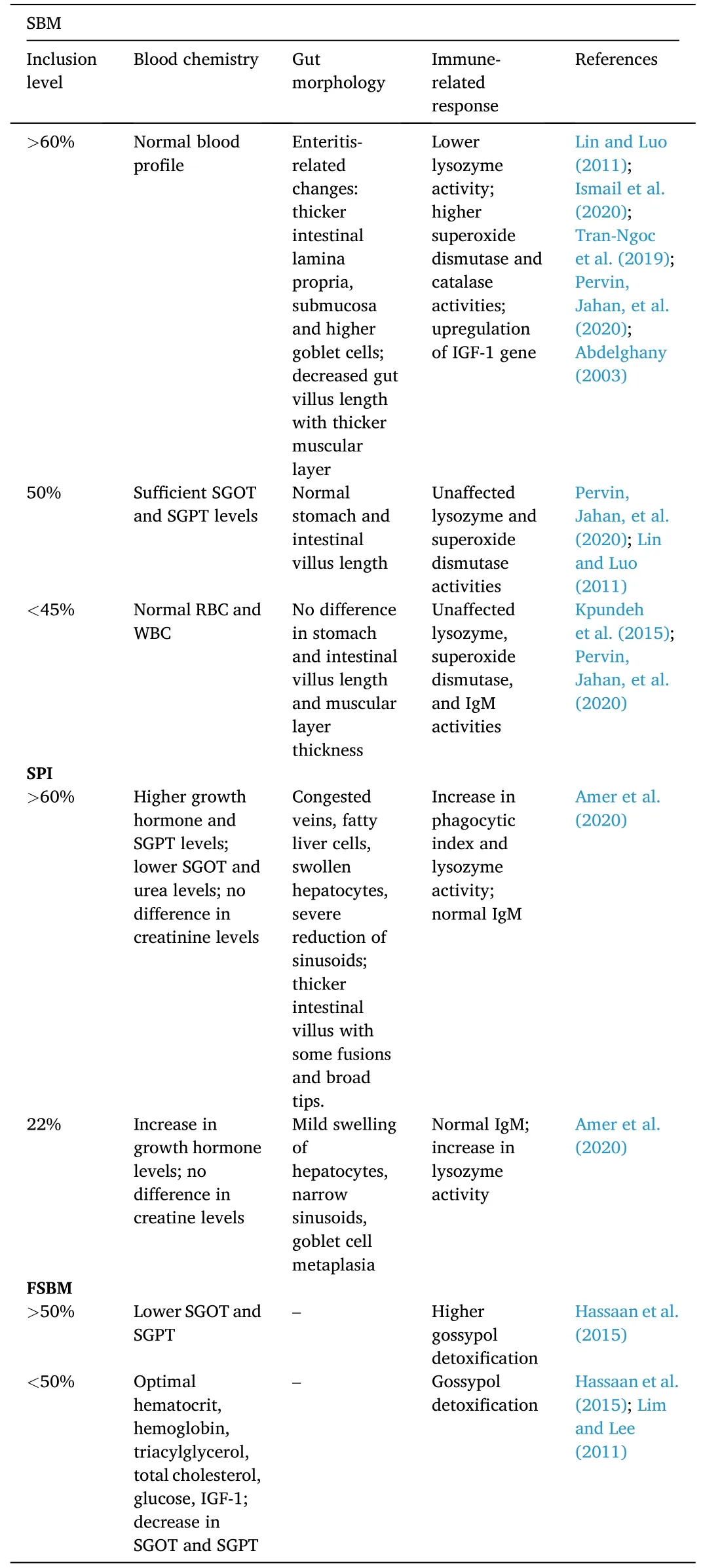
Table 3 Hematological and histological effects of soybean-based protein diet on Nile tilapia O.niloticus.
In a separate study by Pervin,Jahan,et al.(2020),villus height values in the stomach and intestine of Nile tilapia juvenile were higher when fish were fed with SBM replacement up to 50%.This was observed to be inversely proportional to the muscular thickness.This effect was also observed in another study,in which there was an evident goblet cell reduction when soybean meal replaced fishmeal at higher levels (Obirikorang et al.,2020).The histological change may be triggered by the presence of cellulose in plant-based feed as it needs longer time passing through the fish gut (Pervin,Jahan,et al.,2020).Moreover,shortening of villus height and increase in muscular thickness likely indicate the occurrence of induced enteritis commonly caused by soybean meal-based diet (Kumar et al.,2020).There were also higher protease,amylase,and lipase activities when SBM was absent in the diet (Pervin,Jahan,et al.,2020).Liver enzymes such as superoxide dismutase andcatalase were found to be higher in groups fed 100% SBM-based diet(Pervin,Jahan,et al.,2020).These digestive and hepatic enzymes indicate higher oxidative stress defense for the fish (Radhakrishnan et al.,2014).
3.7.2.Soy protein isolate
Methylated soy protein isolates (MSPI) which partially substituted fishmeal (22%–88%) induced higher immune response in Nile tilapia when challenged withAeromonas hydrophila.The post-bacterial challenge showed raised levels of IgM,phagocytic index,and lysozyme activity (Amer et al.,2020).Moreover,several hepatic histopathological changes were observed.Liver sinusoids reduction was least observed when fishmeal was substituted with the lowest level of MSPI (i.e.,22%)while severe reduction with fatty hepatocytes and congested hepatic veins was observed when the highest substitution level (i.e.,88%) was used.Furthermore,intestinal histopathological changes in tilapia fed the least substitution level exhibited a slight increase of goblet cells while intermediate to high levels of MSPI elicited thicker villi,broad tips,and some fusions in the intestinal tissues (Amer at al.,2020).
3.7.3.Fermented soybean meal
Blood chemical profile of the Nile tilapia,specifically levels of hematocrit,hemoglobin,glucose,triacylglycerol,and cholesterol were not altered when FSBM was used as an aquafeed ingredient (Lim &Lee,2011).The fermentation of soybean meal with the use ofAspergillus oryzaeas catalyst decreased the gossypol toxicity that severely affects the liver of the fish (Lim &Lee,2011).In a related study,similar blood chemical parameters plus IGF-1 were also unaffected by FSBM incorporation in Nile tilapia diets at 25%–50% inclusion levels.However,decreases in aspartate aminotransferase and alanine aminotransferase levels were noted (Hassaan et al.,2015).
3.8.Extending beyond the limits of soybean-based proteins
For many years,soybean-based proteins have been one of the major plant-based proteins used in aquafeeds.Some even argue that soybeanbased proteins are no longer mere substitutes to fishmeal but are major protein sources in and of itself.The growing demand for soybean meal is the main driver for its use and sustainability in aquafeeds.However,the presence of several ANFs hinder its use in practical diets for fish and shrimp species.Several processing methods have already been designed to mitigate the problem.Processing steps such as oil extraction,heat treatment,bioprocessing,and even mixing plant-proteins reduce and even at times remove ANFs from soybeans (Chen et al.,2013;Montoya-Camacho et al.,2019).
Like other plant-based proteins,soybean-based proteins fail to completely replace fishmeal not only due to high levels of ANFs (Francis et al.,2001;Hardy,2010) but because of limiting essential amino acids such as methionine,threonine,and lysine (Figueiredo-Silva et al.,2015;Goda et al.,2007;Gonzales et al.,2007).Nonetheless,although an added expense,supplementation with CAA usually compensates for the limiting amino acids.
For example,crystalline methionine supplementation has been extensively used in many plant-based proteins.A minimum dose of 1 g/kg methionine supplemented in plant-based proteins induced weight gain in Nile tilapia (El-Wahab et al.,2016).Clearly,supplementation of methionine and other limiting amino acids in soybean-based diets supported growth and development of Nile tilapia (Michelato et al.,2018).
Lysine is another limiting amino acid in soybean-based diets.It can directly affect fish growth and fillet yield (Furuya &Furuya,2010).About 7.12 g lysine is required in every 100 g protein for Nile tilapia fingerlings (Ove &Eze,2013).Remarkable increase in feed conversion efficiency and weight gain were observed in tilapia fed soybean-based diets with lysine supplementation.Previous studies have shown the necessity for lysine supplementation in practical diets of Nile tilapia to increase growth rate and weight gain (Nguyen &Davis,2016) and to improve muscle formation via hypertrophy and hyperplasia (Michelato et al.,2018).A combined supplementation of lysine and methionine in a plant-based feed forO.niloticusalso resulted in improved feed intake and feed conversion ratio even when plant-based protein levels were increased,and fishmeal levels were decreased in the diets.The improved protein efficiency ratio saved about 30% feed cost (Abdel-Warith et al.,2019).
