Effects of Fungi Fusarium sp. to Rhizosphere Soil and Physiological Characteristics of Camellia oleifera Abel.
Xuejin WANG, Kai YAN, Tianhua YU, Zhannan YANG, Shiqiong LUO
1. Key Laboratory for Information System of Mountainous Areas and Protection of Ecological Environment of Guizhou Province, Guizhou Normal University, Guiyang 550025, China; 2. School of Life Science, Guizhou Normal University, Guiyang 550025, China; 3. Liupanshui Normal University, Liupanshui 553004, China
Abstract [Objectives] To study the effects of fungi Fusarium sp. to rhizosphere soil and physiological characteristics of Camellia oleifera Abel. [Methods] We investigated the effects of Fusarium sp. to rhizosphere soil nutrient element content and metabolites of C. oleifera. C. oleifera was inoculated with the suspension of Fusarium sp. in pot experiments and ammonium-N, available phosphorus, available potassium, organic matter, enzymes and pH of rhizosphere soil, MDA content, activity of SOD, POD of C. oleifera leaves were analyzed. [Results] Fusarium sp. stress significantly inhibited soil enzyme activities and significantly reduced available phosphorus content, especially for phosphatase and sucrase. Antioxidant enzyme activities in C. oleifera tissues showed that Fusarium sp. stress significantly increased MDA and SOD enzyme activities and decreased POD enzyme activity. Especially, SOD enzyme activity was elevated by 53.86% compared with the CK group. In addition, analysis of the content of major metabolites in C. oleifera leaves showed that Fusarium sp. stress significantly reduced the content of total flavonoids, quercetin, isoquercitrin and isoquercitrin in C. oleifera leaves by 7.80%, 50.00% and 75.90%, respectively. [Conclusions] Our results are an important step which showed strong resistance of C .oleifera and can give a novel insight for researches on the effects in the rhizosphere soil enzyme, soil nutrient elements and metabolites of C. oleifera under the Fusarium sp. too.
Key words Camellia oleifera Abel., Fusarium sp., Antioxidant enzymes, Soil enzymes, Soil quality
1 Introduction
In recent years, the demand for food has increased drastically due to the growing population around the world. With the rapid development of hybridization and breeding techniques, food production has increased dramatically, but shortages of food stock still exist in some countries. According to surveys, 828 million people still suffered hunger around the world in 2021. Pathogenic fungi are one of the most significant causes of reducing food production. Because of the deterioration of the ecological environment, plants are inevitably affected by pests and diseases (e.g.,Fusariumsp. andMagnaporthesp.)[1-3]. Crop diseases caused by environmental stress have had a huge effect on world food production, reducing crop yields about a quarter, including about 30% for rice which is considered as one of the most important foods[4]. As an important class of environmental stressors, pathogenic fungal diseases are one of the most important obstacles to crop productivity worldwide, especially in areas where large areas and monoculture crops are grown for long periods of time. The infection of pathogenic fungi impacts the rhizosphere and phyllosphere microbial communities which play an important role in plant growth, such as the secretion of phytohormones and the improvement in plant adaptation. These changes in the microbial communities can impact plant metabolism and soil quality, including the activity of soil enzymes and the content of soil nutrient elements. In addition, most pathogenic fungi have a strong ability to penetrate intact plants, which ultimately leads to wilting and death of the plant and a reduction in yield. This environmental stress is therefore a vital step in humanity’s endeavor to address the reduction of food production.
Soil is an important part of the environment and plays an important role in the growth of plants. Environmental stress factors such as pathogenic fungi and heavy metals are enriched in the soil, which makes the crop more vulnerable to these environmental stresses. Pathogenic fungi spread through the soil and enter the plant by the root surface to cause plant diseases. In this process, certain plant rhizosphere microbial community can improve plant adaptability and host plant can regulate the abundance and structure of the community to help itself growth. However, infection by pathogenic fungi can impact the diversity and structure of the community. The actions of microorganisms also change the soil environment, which is closely related to the growth and nutrient absorption of plants. Infection of pathogenic fungi reduced the diversity of the soil microbial community, resulting in a reduction of microbial action. Then the action impacts the content of soil nutrient elements and soil enzyme activities because microbial activity is the main source of soil enzyme. Soil nutrient elements and soil enzyme activities are usually considered as a reflection of soil fertility.
