Is tuberous sclerosis complex-associated autism a preventable and treatable disorder?
Paolo Curatolo·Mirte Scheper·Leonardo Emberti Gialloreti·Nicola Specchio ·Eleonora Aronica ,5
Abstract Background Tuberous sclerosis complex (TSC) is a genetic disorder caused by inactivating mutations in the TSC1 and TSC2 genes, causing overactivation of the mechanistic (previously referred to as mammalian) target of rapamycin (mTOR)signaling pathway in fetal life.The mTOR pathway plays a crucial role in several brain processes leading to TSC-related epilepsy, intellectual disability, and autism spectrum disorder (ASD).Pre-natal or early post-natal diagnosis of TSC is now possible in a growing number of pre-symptomatic infants.Data sources We searched PubMed for peer-reviewed publications published between January 2010 and April 2023 with the terms “tuberous sclerosis”, “autism”, or “autism spectrum disorder”,” animal models”, “preclinical studies”, “neurobiology”, and “treatment”.Results Prospective studies have highlighted that developmental trajectories in TSC infants who were later diagnosed with ASD already show motor, visual and social communication skills in the first year of life delays.Reliable genetic, cellular,electroencephalography and magnetic resonance imaging biomarkers can identify pre-symptomatic TSC infants at high risk for having autism and epilepsy.Conclusions Preventing epilepsy or improving therapy for seizures associated with prompt and tailored treatment strategies for autism in a sensitive developmental time window could have the potential to mitigate autistic symptoms in infants with TSC.
Keywords Animal model·Autism spectrum disorders·Developmental and epileptic encephalopathy·Mechanisms·mTOR·Risk·Tuberos sclerosis complex
Introduction
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder characterized by deficits in social communication and interactions and restrictive and repetitive patterns of behavior or interests [1].The prevalence of ASD has progressively grown over the last few years, with the last estimation among children aged 8 years in an American monitoring network being 1 in 44 [2].ASD is a lifelong condition, and although there is a lack of definite cure for the core symptoms of ASD, some behaviors can be modified by early intervention strategies and parent-mediated communication-focused treatment [3–5].
Influenced by a combination of genetic and environmental factors, ASD is likely polygenic, with many genes having a minor effect on the overall clinical presentation [6].Progress has been made in understanding genetic underpinnings, and whole-genome sequencing is becoming the first-line test for children with ASD [7].Sequencing technology has improved the capability of identifying possible ASD risk genes, such as synaptic activity-related genes, as well as genes related to molecular regulatory systems, transcription, chromatin modeling, or mechanistic target of rapamycin (mTOR) pathways [8], and shed light on the relative impact of specific biological processes among different autism phenotypes [9].
The discovery of different molecular mechanisms underlying the genetics of autism is now an active area of research[10, 11].However, the disruption of different neurodevelopmental pathways associated with a relatively high number of genes makes it difficult to disentangle the exact mechanisms involved in ASD [12].Only a few ASD-related diseases have monogenic causes [13, 14].
Tuberous sclerosis complex (TSC) is a multisystem genetic disorder characterized by age-related development of benign tumors across several organs, including the brain,skin, kidneys, heart, and eyes, and is caused by inactivating mutations in eitherTSC1orTSC2genes [15, 16].The genetic variant results in an overactivation of the mTOR signaling pathway, which plays a crucial role in several brain development processes.The underlying brain pathology of TSC, including cortical tubers and dysplastic cortex and white matter connectivity abnormalities, predispose patients to multiple overlapping comorbidities, including seizures,intellectual disability and a spectrum of behavioral abnormalities with a high incidence of ASD [16, 17].
Overall, more than 2000 pathogenicTSC1/2variants have been described, making the genotype–phenotype correlation challenging.Identification of a pathogenic variant inTSC1/2is sufficient for the diagnosis of TSC, regardless of clinical findings [18].However, about 10% of TSC individuals meeting clinical diagnostic criteria have no mutation identified by current genetic testing [19].Pre-natal diagnosis or early post-natal diagnosis of infants with TSC by the detection of major TSC criteria, such as cardiac rhabdomyoma, subependymal nodules, tubers, or cortical dysplasia, enables major effective clinical surveillance in pre-symptomatic patients not only for seizures but also for autism [20, 21].This review will discuss new predictive biomarkers for TSC infants with ASD symptoms, the preventive value of early identification of developmental abnormalities, and novel mechanism-based treatment options for TSC-associated ASD.
Biomarkers for autism spectrum disorder and epilepsy
Epidemiological studies of TSC revealed a surprisingly high incidence of ASD ranging between 17% and 63% [10].In a single cohort of 103 patients evaluated by a single neuropsychologist, the prevalence of ASD was found in 40%of children [22].The TOSCA (TuberOus SClerosis registry to increase disease Awareness) database provided a good understanding of the natural course of TSC-associated neuropsychiatric disorders [23], with about 50% of individuals having intellectual disability [16, 24].