4.Copra-based proteins
Copra meal is abundant in and commonly sourced from tropical countries.It is produced from dried kernel nuts of the coconut palmCocos nucifera.In copra meal preparation,the kernel nut is firstly dehusked and dried until it reaches 6%–8% water content.The dried coconuts are then subjected to grounding,flaking,and cooking to reach an even lower moisture content of~3%.The oil is extracted via an expeller machine or through solvent extraction to create the so-called copra cake(Canapi et al.,2005).The resulting copra cake is pelletized.
Coconuts are widely available and distributed in tropic countries such as the Philippines.The Philippines produces almost 42% of the global copra meal,which is at 1.86 million tons copra supply in 2009(FAO,2011).The Philippines also exported 0.5 million tons of copra meal in the same year which accounted to 62% of the total production in the country (Heuzé et al.,2015).
Copra meal is commonly used as a feed ingredient for ruminants and poultry.Compared to soybean meal,copra meal contains a lower protein content,high crude fiber (Hertrampf &Piedad-Pascual,2000),and limiting amino acids (Tacon et al.,2009).Although considered inferior to soybean-based proteins,low inclusions of copra meal have not negatively affected growth response of aquatic animal species including grass carp fry (Hasan,Macintosh,&Jaunceyn,1997) and rohu(Mukhopadhyay,2000).
4.1.Growth response of Nile tilapia when fed copra-based proteins
There are limited studies on the use of copra meal as a fishmeal replacement (Table 4) in aquatic animals.Copra meal has been used as an aquafeed in high value aquatic animal species such as black tiger shrimp (Apines-Amar et al.,2019),grouper (Mamauag et al.,2019) and common carp (Yusup &Nugroho,2017).Unprocessed copra meal has been shown to directly replace 300 g/kg fishmeal for Nile tilapia without any negative effects on feed intake (Obirikorang,Amisah,Fialor,&Skov,2015).High inclusion of copra meal (680 g/kg) supplemented with sesame meal (to make up for the lack in methionine) can also replace fishmeal in Nile tilapia diets without eliciting detrimental effects(Obirikorang et al.,2016).In a separate study,copra meal can be incorporated in tilapia diets up to 30% (Olude et al.,2008).Although copra meal is inexpensive and proteinaceous,copra meal lacks methionine and contains ANFs such as phytic acid,protein inhibitors,and saponins (Francis et al.,2001).Nevertheless,its crude protein,apparent dry matter digestibility,digestible energy,and gross energy were found sufficient for Nile tilapia culture (Santos et al.,2009).As copra meal contains high crude fiber,fermentation usingRhizopussp.efficiently increased its digestibility for saline tilapia seeds (Harlina et al.,2021).
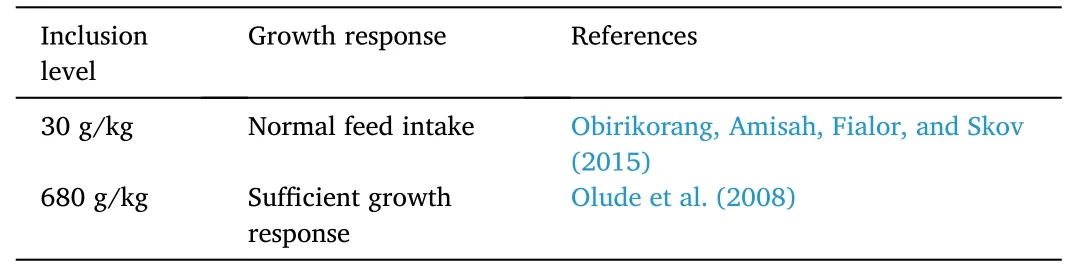
Table 4 Effects of copra-based proteins on growth response of Nile tilapia O.niloticus.
4.2.How do copra-based proteins affect the body indices of Nile tilapia?
In a recent study (Table 5),fermented copra meal and guar meal usingSaccharomyces cerevisiaewas used to replace fishmeal in Nile tilapia diets.Increase in weight gain and lipid and protein contents were observed at 25% replacement (Dileep et al.,2021).The digestiveenzymes (i.e.,protease and amylase) were at normal levels in feeds containing less than 25% copra meal.The liver enzymes (i.e.,superoxide dismutase,catalase,glutathione S-transferase,and thiobarbituric acid reactive substances),and blood serum parameters such as aspartate aminotransferase and alanine aminotransferase were also unaffected by the replacement.
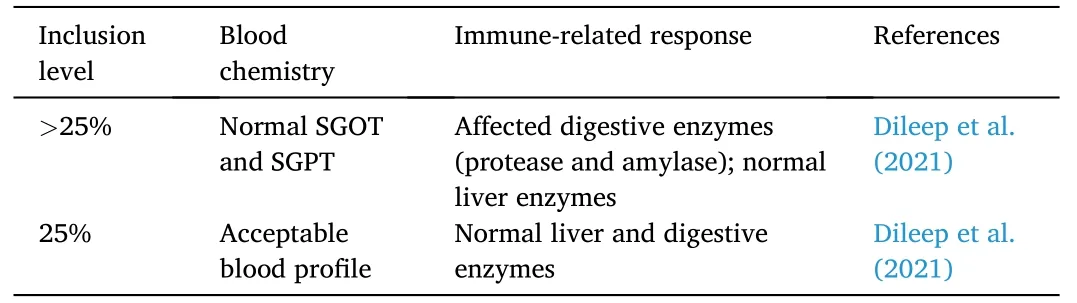
Table 5 Hematological and histological effects of copra-based protein diet on Nile tilapia O.niloticus.
4.3.What hinders the use of copra-based proteins?
Like other plant-based protein diets,the use copra meal as a fishmeal substitute faces challenges due to it imbalanced amino acid profile.Copra meal lacks methionine,lysine,and threonine (Swick,1999;Sundu,Kumar,&Dingle,2005).Moreover,the presence of high arginine content in copra meal results in amino acid antagonism,causing lower utilization of lysine in fish (Heuzé et al.,2015).Moreover,heat treatment during copra meal preparation can decrease the lysine content.Thus,amino acid supplementation can be used to alleviate the problem to improve its potential as a fishmeal replacement in aquafeeds.Also,an improved extraction method that could prevent or reduce amino acid degradation would allow its further use in aquafeeds.
5.Pea-based proteins
Pea (Pisum sativum) is commonly known in agricultural countries as a food and feed source.It is considered as one of the most important legumes together with soybean,other beans,and groundnuts (Muehlbauer &Tullu,1997).Pea production worldwide rose from 1961 to 2008 from 7.3 million tonnes to 9.8 million tonnes with China as the top producer (Watts,2011).Pea meal is considered a good candidate as a fishmeal replacement for marine and freshwater organisms.Pea meal contains moderate amounts of protein with limited amounts of lysine and methionine and high levels of carbohydrates (Gatlin III et al.,2007).It also contains nutrient-limiting compounds like oligosaccharides and alkaloids.Its crude protein is reported to range between 20 and 25%(Abd El-Salam &El-Shibiny,2016),70–80% of which are globulins,namely,convicilin,legumin,and vicilin (González-Pérez &Arellano,2009).Although it has a lower protein profile compared to soybean meal,pea meal contains low fat and high starch content that can provide significant energetic value in fish diets (Glencross,2016).
Pea meal is routinely prepared via a mechanical method,a heattreatment,or a combination of both (Heuzé et al.,2017a).Mechanical method includes grinding and dehulling of peas that reduce indigestible fiber and tannins.In a study,the process of dehulling peas can minimally increase its crude protein from 25.5 to 27.7% and increase its digestibility (Booth et al.,2001).On the other hand,heat treatment includes roasting,autoclaving,and extruding of peas (Poncet et al.,2003).Though heat treatment can decrease ANFs,overheating can also cause protein modification that diminish the suitability of peas as a feed ingredient (Corbett,1997;Perrot,1995).