Fusariumbelongs to the subphylum Deuteromycotina, and the sexual stage belongs to the genusGibberella, and it is a common class of pathogenic fungi found in more than 100 species of plants such as corn and wheat. Widely found in soil, water, and other environments.Fusariumis extremely harmful to the host plant with the ability to cause wilting and death of the host plant, ultimately leading to crop yield reduction or extinction.Fusariumcan be spread through the soil and is one of the pathogens of a soil borne fungi disease. It can cause wilting and death of plants by entering and multiplying in roots and stems, destroying vascular tissues and preventing the transport of water and nutrients[5]. Several species that belong to the genusFusariumcan cause disease in plants.Fusariumoxysporum is the most widely studied and it is one of the most pathogenic pathogens[6-8]. Diseases such as wilt and root rot caused byFusariumare characterized by a wide time span and high infectivity. Plants is infected at any stage from the seedling stage and showed different symptoms between different plants.
CamelliaoleiferaAbel., as an evergreen shrub, is a dicotyledonous plant of the genusCamelliain the family Camelliaceae and has highly strong adaptability.C.oleiferahas a long history of cultivation and can be traced back to 2 300 years ago. It is mainly planted in the mountainous areas of southern China, including Guizhou, Sichuan, Jiangxi and other places with a total planting area of more than 11 million ha, which account for more than half of the oilseed tree species. It is considered to be one of the more disease resistant oilseed crops compared with other crops.C.oleiferais also one of the important pillar industries in Chinese fight against poverty, and it also plays a vital role in ecosystem restoration due to its strong resistance. In addition,C.oleiferahas a strong antibacterial ability, and the lowest concentration reached 1.061 1 μg/mL[9]. After the fruit has been pressed, the production which is seen as tea oil still has strong antimicrobial activity and the cake which was seen as a kind of rubbish can also be used to combat soil-borne diseases in the land[10].C.oleiferais strongly tolerant to various environmental stresses, we therefore considered trying to investigate the reasons for the high adaptability ofC.oleiferato adversity, especially in soil environments with a high abundance of pathogenic fungi. In fact, it is of great importance for the control of soil borne fungal diseases of the crop. The infection ofFusariumsp. may be influential on the physiology and biochemistry ofC.oleiferaplants are able to regulate the secretion of secondary metabolism, which make plants to resist the adverse effects of changes in growth environment when they sense a change in its growth environment.
POD, SOD and MDA are antioxidant enzymes widely present in the plant tissue, which can alleviate the plant cells’ damage caused by free radicals and the changes in enzyme activities can reflect the resistance of the plant to adversity at a certain level. Various environmental stress factors cause certain changes in antioxidant enzyme activities for plant protection. The main source of soil enzymes is the activity of soil microorganisms. The catalytic reactions involved in soil enzymes take place outside the cells of soil microorganisms, which are mainly involved in the synthesis and transformation of various organic substances, the oxidation reduction of inorganic substances and other processes. But low levels of soil enzymes play an important role in maintaining soil nutrient cycling. Their changes to better reflect soil quality[11]. Soil enzyme activity is an important indicator of soil fertility because of its rapid response to environmental changes, which greatly reflects the ability to transform nutrients in the soil. Therefore, it is well suitable for evaluating the effects of environmental stresses, including biotic and abiotic stresses on the soil[12].
In this study,Fusariumsp. isolated fromC.oleiferatissues were used as environmental stress factors to infect root ofC.oleiferain three months. The activities of POD and SOD, the content of MDA, urease, phosphatase and sucrase activities as well as soil nutrient elements were then determined at first, second and third weeks. Our aim was to elucidate whetherC.oleiferaresponds to pathogenic fungi infection and the reasons whyC.oleiferais strongly resistant to pathogens.
2 Materials and methods
2.1Materials
2.1.1Experimental materials. TheC.oleiferaseeds used for the experiment came from Liping County, Qiandongnan Autonomous Prefecture, Guizhou Province. When the fruits ofC.oleiferawere ripe, the fruits with larger particles were selected and then immediately brought back to the laboratory, stored in a cool and dry place. These fruits are allowed to crack naturally and then the seeds were taken out, which could be used for obtainingC.oleiferaseedlings.
TheFusariumsp. used in the experiment was isolated, purified and preserved fromC.oleiferaby Guizhou Normal University. And the strain number is CF-4.
2.1.2Experimental design. To prepare culture medium to activate the experimental strains, 20 g/L glucose, 20 g/L agar, and 200 g/L potato were weighed separately. The potatoes were cut into cubes, stewed and then filtered using 4 layers of gauze, dissolved using the filtrate, naturally pH. And sterilized in an autoclave for 20 min at 121 ℃, and cooled and set aside. The experimental strains were punched and inoculated on fresh PDA medium using a 6 mm punch, and after 7 d the spores were scraped off and placed in sterile water to obtain a spore suspension, which gave a spore concentration of 1×106CFU/mL.