In TSC children with autistic features, epilepsy often precedes the onset of ASD, raising the issue of the effects of the seizures themselves on the developing brain [25].In a systematic review and meta-analysis to investigate the association with ASD, the link between seizures and ASD in children with TSC could not be inferred, but a strong association was found with seizure onset during infancy and specifically with infantile spasms [26].Early onset and persistent seizures, as well as region-specific interictal epileptiform electroencephalography (EEG) activity, may prevent the development of inappropriate neural connectivity leading to autistic-like behaviors.Electrophysiological disturbances may interfere with activity-dependent mechanisms involved in synaptogenesis and dendritic arborization, and these disturbances may disrupt the proper establishment of social cognitive representation, interfering with the process that enables neuronal cells to develop specialization of social functions [27, 28].In a large multicenter observational study evaluating biomarkers predictive of ASD, early seizure onset in these patients negatively impacted neurodevelopmental outcomes at 24 months of age [29].Epilepsy, which often begins in the first years of life, is the most common(80%–90% of patients) of a large spectrum of neurological and psychiatric manifestations of patients with TSC, which also includes intellectual disability, behavioral abnormalities, and ASD [30, 31].
Abnormal behavioral manifestations simulating autisticlike symptoms were first described in 1932 in 29 individuals with impaired social contact, repetitive and stereotyped behaviors, and absence of normal speech [32].However,it was only after the first description of autism that these behaviors were recognized as core characteristics of ASD[33].In addition to the early description, the association between ASD and TSC has only recently been systematically investigated [31].ASD features in TSC may vary from those identified in idiopathic ASD, because they present a higher score on the social communication domain and a lower score on the repetitive domain [34].In addition, the male-to-female proportion in TSC is reportedly approximately equal [24].
Solid evidence now shows that aTSCgene variant could be sufficient to lead to some social deficits; however, the appearance of early onset and persistent seizures could have an additive effect in increasing the likelihood of autistic-like behaviors.This idea is strengthened by the findings that mutations inTSC1andTSC2are associated with both epilepsy and ASD [35].In epilepsy,mTOR activation has been implicated in the development of seizures [36, 37], while in ASD, mTOR dysregulation has been linked to altered neuronal connectivity and synaptic function [38–40].Moreover, mTOR dysregulation has been reported in a clinical series of children with idiopathic autism; therefore, mTOR dysregulation may represent a direct pathway to both epilepsy and autism[41].Interestingly, the variability of the neurological and behavioral phenotypes in TSC infants may be related to the degree of loss of function ofTSC1/2genes, suggesting that mTOR dysregulation could play a direct role in determining susceptibility to both ASD and epilepsy[37, 42].
Molecular and cellular mechanisms
Complex cellular and molecular events play a role in determining a high risk for both epilepsy and the neurodevelopmental disorders associated with TSC, including ASD and intellectual disability [37, 43] (Fig.1).TSC and ASD share molecular and cellular mechanisms, including the dysregulation of signaling pathways such as the mTOR pathway,which plays a critical role in regulating cell growth, proliferation, differentiation, and spine dynamics.The downstream results of mTOR dysfunction include multiple cellular processes in the developing brain, including neuronal cell morphology, number and shape of synapses, and white matter connectivity [38–40, 44].

Fig.1 Mechanisms underlying mTOR-related DEE in TSC and ASD development.Schematic representation of the intricate relationship between genetic mutations, dysregulated mTOR signaling, and downstream mechanisms leading to epilepsy and neurodevelopmental disorders such as ASD.Loss-of-function mutations in TSC1 or TSC2 lead to hyperactivation of the mTOR pathway, resulting in cellular and molecular consequences such as network imbalance, inflammation,oxidative stress, extracellular matrix dysfunction, and myelin pathology.As discussed in “ Molecular and cellular mechanisms”, growing evidence highlights the interconnected nature of multiple pathological mechanisms in the context of TSC-associated ASD, emphasizing the mutual relationships between inflammation/oxidative stress, ECM remodeling, myelin pathology, and network dysfunction.These consequences are in direct interplay with cellular and structural abnormalities in the brain, abnormal brain development, and epileptic seizures.The complex interplay among all these factors plays a crucial role in the development of neurodevelopmental disorders such as ASD.mTOR mechanistic target of rapamycin, DEE developmental and epileptic encephalopathy, TSC tuberous sclerosis complex, ASD autism spectrum disorder, ECM extracellular matrix, OS oxidative stress, ROS reactive oxygen species, EEG electroencephalography, GABA gammaaminobutyric acid
One of the principal shared mechanisms that has been proposed between epilepsy and ASD is an abnormal balance between excitatory and inhibitory neurotransmission [45].Multiple aspects play a role in this imbalance, starting with microtubule cytoskeletal network alterations and abnormal synaptic function [39, 46].Czapski et al.reported abnormalities in the microtubule-associated protein Tau (MAPTau) and reduced levels of MAP1B and neurofilament light proteins, as well as nerve ending swelling in a TSC mouse model of ASD (Tsc2± mice) [39].Tsc2haploinsufficiency also produces alterations in the expression of synaptic proteins (VAMP1/2 and phospho-synapsin-1), synaptic density,synaptic vesicles, and synaptic mitochondria [46].Abnormalities in synaptic structure, including synaptic density and mitochondrial ultrastructure, have also been reported in different neuronal in vitro and in vivo models of TSC(Tsc1/2-deficient neurons) [47].
Several lines of evidence have also suggested that perturbation of mTOR signaling can lead to a developmental disruption of GABAergic interneurons and enhanced glutamatergic function, leading to abnormal synaptic homeostasis and explaining the concomitant presence of epilepsy and autism in infants with TSC [48–52].In a recent study, organoids derived from patients with heterozygousTSC2mutations were examined, revealing excessive production of mid-gestational human interneurons.This finding further strengthens the association between mTOR dysregulation and the development of GABAergic interneurons [53].GABAergic deficits are implicated in the dysfunctional neural circuits underlying both neurodevelopmental disorders and epileptogenesis [54–56].A developmental disruption of GABAergic interneurons linked to abnormal tangential migration can also possibly explain the concomitant occurrence of epilepsy and autism [57–60].Interestingly, additional studies using resected brain samples from pediatric epilepsy patients,including patients with TSC, have provided compelling evidence of a delay in the physiological maturation of GABAergic signaling (GABAergic immaturity) leading to alterations in excitatory/inhibitory (E/I) balance at the network level [37, 61].