5.1.Growth response of Nile tilapia when fed pea-based proteins
Only a number of studies have evaluated the effects of pea meal as a fishmeal replacement for Nile tilapia diets (Table 6).In a study bySantiago et al.(2002),pea meal can substitute 10%–50% fishmeal without negatively affecting feed efficiency and feeding activity.Moreover,weight gain and survival rate of tilapia were seen similar across the treatment groups.Feed conversion ratio and protein efficiency ratio were also found unaffected by replacing 92% of fishmeal with soybean meal,copra,and rice bran in a 2:1:1 ratio and supplemented with pea meal in increasing concentrations.The same results on growth performance and feed utilization were observed in separate studies when soaked pigeon pea and cow pea seed meal replaced fishmeal up to 45% (Ndau &Madalla,2015) and 50% (El-Saidy &Saad,2008),respectively.Moreover,tilapia juveniles grew unimpeded when pea meal replaced 30% of the fishmeal (Schulz et al.,2007).
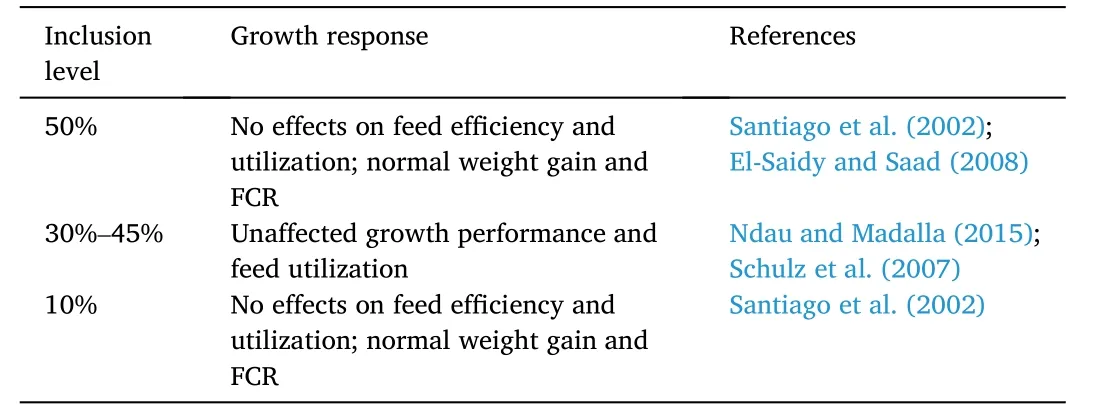
Table 6 Effects of pea-based proteins on growth response of Nile tilapia O.niloticus.
5.2.How do pea-based proteins affect the body indices of Nile tilapia?
Varying the feeding frequencies of pea-based protein diets seems to impact the growth and immune response of Nile tilapia.Although no significant differences in tilapia growth,cortisol levels,respiratory burst,and phagocytic activities of renal macrophages extracted postinfection withYersinia ruckeriwere recorded between tilapia fed twice and eight times a day with pea-based protein diets,the renal macrophages from fish fed the latter were more efficient in combatting the bacterial cells within 24 h compared to fish fed the former (Garcia &Villarroel,2009).Thus,the presence of various stressors in fish such as feed suppression can lead to improper immune response and pathogen invasion.Moreover,the partial substitution of raw and treated cowpea meal at 200 g/kg showed no structural effects on tilapia fingerling pancreatic cells,although there were vacuoles observed (Olivera-Castillo et al.,2011).The presence of vacuoles commonly denotes fatty degeneration in fish liver (Velisek et al.,2011).
5.3.What hinders the use of pea-based proteins?
Pea meal is a cheap and readily available fish and poultry raw feed ingredient.It is proteinaceous enough to be used as a partial fishmeal replacement.However,it also contains ANFs that can decrease fish performance.There are only few studies that evaluated pea-based proteins as an aquafeed ingredient for Nile tilapia diet.Moreover,the available studies,if any,have only focused on how processing affects crude protein and ANF contents of pea-based proteins.Further studies involving efficient and cost-effective pea meal preparation methods,optimization of the dietary inclusion levels of pea meal,variation and combination of pea meal with other plant-based proteins,and evaluation of the effects of pea meal on gut and hepatic morphology and immune response of Nile tilapia are necessary.
6.Corn-based proteins
Corn (Zea maysL.) is another agricultural and food product used as a fishmeal replacement in aquafeeds.Corn is cultivated around the world with the United States as the top producer.Corn production had increased from 1991 to 2001.Global corn production was around 875,226,630 tons in 2012 (FAO,2012;Ranum et al.,2014).The total production was dominated by the USA at 31%,followed by China and Brazil at 24% and 8%,respectively (Ranum et al.,2014).There are different types of processed and unprocessed corn being sold in the market today,such as whole grain,cracked,steamed-rolled,ground,steamed-flaked,and wet-milled,otherwise known as corn gluten.
Corn meal contains high crude protein (60%–70% in dry matter basis) and low ANFs (Wu et al.,2022;Hernández et al.,2020).However,it is deficient in essential amino acids,specifically,lysine and arginine(Hardy,2010;Wu et al.,2022;Hernández et al.,2020).As a candidate fishmeal replacement,earlier studies reported that corn gluten meal is highly digestible for gilthead sea bream and European sea bass at 20%–25% inclusion levels (Gatlin et al.,2007).
In a recent study,corn protein concentrate was tested as a partial fishmeal replacement (30–120 g/kg) in the diets of rainbow trout(Oncorhynchus mykiss).Corn protein concentrate is a processed corn byproduct in which the non-protein nutritional contents of corn are reduced enzymatically.Growth parameters such as weight gain,SGR,CF,and feed utilization were not affected by corn protein concentrate inclusion up to 90 g/kg.Beyond 90 g/kg inclusion level,growth reduction was evident.Similarly,filler proximate composition was not altered up to 90 g/kg inclusion level.However,blood white blood cell count,alanine aminotransferase,lactate dehydrogenase,and lysozyme activity increased in rainbow trout fed high inclusion levels (i.e.,90–120 g/kg).Hence,an optimum inclusion range between 81 and 82.2 g/kg corn protein concentrate was recommended (Hosseini Shekarabi et al.,2021).
When supplemented with probiotics,corn gluten more efficiently enhanced growth and health of certain fish species.A 30% inclusion of corn gluten meal supplemented with 2 g/kg of probiotic improved the overall performance of common carp (Cyprinus carpio) fingerlings.Optimal growth and feed performance,blood chemistry (i.e.,hematocrit,hemoglobin,red blood cells,and white blood cells),and carcass proximate composition were noted (Hussain et al.,2021).
Similar results were also associated with combining corn-based proteins with other plant protein sources.When fishmeal was partially replaced by soybean meal,corn gluten meal (CGM),combination of soybean and corn gluten meal,and combined soybean meal and wheat gluten meal in diets of Atlantic cod,the plant protein combination supported adequate growth rate,feed utilization,and apparent digestibility coefficients (Hansen et al.,2006).Moreover,unlike other plant proteins especially soybean-based proteins,the plant protein combination did not induce enteritis-related changes in the intestinal tissue of the cod.The hepatic tissues were also unaffected while the heat shock protein expression (i.e.,HSP70 and HSP90) remained at normal levels.Hence,even at high inclusion levels,combining different plant-based proteins did not trigger stress-related response in Atlantic cod (Hansen et al.,2006).
6.1.Growth response of Nile tilapia when fed corn-based proteins
Several studies observed that even at high dietary inclusion levels,corn-based proteins did not adversely affect fish response (Table 7).Growth,feed conversion ratio,and carcass proximate composition of Nile tilapia remained unaltered when fed diets incorporated with high levels corn protein concentrate (i.e.,50–190 g/kg).Nonetheless,growthresponse seemed to flatten at levels more than 100 g/kg dietary corn protein concentrate (Khalifa,Belal,El-Tarabily,Tariq,&Kassab,2018).
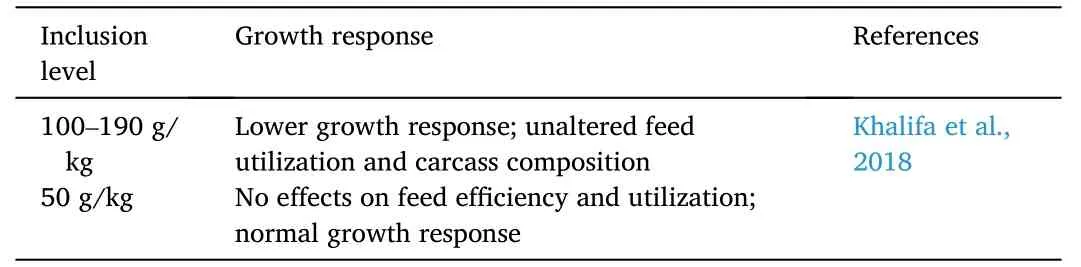
Table 7 Effects of corn-based proteins on growth response of Nile tilapia O.niloticus.
Studies have also compared various corn-based raw materials in Nile tilapia aquafeeds.In a long-term feeding trial,the fish were fed isonitrogenous diets containing four corn co-products,namely,highprotein distiller’s dried grains,corn protein concentrate.distillers’dried grains with solubles,and corn gluten meal that replaced fishmeal.The two distiller’s dried grains provided better growth response while lowering FCR (Herath et al.,2016).Although the fish fillet quality and composition were unaltered,higher fillet lipid content was attributed to polyunsaturated fatty acids and linoleic acids (18:2n6) in distiller’s dried grains.On the other hand,corn protein concentrates and corn gluten meal induced formation of higher levels of saturated fatty acids like palmitic acid (16:0) (Herath et al.,2016).
6.2.How do corn-based proteins affect the body indices of Nile tilapia?
The effect of corn-based proteins on carcass composition and indices seems to vary.Increasing the levels of dietary corn gluten meal even up to 100% did not alter growth,feed utilization,carcass composition,and blood chemical profile of Nile tilapia (Metwalli,2013).In a recent study,although growth was not affected,high levels (i.e.,50 g/kg) of dietary corn husk resulted in higher carcass protein.Liver enzymes such as superoxide dismutase and glutathione peroxidase with exemption to catalase,were also affected (Galeana-López et al.,2021).However,there were no differences observed in the fish gut microbiota.The gut microbiota was dominated by Proteobacteria,Bacteroides,and Fusobacteria In a separate study,the total coliform in the intestines of tilapia fed corn protein concentrate decreased with increasing dietary protein concentrate (Khalifa et al.,2018).Moreover,compared to tilapia fed fishmeal-based diets,the stomach size was bigger while the stomach walls were thicker,which could indicate increased requirements for gastric acids and digestive enzymes.
Extruded corn-based proteins combined with other plant protein sources and supplied with probiotics can promote intestinal health in Nile tilapia.When combined plant-based proteins (i.e.,soybean,wheat,and corn) supplemented with probiotics are primarily used as dietary protein source,tilapia exhibited thicker epithelial layer in the middle intestine,which could imply higher absorption rate due to the increased surface area (Nakandakare et al.,2013).
6.3.What hinders the use of corn-based proteins?
The demand of corn as a food product competes with the demand as an animal feed,resulting in inflated corn prices through the years.Until 2007,corn is priced at USD 257 per tonne but the year after,it increased to USD 575 (Hardy,2010).The economics of corn demand,supply,and market price are not the only factors limiting its full potential as an aquafeed.
High inclusion levels of corn-based proteins can trigger enteritis,resulting in a lowered immune response (Bai et al.,2019).Moreover,the deficiency of essential amino acids such as lysine and arginine hinder its suitability as a complete fishmeal replacement.Optimized corn meal production,combining corn-based proteins with other plant protein sources,and supplementing with crystalline amino acids or probiotics may optimize its use as a functional feed ingredient for Nile tilapia.
7.Palm kernel-based proteins
Palm kernels are usually produced from twoElaeisspecies,Elaeis guineensisandElaeis oleifera.E.guineensisgenerates 0.5 t oil per hectare per year (Heuzé et al.,2016),making it the mainElaeisspecies used for palm oil production.Its production increased from 1996 to 2005 by 10%annually since palm oil is a major staple for cooking in Africa and Southeast Asia.Almost 80% of palm oil is produced from Indonesia and Malaysia and contributed 21.5 million tons and 17 million tons in 2010,respectively (FAO,2012).
The palm fruit is used to produce two types of oils: palm oil and palm kernel oil.The fruit’s mesocarp is used to extract palm oil,which contains high levels of oleic and palmitic acids (Basiron,2005).On the other hand,palm kernel oil,which is produced from the fruit’s kernel,contains high amounts of lauric acid (Gervajio,2005).Traditional extraction process starts from boiling and pounding of the fruit,followed by soaking the pulp in water until the oil precipitates.The oil is collected and boiled to remove the remaining water content (Heuzé et al.,2016).The extraction of the kernel also produces palm kernel meal that is mainly used for animal feeds.The kernels are subjected to solvent extraction and results in a fibrous and protein-rich feed that contains 0.5–3% oil (Chin,2001;Pickard,2005).
Ng and Chen (2002) tested the effects of palm kernel meal (PKM) as a partial substitute of soybean meal in the diets of hybrid Asian-African catfish (Clarias macrocephalus×C.gariepinus) fingerlings.Soybean was partially replaced by PKM at levels of 0,10%,20%,and 40%.The growth performance was not altered when the catfish was fed diets with inclusion up to 20% PKM.However,beyond 20%,the catfish experienced lower growth and feed utilization.Although the lipid carcass and hepatosomatic indices of fish fed high PKM supplementation were lower,the hematocrit level across test groups were not affected.
In another study,using the same dietary levels,PKM replaced fishmeal in the diets of Malaysian prawn (Macrobrachium rosenbergii).There were no detrimental effects in weight gain,specific growth rate,and feed utilization in prawns fed diets with less than 40% PKM.Moreover,the addition of feed attractants such as shrimp meal and squid meal could enable PKM supplementation levels that are higher than 40%(Kader et al.,2018).
PKM was also found to be a possible fishmeal replacement for Pacific white shrimp (Litopenaues vannamei) feed.Isonitrogenous diets (35%crude protein) containing 0,25%,50%,and 75% of PKM were formulated.Generally,diets with more than 25% PKM had a negative effect on shrimp growth performance.Nonetheless,the carcass composition of shrimps fed 25% PKM showed comparable protein and lipid values with the control group (Shamsuddin et al.,2021).
7.1.Growth response of Nile tilapia when fed palm kernel-based proteins
In an early study on Nile tilapia,when PKM replaced fishmeal at 0–30% levels,the growth and feed utilization parameters,such as,final weight,weight gain,SGR,FCR,and protein efficiency ratio (PER),were improved in fish fed diets with 15% PKM.Higher or lower than 15%PKM supplementation led to poor fish growth performance and feed conversion (Omoregie &Ogbemudia,1993).Similarly,Obirikorang,Amisah,Agbo,et al.(2015);reported that high inclusions of PKM can negatively affect apparent digestibility coefficients,growth performance,and carcass composition of Nile tilapia.To alleviate negative effects,the optimum dietary inclusion level of PKM was estimated at 20% (Table 8).
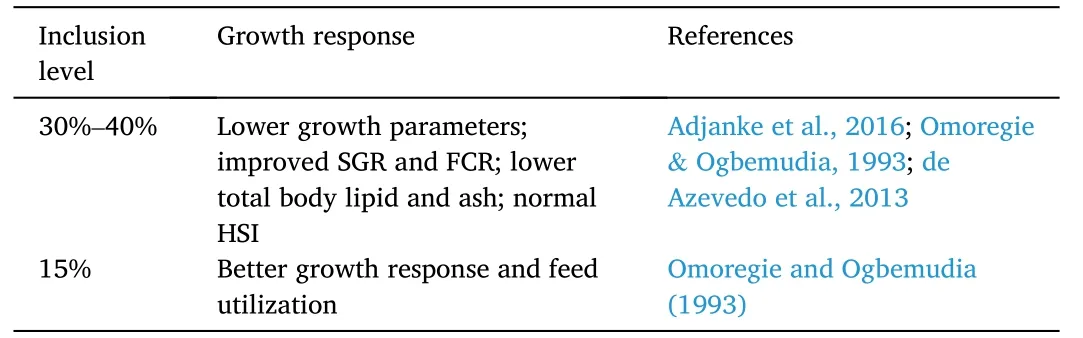
Table 8 Effects of palm kernel-based proteins on growth response of Nile tilapia O.niloticus.