In epilepsy, seizures are associated with an increase in pro-inflammatory cytokines [62, 63], while in ASD, immune dysregulation has been linked to altered neural connectivity,synaptic function, and behavior [64, 65].Several genes associated with immune function have been implicated in ASD epilepsy, further highlighting the overlap between the two disorders [66].The mTOR signaling pathway is known to be a central regulator of immune responses [67, 68].Immunohistochemical and large-scale transcriptomic studies in brain tissue from TSC patients have shown enrichment of reactive astrocytes and microglia associated with activation of both innate and adaptive immune responses and induction of different inflammatory pathways, which may contribute to epileptogenesis and associated comorbidities (Fig.1) [69–74].Interestingly, pre-natal activation of inflammatory pathways has been observed even in developing TSC brain lesions [72,75].Notably, functional studies have provided compelling evidence that neuroinflammatory processes and disrupted chloride homeostasis can synergistically contribute to increased brain excitability, shedding light on the intriguing connection between neuroinflammation and the abnormal balance of excitatory and inhibitory neurotransmission in TSC.Indeed, cytokines, such as interleukin-beta, have been recognized as neuromodulators that exert specific effects on GABAergic neurotransmission [76–78].These findings highlight the interconnected nature of multiple pathological mechanisms in the context of TSC-associated ASD (Fig.1).Furthermore, oxidative stress, a state of imbalance between the production of reactive oxygen species and the antioxidant defense system, has been implicated in the pathogenesis of various neurological disorders, including epilepsy and ASD [79, 80].Recent studies have provided evidence of early and sustained oxidative stress in the TSC brain.Interestingly, the extent of oxidative stress is suggested to predict the neuroinflammatory state of the brain [72, 73, 81].Thus,inflammation and oxidative stress may act synergistically to support the development of a dysfunction underlying both epilepsy and ASD (Fig.1).
The extracellular matrix (ECM) is a complex network of molecules that surrounds cells and plays a crucial role in regulating cell behavior, including cell adhesion, migration,and signaling [82, 83].Growing evidence suggests that dysregulation of the ECM may contribute to the pathogenesis of both epilepsy and ASD.For example, studies have shown alterations in the expression and distribution of ECM molecules in the brains of individuals with epilepsy and ASD,suggesting that ECM remodeling may be involved in the development and progression of these disorders [84, 85].In particular, neuropathological and molecular studies provided evidence of ECM remodeling and dysfunctional cell adhesion molecules in brain tissue from TSC patients [69, 70,86] (Fig.1).Higher expression of matrix metalloproteinases(MMPs) has been shown in TSC tubers compared to normal neocortical brain tissue and perituberal cortex [87].Reactive astrocytes represent an import source of MMPs, and their release may critically contribute to ECM remodeling and pathological synaptic plasticity [84, 88].Dysregulation of MMP may contribute to the degradation of perineuronal nets around fast-spiking inhibitory interneurons, which is associated with network dysfunction [89, 90].These observations further emphasize the mutual relationships between neuroinflammation, ECM remodeling, and network dysfunction (Fig.1).
Finally, myelin, the fatty substance that surrounds and insulates axons in the nervous system, is critical for proper neuronal communication and signaling.Abnormalities in myelin structure and function have been implicated in the pathogenesis of ASD and epilepsy [91].Growing evidence from neuropathological and in vitro studies strongly suggests that myelin reduction is an intrinsic component of the pathological mechanisms linked to mechanistic target of rapamycin complex 1 (mTORC1) deregulation during brain development [92].Interestingly, myelin pathology extends beyond white matter, also impacting gray matter myelination.There is compelling evidence that a decrease in the number of oligodendrocytes is associated with lower cognitive functioning, and lower levels of myelin-associated oligodendrocyte basic protein have been linked to the presence of ASD [93].Furthermore, myelin pathology in TSC has been shown to be associated with the presence of inflammatory cells (T-lymphocytes) [93].Additionally, recent studies propose that disrupted communication between oligodendrocytes and inhibitory interneurons could contribute to the formation of the pathological network, warranting further investigation in relation to TSC-associated epilepsy and ASD [94, 95].Taken together, these findings suggest a complex interrelationship between inflammation, myelin pathology, and network dysfunction, highlighting their role in the development of associated neuropsychiatric comorbidities (Fig.1).
In ASD, myelin dysfunction may lead to impaired communication between neurons and contribute to the social and communication deficits characteristic of the disorder [96].In epilepsy, myelin abnormalities may contribute to the development and propagation of seizures by disrupting the balance between excitatory and inhibitory signaling in the brain[97].TSC can be considered a model of genetically determined developmental and epileptic encephalopathy (DEE), a group of severe epilepsies that are characterized by seizures that are often drug resistant and encephalopathy [98].DEEs represent a heterogeneous group of disorders characterized by early onset, often difficult-to-treat epileptic seizures and EEG abnormalities associated with developmental impairment, which is related to the underlying genetic defects and may possibly worsen as a consequence of epileptiform activity [98–100].