Another study investigated the growth response,feed utilization,and carcass composition of Nile tilapia fingerlings when fed dietary palmkernel meal.There were five isonitrogenous diets formulated with increasing PKM supplementation (0–40%).Remarkably,improved SGR and FCR values were observed in fish fed 30 and 40% PKM.The total body protein values of Nile tilapia fingerlings increased with dietary PKM.However,lipid and ash content decreased with PKM inclusion(Adjanke et al.,2016).
In contrast to other oilseed-based products,the fermentation of palm kernel may negatively affect fish growth.High inclusions of fermented palm kernel cake (fermented withTrichoderma longibrachium) in red tilapia diets led to lower protein digestibility,growth response,and carcass composition values (Iluyemi et al.,2010).The lower digestibility in the Mozambique tilapiaO.mossambicusfeed was attributed to the presence of aflatoxin B1in the fermented palm kernel cake (Lim et al.,2001).Mycotoxins such as aflatoxins can cause poor fish performance and health (Magbanua &Ragaza,2021).
7.2.How do palm kernel-based proteins affect the body indices of Nile tilapia?
Cooking PKM in water at 30% inclusion level can increase its palatability and consequently improve digestibility and serum indices of the cultured species (Table 9).In a study,after feeding the Nile tilapia with cooked palm kernel meal and then subjecting to two days of starvation,the fish were dissected at different timestamps (0–24 h) to evaluate changes in the intestinal histology.The digestive transit was calculated by comparing the content weights of the gut compartments during and after the fasting period.The fish fed control and cooked PKM had slower food digestive transit,resulting in improved nitrogenous excretion and total protein in the serum (Adjanke et al.,2021).An increase in the digestive transit reduces absorption as its contact time with the intestinal mucosa and digestive enzymes are decreased (Burel &Médale,2014).In a separate study,30% of palm kernel cake (PKC) did not affect FCR and survival rate of juvenile Nile tilapia.Moreover,feed intake,SGR,and CF were increased upon PKC supplementation.The carcass composition,plasma cholesterol,and hepatosomatic indices were also unaffected by PKC inclusion (de Azevedo et al.,2013).
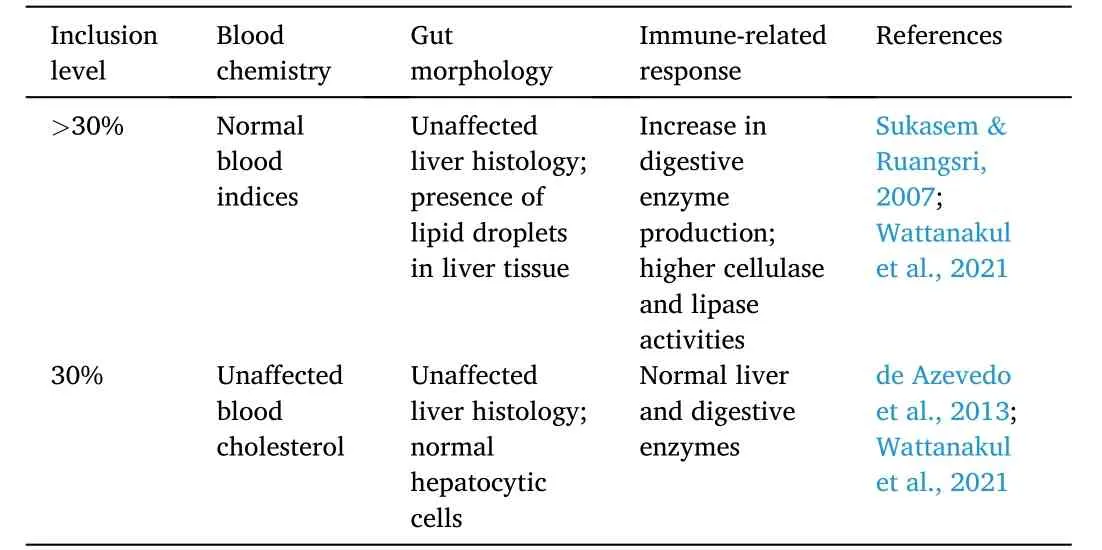
Table 9 Hematological and histological effects of palm-based protein diet on Nile tilapia O.niloticus.
Fermented palm kernel meal (FPKM) was found to be a sufficient partial replacement in soybean meal-based diets of sex reversed red tilapia (Oreochromis niloticus×O.mossambicus).SBM was partially replaced with FPKM (0–100%) in a 12-week feeding trial.The feed utilization parameters were unaffected by FPKM inclusion across test groups and resulted in a better growth performance and protein synthesis capacity at 50% FPKM.Fish fed more than 25% FPKM had increased digestive enzyme activities,such as,pepsin,trypsin,lipase,and cellulase.Moreover,actin and myosin qualities were not affected across test groups.Fish fed 50% FPKM showed normal carcass composition,liver histology,and hematological indices.Fish fed FPKM higher than 50% showed increased cellulase and lipase activities,decreased actin-myosin ratio,and lower mean cell hemoglobin concentration(Wattanakul et al.,2021).Similarly,45% inclusion of PKC led to normal growth performance,survival rate,hepatocytic cells,and blood parameters of sex-reversed red tilapia.However,the liver histology showed lipid droplets in fish fed 45% PKC (Sukasem &Ruangsri,2007).
7.3.What hinders the use of palm kernel-based proteins?
Studies have shown that palm kernel meal can partially replace fishmeal.Relatively high inclusions of palm kernel meal often result in poor fish growth.Like other plant-based proteins,it faces many factors that lowers its efficiency.Palm kernel meal has limiting amino acids,anti-nutritional factors,and comparatively low crude protein content(Chakaborty et al.,2019).Further feed ingredient processing may enable higher inclusion levels and replace more fishmeal in the diet.
8.Microalgae-based proteins
Microalgae are unicellular aquatic organisms that may exist in colony or independently.Microalgae are photosynthetic and produce byproducts such as hydrocarbons which can be used for fuel production(Randrianarison &Ashraf,2017).These autotrophic organisms measure from 5 μm likeChlorellato the size ofSpirulinaat 100 μm (Becker,2013).Aside from industrial use,microalgae are also used as dietary aquafeed ingredients.
Chlorellais the first microalga ever cultured in the 1960s in Japan followed bySpirulinain the 1970s in USA and Mexico (Heuzé et al.,2017b).Microalgae production in 2010 was at 97104 t,a marked increase from the 16483 t produced in 2003.In aquaculture,microalgae are fed in intensive and extensive culture of shrimp,bivalves,and carp.Microalgae are either fed alive or processed.These organisms are first preserved for a longer shelf-life.The preservation process includes spray-drying,freeze-drying,refrigeration,and microencapsulation.Although the process helps in prolonging storage,it degrades some nutrients including unsaturated fatty acids that may affect fish growth(Becker,2013).
A study reported that defatted microalgae meal (DMM) produced fromHaematococcus pluvialisis a feasible fishmeal replacement for Pacific white shrimp (Litopenaeus vannamei) diets at 12.5% inclusion level.Dietary DMM inclusion resulted in higher growth rate and lower FCR (Ju et al.,2012).Moreover,in a separate study,a brighter pigmentation together with an increase in growth,survival,and feed utilization was observed in the giant freshwater shrimp (Macrobrachium rosenbergii) fed 5%–20% of dietarySpirulina(Nakagawa &Gomez-Diaz,1995).Spirulinawas reported to completely replace fishmeal for Indian carps such as catla (Catla catla) and rohu (Labeo rohita) and Gibel carp (Carassius auratus gibelio) (Cao et al.,2018;Nandeesha et al.,2001) diets.A recent study reported that juvenile blunt snout bream had varied responses to dietary Spirulina (Arthrospira platensis) supplementation.Enhanced growth performance and feed utilization efficiency was observed in fish fed 3% Spirulina.Moreover,improvement in apparent digestibility and antioxidant capacity was observed in fish fed 9 and 1.5% Spirulina,respectively (Jiang et al.,2022).
8.1.Growth response of Nile tilapia when fed microalgae-based proteins
Studies have shown that microalgae can efficiently replace fishmeal for Nile tilapia diets (Table 10).Growth performance parameters such as SGR,percent average weight gain,and feed efficiency ratio increased at 30%A.platensisinclusion level in juvenile Nile tilapia diets.Moreover,carcass composition and liver indices (hepatosomatic and viscerasomatic indices) were improved at the same replacement level.However,significant growth retardation was observed in fish fed 75%Spirulina (Velasquez et al.,2016).
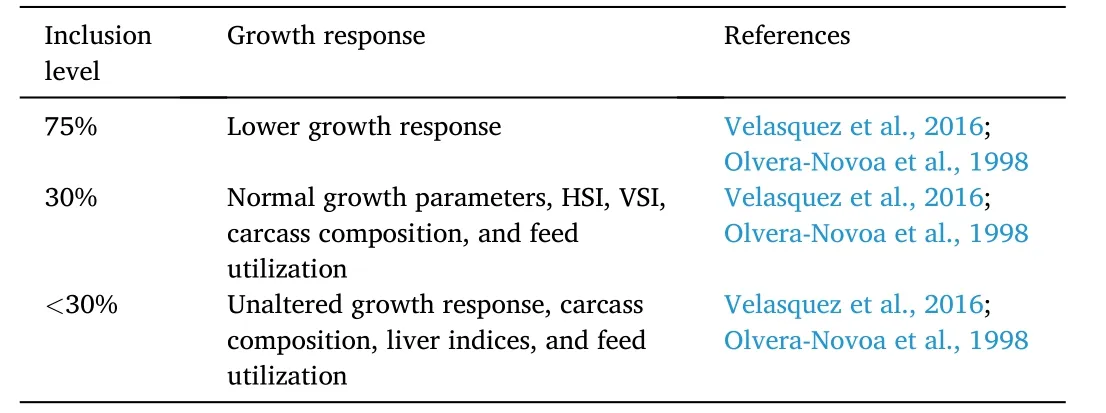
Table 10 Effects of microalgae-based proteins on growth response of Nile tilapia O.niloticus.
Spirulina maximacan partially replace fishmeal inO.mossambicusfry diets.In a study,growth and feed utilization parameters of fish fed 20 and 40%Spirulina maximawere on par with fish fed fishmeal-based diet.Albeit the growth response decreased in fish fed 60–80%,the carcass composition of fish across test diets remained unaffected (Olvera-Novoa et al.,1998).
Another study utilizedNannochloropsis salinaas the sole dietary protein source in diets of Nile tilapia.N.salinafed tilapia were compared to those which were fed fishmeal-based and soybean meal-based diets.Fish fed dietaryN.salinahad similar growth performance with fish fed fishmeal-based and soybean meal-based diets.Remarkably,fish fedN.salinashowed better FCR and PER than fish fed soybean meal.Moreover,the total n-3 polyunsaturated acids and n-3/n-6 fatty acid ratios ofN.salinafed fish were observed to be similar with those in fish fed fishmeal diet and higher than those in fish fed soybean meal-based diet (Gbadamosi &Lupatsch,2018).
8.2.How do microalgae-based proteins affect the body indices of Nile tilapia?
In several studies,microalgae supplementation in Nile tilapia diets has improved hematological indices in varying inclusion levels(Table 11).At a 60-day feeding trial,majority of the blood chemicalindices,such as,cholesterol,glucose,aspartate aminotransferase,alanine aminotransferase,creatinine,high-density lipoprotein cholesterol,and uric acid values were found to be unaffected by Spirulina supplementation up to 75% inclusion level (Velasquez et al.,2016).
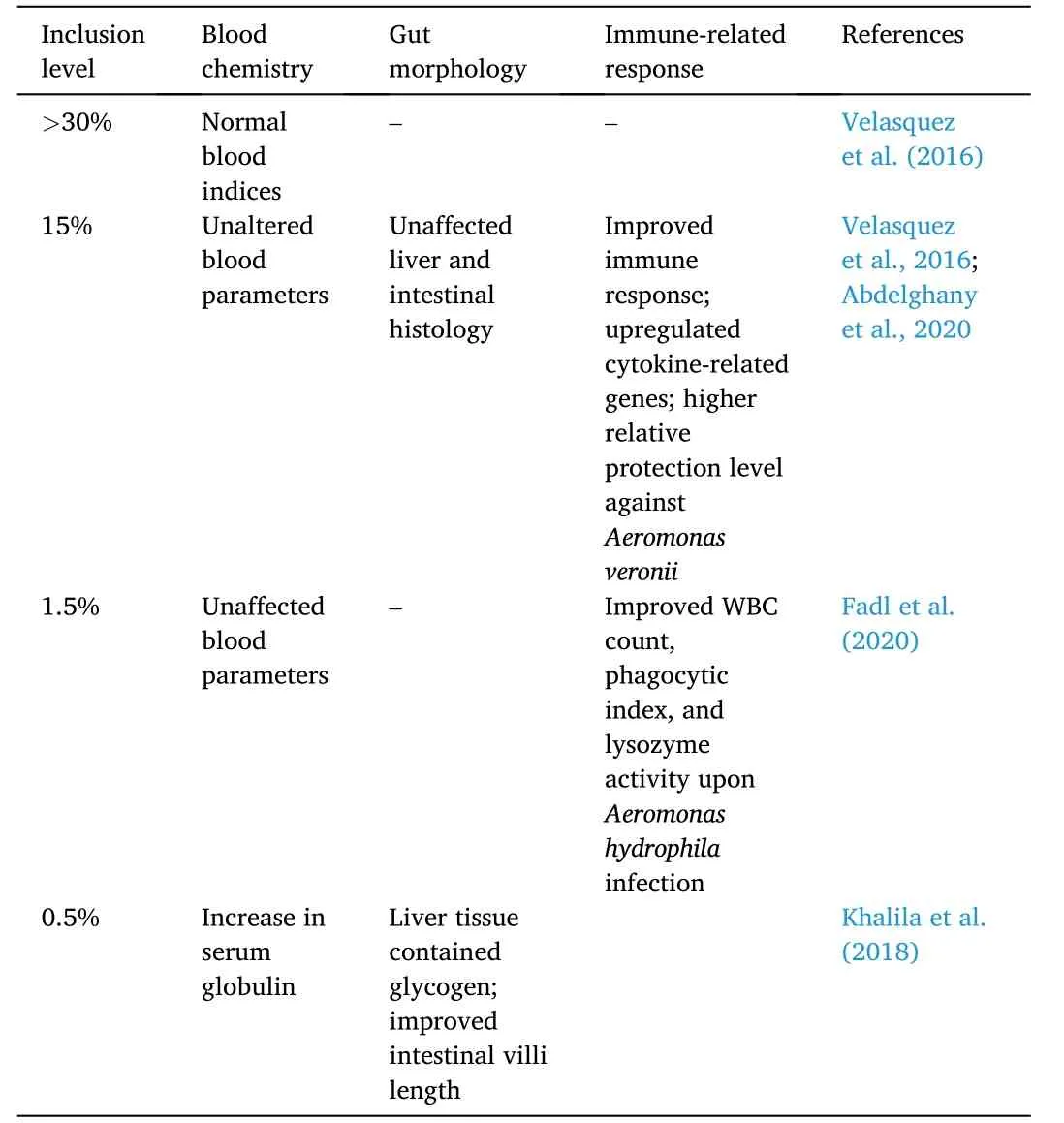
Table 11 Hematological and histological effects of microalgae-based protein diet on Nile tilapia O.niloticus.
Nannochloropsis oculatawas also tested as a partial fishmeal replacement for Nile tilapia juveniles.Growth parameters such as SGR,weight gain,and feed intake increased in fish fed 5%N.oculatawhile FCR decreased as supplementation was increased to 10 and 15%.Moreover,the serum indices were decreased at 15% supplementation.However,among the serum indices,aspartate aminotransferase and alanine aminotransferase values remained unaffected.Notably,nitric oxide,nitro blue tetrazolium,and serum lysozyme activities were improved in fish fed 5%N.oculata.Moreover,the same fish group showed normal intestinal and hepatopancreatic tissue morphology.Cytokine-related genes (i.e.,il-8,ifn-γ,tgf-ß,andtnf-α) were upregulated in fish fed diets 10% and 15% supplementation while thesodantioxidant gene was downregulated.The immune response of Nile tilapia improved in fish fed 10% and 15% as the relative protection level of these fish challenged withAeromonas veroniiwas observed to be higher across test groups.The higher relative protection was attributed to the short-chain fatty acids inN.oculatawhich are reported to possess antimicrobial properties (Abdelghany et al.,2020).