Pre-clinical studies evaluating the efficacy of mTOR inhibitors in epilepsy and autism
A variety of animal models of TSC, including mice, rats,zebrafish, and non-human primates, each have unique advantages and limitations [37, 101].These models have been used to investigate the molecular mechanisms underlying TSC, test the efficacy of potential therapies, and explore the natural history of the disease.Studies involving the cooccurrence of epilepsy and ASD in TSC have predominantly been conducted using rodents [37, 101].Several animal models have been generated to identify the molecular and cellular mechanisms underlying the development of epilepsy and ASD.These animal models have been utilized not only to investigate the pathophysiology of TSC and ASD but also to test the efficacy of potential treatments (summarized in Table 1).
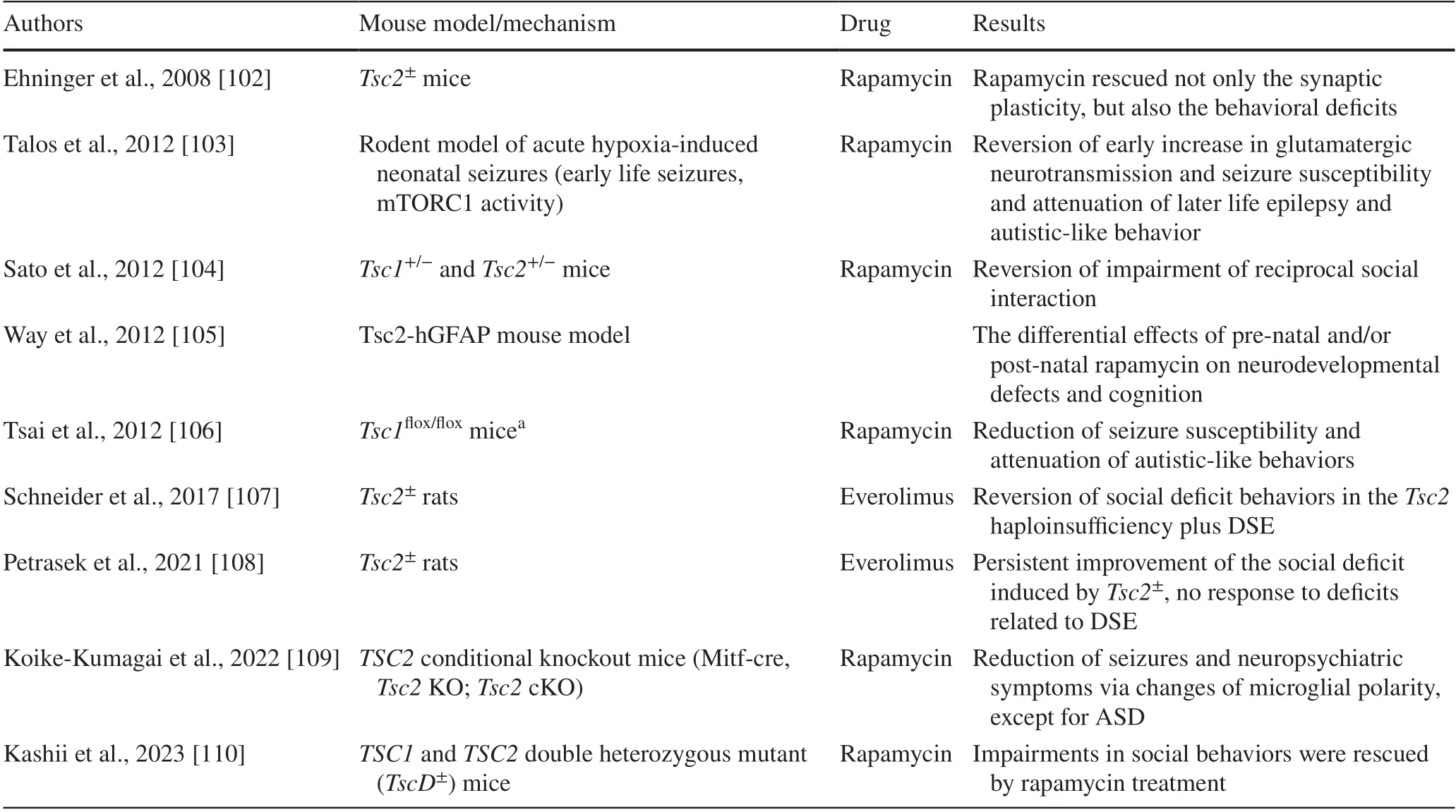
Table 1 Summary of the main preclinical studies evaluating the efficacy of the mTOR inhibitors on epilepsy and behavioral deficits
There is a significant role for the overactivation of the mTOR signaling pathway in linking the pathogenesis of these disorders [35, 37, 111, 112].The inhibition of this pathway can be achieved by pharmacological agents such as rapamycin and its analogs, known as mTOR inhibitors, which target mTORC1 and lead to a reduction in the hyperactivity of the pathway [37, 43, 101].The use of these mTOR inhibitors has shown promise in preclinical studies for the treatment of epilepsy, autism, and related neuropsychiatric disorders and could prevent susceptibility to autisticlike behaviors and epilepsy [16, 37].
Rapamycin, a selective inhibitor of the mTOR complex,was observed to reverse impaired social interactions in a mouse model of TSC (Tsc2± mouse model).Additionally,treatment with rapamycin immediately before and after seizures reversed the increased glutamatergic neurotransmission and attenuated later epilepsy and autistic-like behaviors,indicating that mTOR overactivation is involved in both epileptogenesis and altered social behaviors in a rodent model of acute hypoxia-induced neonatal seizures.Further studies have shown that rapamycin was able to not only reduce seizure susceptibility but also attenuate autistic-like behaviors in rodent models of TSC-ASD (Table 1).
Sato et al.[104] reported that rapamycin treatment alleviated impaired social interaction in bothTsc1± andTsc2±mice.In the Tsc2-hGFAP mouse model, which is characterized by cortical abnormalities, seizures, and cognitive deficits resulting from Tsc2 removal in embryonic neural progenitor cells, the effects of rapamycin treatment on neurodevelopmental defects were found to vary depending on pre-natal and/or post-natal administration [105].
In a rat model withTsc2haploinsufficiency and induced developmental status epilepticus, social deficits were observed.Treatment with the mTOR inhibitor everolimus successfully reversed these deficits, bringing the behavior of the experimental group to control levels [106].Early epileptic activity had an additional effect on the ASD-like phenotype in an animal model withTsc2haploinsufficiency.However, the ASD-like social impairment induced byTsc2± responded well to everolimus therapy, while the behavioral consequences of prolonged epileptic activity were not ameliorated by the drug [108].In aTSC2conditional knockout mouse model, sirolimus wasobserved to change the polarity of microglia via inhibition of mTORC1 and reduce multiple neuropsychiatric symptoms in TSC mice, except for ASD [109].Finally, Kashii et al.investigated social behaviors inTsc1andTsc2double heterozygous mutant mice by the social interaction test and three-chambered sociability tests [110]; impairments in social behaviors were rescued by rapamycin treatment.Interestingly, the gene expression changes compared with wild-type mice were enriched in the inflammation-related canonical pathway.
Collectively, these findings suggest that mTOR pathway dysregulation plays a significant role in the interaction between epilepsy and autism and highlight the potential of mTOR inhibitors as a treatment option for these disorders.Pharmacological targeting of this pathway with mTOR inhibitors such as rapamycin has shown promise in both preventing susceptibility to the development of autistic-like behaviors and epilepsy and reversing these symptoms in animal models.While there are limitations to the use of animal models, the knowledge gained from these studies is critical to the development of new treatments and a better understanding of the underlying mechanisms of these disorders.
Factors for an increased risk of autism spectrum disorder
Potential risk factors for the co-occurrence of ASD in TSC infants include genetic mutations, structural brain abnormalities, and epileptic activity [24].In the multicenter Tuberous Sclerosis Complex Autism Center of Excellence Research Network study group, having aTSC2pathogenic variant was associated with significantly lower Mullen Scales of Early Learning scores at 24 months, independent of seizures [113].Recent evidence has shown thatTSC2genetic variants may cause a high risk of developing ASD in comparison toTSC1gene variants [114].
The detection of fetal brain lesions on magnetic resonance imaging is associated with subsequent poor neurodevelopmental outcomes [115].Structural brain abnormalities, including tuber burden, cyst-like tubers, and abnormal connectivity, have been observed in children with ASD-associated autism [22, 116].Abnormal fusiform gyrus connectivity captures the risk of developing ASD as early as 12 months of age [117].A recent study provided evidence of a strong association between tuber involvement of the right fusiform face area and ASD diagnosis[118].Moreover, imaging studies examining brain–behavior relations have shown early abnormalities in white matter in infants with TSC who later develop ASD [91, 119,120].White matter diffusion tensor imaging abnormalities have been detected in children with ASD in comparison with children without ASD, suggesting microstructural changes in myelination and axonal integrity [121].Furthermore, cerebellar volume loss, perhaps reflecting Purkinje cell degeneration, may predict neurodevelopmental severity in patients withTSC2mutations [122].
In an early developmental stage during the critical period of synaptogenesis, early onset refractory seizures may contribute to autistic-like behaviors.Persistent seizure activity may disrupt connectivity in cortical areas that are crucial for the normal development of emotional and cognitive functions, including the cingulum [123] and the temporal areas [22].Furthermore, the presence of multifocal epileptiform abnormalities on EEG and relative alpha band power could enhance the detection of risk for ASD, since it was significantly elevated in an ASD group of TSC infants at 24 months of age [124].Sleep dysfunction is strictly connected with ASD in infants with TSC and is dependent on mTOR overactivation [125].Sleep disturbances and perturbation or disruption of the sleep–wake cycle could be correlated with ASD symptoms [126].In a multicenter prospective observational study, language variables by 12 months predicted an ASD diagnosis at 36 months [127].Serum-based biomarkers such as microRNAs have shown potential for early risk assessment of ASD [128].Early predictors of impending ASD are summarized in Table 2.Identifying early indicators of impending autism and epilepsy can have a clear impact on prevention and early intervention efforts [130].