A minute supplementation ofAnabaenasp.(i.e.,1.5%) was reported to have increased Nile tilapia immune response.The cyanobacterium supplementation improved the growth performance,total body protein composition,and serum profile of Nile tilapia.When the tilapia fedAnabaenadiets were challenged withA.hydrophila,higher white blood cell count and lysozyme and phagocytic activities were evident and attributed to the antibacterial properties of substances in the cyanobacteria (Fadl et al.,2020).
Combining microalgae with other plant proteins has shown a synergistic effect.Alternative plant proteins such as SBM,CGM,and distiller dried grains were supplemented with 0.5% dietary Spirulina in Nile tilapia diets.There was better growth response,survival,FCR,and nutrient utilization in fish fed SBM supplemented with dietary Spirulina.Additionally,the same test group exhibited an increase in serum globulin,glycogen-rich hepatic histopathology,and a larger area of absorption due to improved intestinal villi length (Khalila et al.,2018).
8.3.What hinders the use of microalgae-based proteins?
Microalgae are rich in biochemical constituents needed by fish.Nonetheless,some microalgae species accumulate heavy metals from their environments (Lum et al.,2013),which can consequently affect fish growth.Moreover,industrial scale microalgal production is still limited by several factors such as contamination issues,high production costs,and excretion of industrial wastes and effluents (Ansari et al.,2021).These issues also pose health risks for both fish and human consumption.There is a need to investigate high inclusion levels of microalgae as fishmeal replacers in aquafeeds as most of the studies available only focus on low microalgal inclusions or supplementation.
9.Seaweed-based proteins
Seaweeds are commonly utilized for human and animal consumption.It contains amino acid,vitamins,minerals,and bioactive compounds.Seaweeds gained popularity in aquaculture due to its protein content (Wassef et al.,2013) and immense productivity over terrestrial plants such as soybean.Reports described that seaweed production can reach up to 50 t per hectare per year (Neori et al.,2004).Moreover,recent data showed that seaweed production increased from 3.8 million t to 19 million t between 1990 and 2010 (Heuzé et al.,2017c).Commonly used seaweeds in aquaculture are brown algae (Phaeophyceae),green algae (Chlorophyceae),and red algae (Rhodophyceae).
In seaweed meal production,the harvested wet seaweeds are passed through a hammer mill with a small screen or mesh for fine grinding.The fine particles are then dried from 700 to 800◦C before cooling down to 70◦C.The stored seaweeds contain at least 15% moisture (Heuzé et al.,2017c).
Although the protein content is low,seaweeds have been utilized as a partial fishmeal replacement for various aquatic organisms like Japanese flounder (Ragaza et al.,2021),Atlantic cod (Walker &Berlinsky,2011),black tiger shrimp (Anh et al.,2018),Pacific white shrimp (Qiu,Neori,Kim et al.,2018),freshwater prawn (Felix &Brindo,2014),and European seabass (Batista et al.,2020).Studies have shown that seaweed supplementation supports the overall growth and health of cultured fish species.Ragunath and Ramasubramanian (2022) recently reported that 4.5% inclusion of the brown leafy rolled-blade algaePadina boergenesiiimproved the growth and feed utilization parameters,carcass composition,and blood serum parameters of mringal or white carp (Cirrhinus mrigala).When challenged withPseudomonas aeruginosa,fish fed dietaryP.boergenesiiexhibited higher survival rate,white blood cell count,and lysozyme activity due to its inhibitory activities.In a separate study,two brown seaweeds,Saccharina japonicaandUndaria pinnatifidaand two red seaweeds,Gracilia lemaneiformisandPorphyra haitanensis,were supplemented in the diets of golden pompanoTrachinotus ovatus.Growth parameters such as weight gain and final body weight were improved in fish fedU.pinnatifidaandP.haitanensis.Furthermore,the latter had lower FCR compared to the control group.The expression of digestion related genes (cartinine palmitoyltransferase 1andchymotrypsinogen),antioxidant-related gene (catalase),and non-specific immunity related-gene (lysozyme) were also higher in fish fed dietaryP.haitanlensis.The intestinal villi length and muscular thickness were improved in the same fish group (Xie &Niu,2022).
9.1.Growth response of Nile tilapia when fed seaweed-based proteins
Several studies have shown that seaweeds can partially replace fishmeal in Nile tilapia diets without adverse effects (Table 12).In a study,three seaweeds,namely,Gracilaria vermiculophylla,Porphyra dioica,andUlvaspp.partially replaced fishmeal by 10% in the diet.ExcludingG.vermiculophylla,the growth and feed utilization parameters,such as,final body weight,SGR,PER,FCR,and feed efficiency were similar across test groups with normal carcass composition.On the other hand,G.vermiculophyllafed fish showed lower growth response and feed utilization (Silva et al.,2015).Similar results were reported in a separate study when 20%Enteromorpha intestinaliswas used for monosex Nile tilapia fry diets.There were no detrimental effects on final body weight,SGR,weight gain,FCR,and PER in fish fed 20%E.intestinalis.Beyond the optimum level,the parameters slightly decreased.The whole body proximate was also unaffected in fish fed 10%–50%E.intestinalis(Siddik et al.,2015).On the contrary,the incorporation of dietaryGracilaria arcuatain Nile tilapia diets reduced growth performance of the fish beyond the 20% inclusion level without negatively affecting carcass composition and feed utilization (Younis,Al-Quffail,Al-Asgah,Abdel-Warith,&Al-Hafedh,2018).
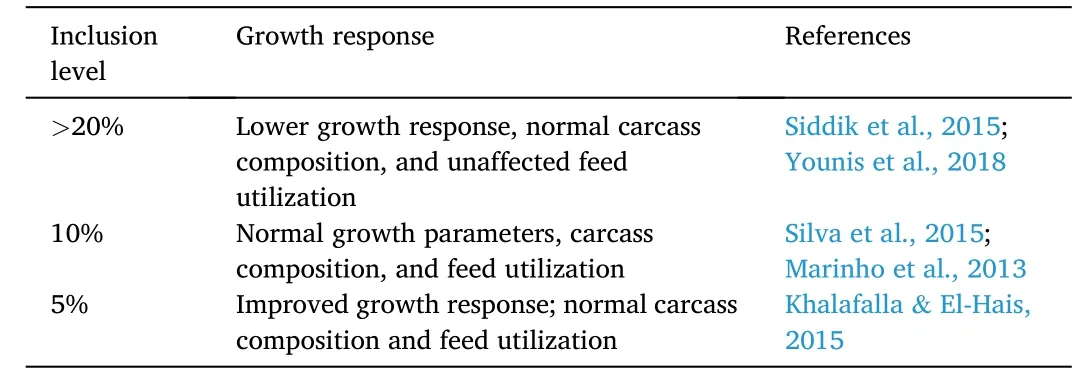
Table 12 Effects of seaweed-based proteins on growth response of Nile tilapia O.niloticus.
Supplementation ofUlvaspp.promoted growth and feed utilization parameters in Nile tilapia.The final body weight and SGR were highest at 15 and 20% inclusion levels.The FCR increased together with inclusion level while the PER was highest at 10% inclusion.Although fishfed fishmeal-based diet showed better carcass composition,the total body lipid composition was highest at 20% inclusion level of the seaweed (Marinho et al.,2013).Low inclusion (i.e.,5%) ofUlva rigadain Nile tilapia fingerlings diet improved growth performance and carcass composition (Khalafalla &El-Hais,2015).
9.2.How do seaweed-based proteins affect the body indices of Nile tilapia?