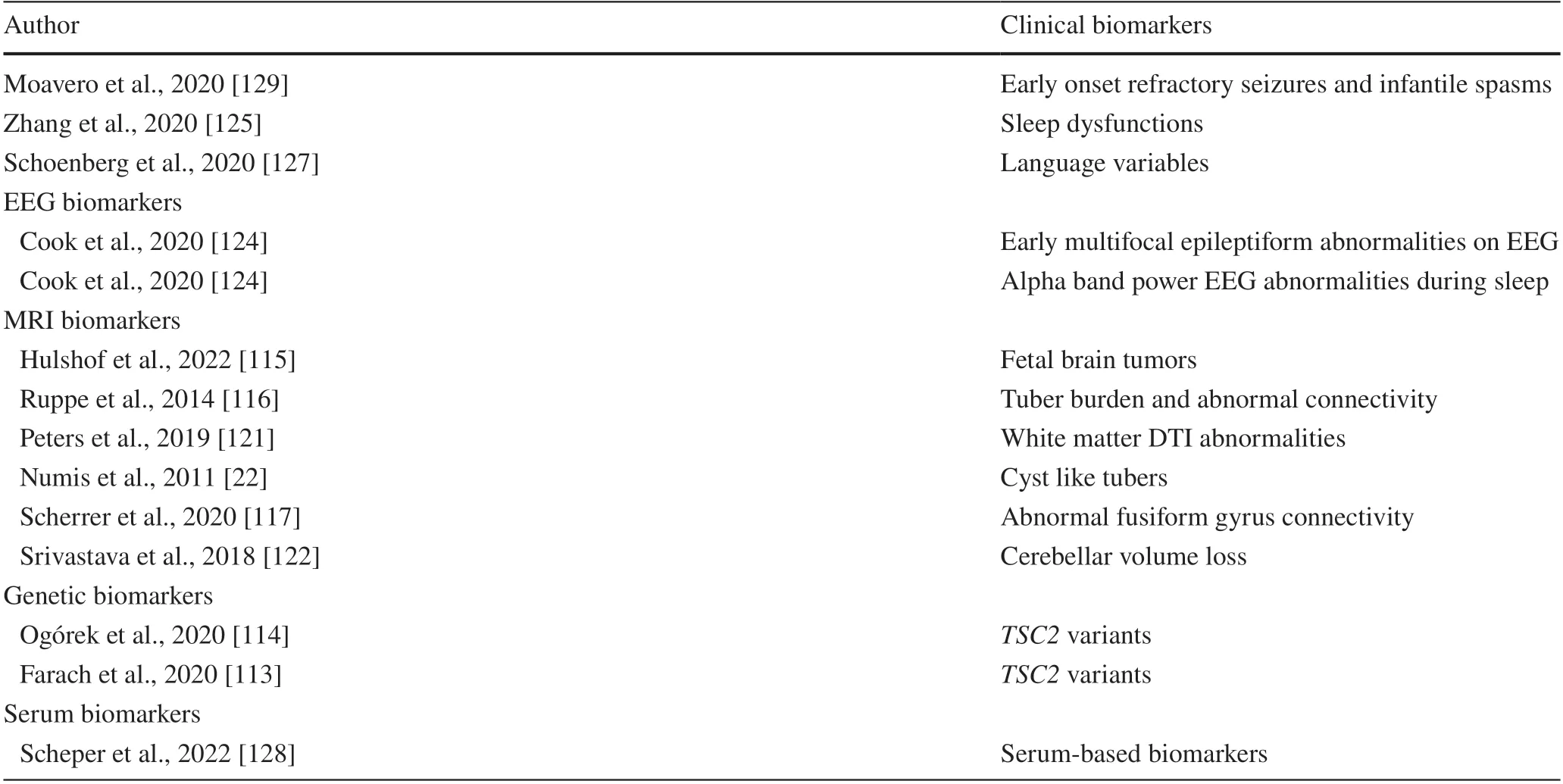
Table 2 Clinical, electroencephalography, magnetic resonance imaging, and genetic and serum biomarkers of ASD
Advances in early recognition of autism spectrum disorder
The Autism Diagnostic Observation Scale (ADOS) combined with expert clinical assessment is considered the gold standard for the diagnosis of ASD even before 24 months[131].However, behavioral markers detected from the autism observation scale of infants were consistent with ADOS items at 24 months [132].Recently, we gained a deeper understanding of earlier developmental manifestations of TSC that precede ASD or intellectual disability [29,133].Already at 6 months, infants with TSC that will be classified as having ASD at the age of 24 months tend to diverge from a typical neurodevelopmental trajectory and present a lower level of developmental skills when compared with infants classified with TSC and not ASD at 24 months[134].Rigorous prospective studies of the developmental trajectories of high-risk infants are needed [135].However,although TSC is diagnosed early during development, only a few clinical studies have evaluated the early trajectory deviation leading to developmental delay and ASD, monitoring infants with TSC prospectively at multiple time points.Inthe epileptogenesis in a genetic model of epilepsy–tuberous sclerosis complex (EPISTOP) trial, there was evidence that in TSC infants receiving a diagnosis of ASD at 2 years of age, some developmental abnormalities were already evident at 6 months of age, expanding to all developmental domains at 12 months [133].
Clinical trials for tuberous sclerosis complex-associated autism spectrum disorder
Targeting E/I balance with GABAergic drugs as possible secondary prevention of ASD
Among the mentioned factors that increase the risk for ASD in TSC, only seizures may be potentially preventable, and prompt detection and treatment of both interictal epileptiform discharges on EEG and clinical seizures could improve the course of the disorder, reducing the negative impact of early seizures on neurodevelopment [29].
Seizures are preceded by a latent period of epileptogenesis in which interictal epileptiform discharges may appear in the EEG [136].Prompt control of EEG abnormalities and seizures plays a crucial role in preventing subsequent epileptic encephalopathy and in reducing the cognitive and behavioral consequences of epilepsy [124, 137].Vigabatrin is known to be particularly beneficial in TSC-associated infantile spasms and may have disease-specific efficacy,particularly due to the GABAergic effect [138, 139].Therefore, pre- and postepileptic windows for secondary prevention of ASD may exist.A shorter gap between seizure onset and the start of vigabatrin treatment could reduce the risk of epileptic encephalopathy and the severity of intellectual disability and ASD, minimizing the negative impact of early life seizures [140].Pre-symptomatic treatment with vigabatrin given during the period of epileptogenesis and before any clinically detectable seizure may show even better effi-cacy.In an international randomized-controlled trial, preventive treatment with vigabatrin resulted in a reduced risk of developing infantile spasms and drug-resistant epilepsy in comparison with treatment initiated after seizure onset[141].However, in the EPISTOP trial, autistic features at 24 months of age were detected irrespective of treatment strategy, although preventive treatment resulted in a better neuropsychological result than in other historical cohorts of TSC children [141].
Bumetanide has been reported to alter the synaptic excitation/inhibition balance by strengthening the action of GABA, thereby attenuating the severity of ASD in animal models [142].Bumetanide treatment is a potential treatment to ameliorate the behavioral burden and quality of life associated with TSC [143].Furthermore, bumetanide has been reported to have effects on EEG, reflected in increased absolute and relative alpha power and functional E/I ratio and in decreased central frequency; these EEG findings were related to medium-to-high repetitive behavior scale-revised improvement [144].
Targeting translated and epigenetic regulation with mTOR inhibitors
The involvement of mTOR signaling in determining the behavioral phenotype associated with TSC led to the hypothesis that mTOR inhibitors could also have benefits in ASD symptoms.The negative effect of mTOR overactivity on white matter microstructural integrity was improved by mTOR inhibition with everolimus, and rapid white matter maturation was observed during long-term treatment in younger patients [121].Clinical evidence based on anecdotal cases showed contradictory results about the beneficial effects of mTOR inhibitors, including rapamycin and its derivative everolimus, on autistic symptoms in a few patients[145].
In a prospective double-blind randomized and placebocontrolled study to evaluate treatment efficacy with everolimus on neurocognition and behavior in children with TSC,no significant improvement in neurocognitive functions was observed in 47 individuals with TSC [146].However, no definite conclusions can be achieved from this study considering that the drug was used in individuals older than 6 years of age and already with symptoms.What is still unclear is the right timing of everolimus.However, the safety of everolimus has been proven in young children under the age of 24 months [147].After the promising results from preclinical studies, clinical trials with mTOR inhibitors are now underway, even if the right timing to start everolimus is still unclear.Since the dysregulation of the mTOR pathway is a possible pathological substrate for TSC-related seizures and developmental encephalopathy, different windows of opportunity for the prevention of the development of seizure and co-occurring cognitive and behavioral disorders could be possible [36].mTOR signaling is already activated in fetal tubers in various brain cell populations, including dysmorphic neurons within the dysplastic cortex and giant cells within the subcortical white matter, providing a likely pathological substrate for early onset epilepsy [43, 75, 148].These findings raise the possibility that modifying epileptogenesis in the immature brain by mTOR inhibition may also prevent other manifestations of network dysfunctions leading to autism and other neurobehavioral comorbidities.
In a single case report, a fetus diagnosed with TSC at the age of 19 weeks of gestation who presented cardiac and brain tumors on pre-natal ultrasound and underwent intrauterine treatment with everolimus presented regression and subsequent stabilization of the cardiac and brain lesions.Additionally, the patient did not develop seizures or autism and presented good psychomotor development [149].
Non-pharmacological treatments
Brain social skills develop through an active process of post-natal interaction with the environment.The efficacy of environmental enrichment with enhanced somatosensory,cognitive, visual, and motor stimulation in the critical developmental window of synaptogenesis has been reported to improve joint engagement, joint attention, and social interaction in toddlers at high risk of ASD [150–152].
Due to the greater plasticity of developmental neural systems, intensive and targeted parent-mediated behavioral interventions can speed up the developmental trajectories and ameliorate the disabling effects of ASD [152].In TSC infants, new evidence shows that early environmental enrichment may restore functional and structural changes and improve impaired synaptic plasticity.In a pilot study on five children with TSC aged 1–3 years, early play-based, parent-mediated behavioral interventions were able to improve communications and social skills [151].
Clinical trials on early intervention in toddlers aged 12–16 months are in progress, yet standardized measures to capture the effect of clinical interventions are needed.The recent TSC consensus workshop for clinical recommendations emphasized the need to offer early intervention services focused on building social communication skills in children with ASD concerns (Specchio et al., submitted to EJPN).Early screening for sleep problems and personalized approaches with sleep interventions may improve not only sleep but also daytime behaviors in TSC children with ASD.Melatonin,a hormone involved in the endogenous synchronization of internal biological clocks, may facilitate falling asleep and maintaining sleep in children with ASD [153, 154].
Epilepsy surgery in TSC typically involves the resection or disconnection of the epileptogenic tubers or the placement of neuromodulation devices, such as vagus nerve stimulation or responsive neurostimulation systems.Multiple studies have demonstrated the effectiveness of epilepsy surgery in TSC patients with refractory seizures.A study by Jansen et al.[155] reported a significant reduction in seizure frequency and improved seizure control in 70% of TSC patients who underwent epilepsy surgery.Another study by Specchio et al.[156] showed that surgical interventions, including focal resections and hemispherectomies, resulted in a favorable seizure outcome in TSC patients, with over 60% achieving seizure freedom or a significant reduction in seizure frequency.The potential risks and benefits of surgery should be carefully considered, as TSC patients may have multiple tubers or cortical abnormalities, making the surgical approach challenging.Epilepsy surgery can be a valuable therapeutic option for individuals with TSC who have medically refractory seizures.It offers the potential for improved seizure control and enhanced quality of life.