Low inclusion of seaweeds has improved health indices of Nile tilapia(Table 13).In a study,a 10% inclusion ofUlvaspp.andPorphyra dioicaresulted in intact intestinal villi lengths and diameters in Nile tilapia juveniles (Silva et al.,2015).The innate immunological parameters such as lysozyme and peroxidase activities were unaltered upon 10% supplementation ofUlvaspp.(Valente et al.,2016).Moreover,alternative complement pathway (ACH50) increased in fish fed the dietary seaweed.Aside from normal intestinal health,5%Ulva rigadasupplementation in Nile tilapia diets promoted fish liver health as aspartate aminotransferase and alanine aminotransferase remained stable (Khalafalla &El-Hais,2015).Although there were slight increases in blood cells and hemoglobin values,blood protein fractions were similar with fish fed fishmeal-based diet.
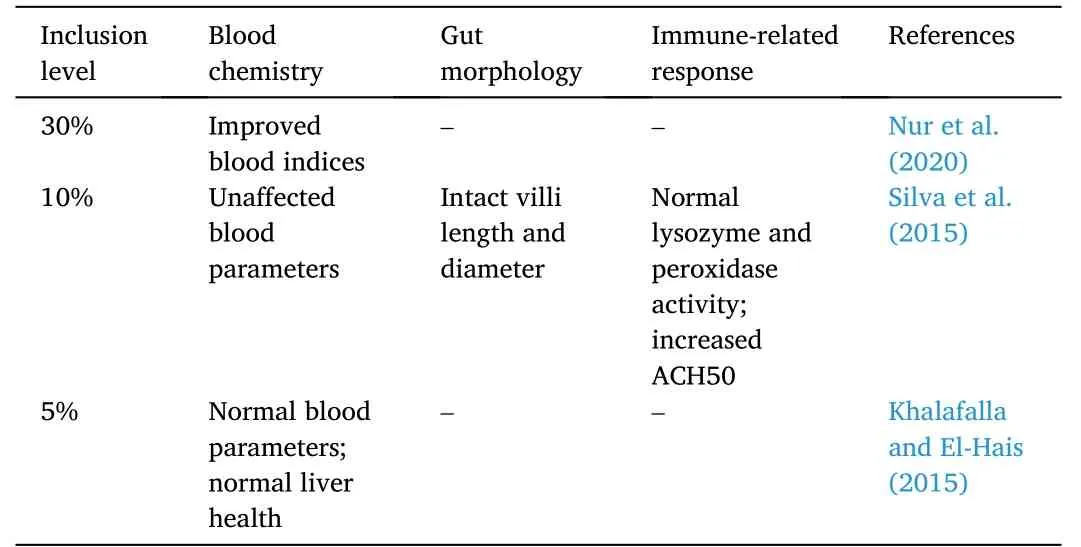
Table 13 Hematological and histological effects of seaweed-based protein diet on Nile Tilapia O.niloticus.
In a separate study,hematological data were reported to be unaffected up to 30% inclusion ofUlvaspp.but with slight detrimental effects on growth response and whole-body proximate values(Suryaningrum &Samsudin,2020).In contrast,Nur et al.(2020),increasingHypnea musciformissupplementation up to 30% resulted in better growth response and hematological profile.
9.3.What hinders the use of seaweed-based proteins?
Unlike other plant-based proteins that usually contain limiting amino acids like methionine,threonine,and lysine,seaweeds or macroalgae contain sufficient essential amino acids needed by fish (Hua et al.,2019).However,the total amino acid concentration of seaweeds is markedly lower than fishmeal (Angell et al.,2016).Moreover,the high carbohydrate content of seaweeds limits its adequacy as a total fishmeal replacement.Innovations in seaweed preparation,such as,extraction and isolation of non-protein components,is necessary to maximize seaweed use in aquaculture feeds (Hua et al.,2019).
10.Possible approaches for accentuating the use of plant-based proteins
The key direction of finding fishmeal alternatives is to improve fish performance while reducing feed costs.The transition of using plantbased proteins has been a challenging quest as fish response seems to be species-specific.Hence,the utilization of alternative protein sourcesshould be optimized depending on the cultured fish species.In most studies within this review,the complete replacement of fishmeal via plant-based proteins negatively affected the overall fish growth and health.With the currently available technology,genetic engineering of selected plant species in accordance with fish nutritional requirements can specifically address the problem of fishmeal replacement.Although this is a long-term pursuit,genetic manipulation can potentially maximize the use of plant proteins as aquafeeds.
Physico-chemical approaches in feed preparation can also alleviate the concern on using plant proteins.The presence of ANFs greatly affects the overall fish performance.Thus,reduction or removal of ANFs can improve fish growth and health.For example,soaking (Olude at al.,2008;Ndau &Madalla,2015) and irradiation (Thongprajukaew et al.,2015) of plant proteins positively affected the growth performance of Nile tilapia (Thongprajukaew et al.,2015).Dehulling and autoclaving may also lead to reduction of ANFs in plant-based ingredients (Ganzon Naret,2019).Fermentation technologies can also increase the suitability of plant-based proteins as a fishmeal replacement.
Another major problem in utilizing the full potential of plant-based proteins in aquafeeds is the limiting amino acids in plant proteins.A potential solution is dietary supplementation with CAA that is proven to compensate the lacking amino acids and to increase fish performance(Ove &Eze,2013;Nguyen &Davis,2016;El-Wahab et al.,2016).Limiting amino acids vary depending on plant species.Amino acid imbalance,especially in diets using different plant proteins may lead to amino acid antagonism.
Supplementing dietary plant-based proteins with probiotics can help in enhancing the immune response of fish especially against disease.Probiotics contribute to the microbial balance in the gut of the fish.For example,the administration of probiotics likeBacillusspp.improved the immune response of Nile tilapia (Aly et al.,2008).
11.Beyond the horizon: plant-based proteins as primary protein sources,not just substitutes
Plant-based proteins are low-cost and nutrient-rich sources that can partially or completely replace fishmeal.The inclusion of dietary plantbased proteins provides sufficient fish growth and enhanced immune health and reduces the total feed costs.
Currently,soybean,copra,pea,corn crops,palm kernel,microalgae,and seaweeds have been efficiently used to replace fishmeal in aquafeeds for Nile tilapia and other aquatic animal species.These crops have been shown to promote adequate growth and immune response in Nile tilapia.There are still many unexplored plant-based proteins.Since these plant protein sources generally lack some essential amino acids and are rich in anti-nutritional factors,innovative preparation methods and supplementation protocols could fully exploit and optimize their nutritional quality.
These plant-based proteins,especially soybean,are not just substitutes,replacements,or supplements for fishmeal.These readily available crops should be considered as primary protein sources for aquaculture.In the coming years,global aquaculture would certainly move from fishmeal to plant protein use in aquafeeds.The only hindrance for plant-based proteins to reach full potential as primary protein source is their demand and use for human consumption.Their demand as human food counterbalances their importance as an animal feed.
CRediT authorship contribution statement
Tzodoq Obrero Magbanua:Writing– original draft,preparation,Visualization.Janice Alano Ragaza:Conceptualization,Methodology,design of Methodology,Writing– review &editing,Supervision.
Acknowledgments
The first author would like to thank the Accelerated Science and Technology Human Resource Development Program of the Department of Science and Technology (DOST),the Loyola Schools (LS) Office of the Associate Dean for Graduate Programs,and the LS School of Science and Engineering Dean’s Office for generously funding his graduate research studies.The second author wishes to acknowledge the Hubert Curien French-Filipino Partnership-Science for the People Researchers’Mobility Program under the DOST-Philippine Council for Agriculture,Aquatic and Natural Resources Research and Development.
 Aquaculture and Fisheries2024年1期
Aquaculture and Fisheries2024年1期
- Aquaculture and Fisheries的其它文章
- The roles of polysaccharides in tilapia farming: A review
- The effects of mixed prebiotics in aquaculture: A review
- Growth performance,fatty acid profile,gut,and muscle histo-morphology of Malaysian mahseer,Tor tambroides post larvae fed short-term host associated probiotics
- Evaluation of growth performance,feed efficiency and nutrient digestibility of red hybrid tilapia fed dietary inclusion of black soldier fly larvae(Hermetia illucens)
- Effects of dietary astaxanthin enrichment on enhancing the colour and growth of red tilapia,Oreochromis sp.
- Microbial,immune and antioxidant responses of Nile tilapia with dietary nano-curcumin supplements under chronic low temperatures