Future directions and conclusions
The emerging clinical neuroscience of TSC-associated ASD, possible prevention, and early intervention are shown in Table 3.Significant advances made in the neurobiology of TSC and animal models have clearly suggested that the neurodevelopmental manifestations of TSC, including ASD,may be a direct consequence of gene variants and cell signaling abnormalities.Therefore, mTOR signaling overactivity through both direct and indirect pathways plays a role in the disruption of GABAergic interneurons during early critical stages of neurodevelopment and confers a high susceptibility to autism in infants and young children with TSC.
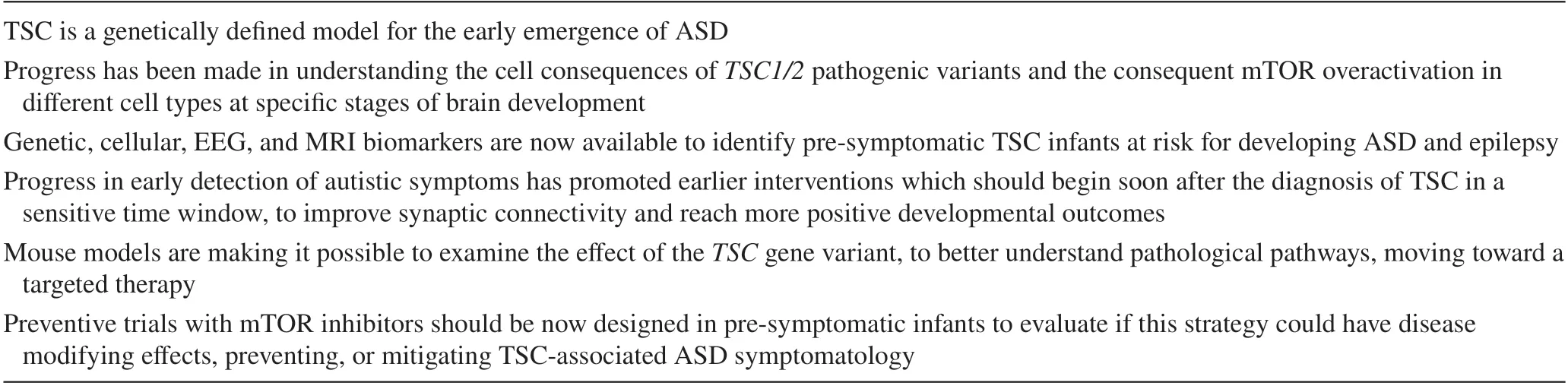
Table 3 The emerging clinical neuroscience of TSC-associated ASD and possible prevention and early intervention
Several risk factors for both autism and epilepsy have been described, and progress in the early identification of prodromic signs has been made.These findings are changing our clinical practice and have provided new insights for behavioral and pharmacological therapeutic options.However, in this specific context, it is difficult to separate treatment from prevention, particularly from tertiary prevention.Especially, when dealing with chronic conditions, tertiary prevention aims to diminish the effects of a disease or disorder by reducing or minimizing disability while boosting the person’s quality of life.In epidemiological terms, the aim of tertiary prevention is to lessen the impact of complications.An increase in the knowledge and identification of subclinical ASD endophenotypes might even allow interference with the progression toward a full-blown ASD developmental trajectory.From this perspective, future advancements might lead to the possibility of developing secondary prevention strategies, further highlighting the preventive value of early detection.
Even though animal models demonstrated promising results in improving or reversing behavioral symptoms, recent clinical findings showed that no therapeutic options targeting either GABAergic or mTOR inhibition with everolimus were able to determine clinically significant behavioral gains even in children with a concomitant decrease in seizure frequency after targeted therapy.
Elucidating the cellular and molecular basis of TSC-associated ASD has shown a close link between the encephalopathic process and the epileptogenesis leading to ASD or epilepsy.Predicting, preventing, and improving treatment of TSC-associated seizures, including pharmacological and surgical intervention, could reduce the burden of autistic symptoms in infants with TSC.Future studies should clarify whether early parent-mediated behavioral intervention in a developmental sensitive time window associated with early prescription of mTOR inhibitors has the potential to mitigate the severity of ASD symptoms.
AcknowledgementsS N was supported by Next-Generation EU(NGEU) and funded by the Ministry of University and Research(MUR), National Recovery and Resilience Plan (NRRP), under project No.MNESYS (PE0000006)–a multiscale integrated approach to the study of the nervous system in health and disease (DN.1553 11.10.2022).
Author contributionsCP conceived the review.SM and AE contributed particularly to “ Molecular and cellular mechanisms” and “ Preclinical studies evaluating the efficacy of mTOR inhibitors in epilepsy and autism” and the preparation of Fig.1.All authors drafted and prepared the manuscript.All authors read, revised, and approved the final manuscript.
FundingA E and SM were supported by Healthcare Research and Medical Sciences (ZonMw; No.09120012010007).
Data availabilityData sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethical approvalNot required.
Conflict of interestNo financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.The authors have no conflict of interest to declare.
 World Journal of Pediatrics2024年1期
World Journal of Pediatrics2024年1期
- World Journal of Pediatrics的其它文章
- Editors
- Information for Readers
- Instructions for Authors
- Current status of Mycoplasma pneumoniae infection in China
- Coinfection of SARS-CoV-2 Omicron variant and other respiratory pathogens in children
- PACS gene family-related neurological diseases: limited genotypes and diverse phenotypes