2.1.3Infection of pathogenic fungi. QualityC.oleiferaseeds without visible diseases were selected, shelled and the seed hulls were carefully removed using a utility knife. In order to obtain a higher germination rate, the seeds were soaked in a 2 mg/L GA3solution for 2 h. Then, the seeds were sterilized on a sterile table under the conditions of 75% alcohol for 30 seconds and 0.2% HgCl2(V/V) solution for 8 min. The seeds were sown in sterile soil and after germination, theC.oleiferaseedlings were transplanted into pots. Each treatment group has three parallels. Then, the fungal spore suspension was inoculated into theC.oleiferaseedlings by irrigating root and plant tissue and rhizosphere soil samples were collected for determination and analyses every 7 d. The incubation conditions were 23-25 ℃, 1 500-2 000 Lx of light, 12 h of photoperiod and 12 h each of light and darkness.
2.2Methods
2.2.1Determination and analysis of urease. The indophenol blue colorimetric method was used for determination of soil urease. 5 g of air-dried soil was weighed and 1mL of toluene was added. After 15 min of treatment, 5 mL of 10% urea (5 mL of buffer was added to the soil blank) as well as 10 mL of citric acid buffer were added and incubated for 3 h at 37 ℃ with shaking. To determine the amount of ammonia produced by urease, 1 mL of filtrate was placed in a 10 mL colorimetric tube, 4 mL of sodium phenol solution was added and 3 mL of sodium hypochlorite solution was immediately injected. Then, water was added to dilute this scale. Finally, the absorbance value of the cyan color of the solution was measured at 578 nm. The soil urease activity (Ure) was expressed by milligrams of NH3-N in 1 g of soil after 24 h. The urease activity is calculated as follows:
Activity of urease=A×V×n/m(mg/(g·d))
whereV(mL) represents the volume of measured extracts,nrepresents the ratio of the leachate volume to the volume of suction, andm(g) represents soil dry weight.
2.2.2Determination and analysis of phosphatase. 1.00 g of soil sample was weighed, 0.2 mL of toluene, 4 mL of pH 6.5 buffer solution and 1 mL of 1g/L 4-Nitrophenyl phosphate solution were added, shaken well and sealed, and incubated at 37 ℃ for 1 h. Then 4 mL of 0.5 mol/L NaOH and 1 mL of 0.5 mol/L calcium chloride solution were added, shaken well and filtered using filter paper. The absorbance value of the yellow solution was determined using UV at 420 nm. In order to eliminate the effect of soil and reagent colors, a no-soil control and a no-substrate control were required for each soil portion. Soil phosphatase activity was expressed in 4-Nitrophenol production per unit of time.
Activity of phosphatase=m1/m2(mg/(g·h)).
wherem1represents the weight of 4-Nitrophenol produced (mg), andm2represents the weight of the soil sample.
2.2.3Determination and analysis of sucrose. Sucrase activity was determined by colorimetric method using 3, 5 dinitrosalicylic acid. 1.00 g of soil sample was weighed and placed in a 20 mL sample bottle, injected with 7.5 mL of 10% sucrose solution, 2.5 mL of phosphate buffer solution with pH 5.5 and 0.25 mL of toluene. Vigorous shaking and then put into 37 constant temperature oven to incubate for 24 h. Quickly took out and filtered. 1mL of the filtrate was pipetted into a 25 mL volumetric flask, 3 mL of 3,5-dinitrosalicylic acid was added and heated in a water bath of boiling water for 5 min and then removed and cooled when the time came. Finally, dilute with water to the scale, measure the absorbance value at 508 nm, and calculate the glucose content according to the standard working curve. In order to eliminate the error arising from the original glucose in the soil, each soil sample needs to be done without substrate and without soil control. The activity of soil sucrase was expressed by milligrams of glucose per gram of soil after one day.
2.2.4Determination of soil physicochemical properties. Soil ammonium nitrogen, quick potassium and available phosphorus were determined using LD-TYC soil nutrient tester. The rhizosphere soil was shade-dried under indoor conditions away from direct sunlight according to the instrument manufacturer’s instructions. The soil was crushed and passed through a 2 mm sieve, 0.500 g of soil was weighed into a 20 mL sample bottle and 10 mL of soil leachate and soil decolourant were added at one time and shaken vigorously for 15 min. After the reaction is sufficient, the solution is filtered to obtain the soil nutrient solution to be measured. Then the content of ammonium nitrogen, available phosphorus and quick potassium in the soil was determined according to the instruction manual of the instrument.
A pH meter was used to determine the acidity of the soil. 2 g of air-dried soil was weighed in a glass jar, added to 5 mL of distilled water (soil:water=1:2.5), shaken vigorously for 2 min using a horizontal shaker and allowed to stand for 30 min to be determined within 1 h.
2.2.5Determination and analysis of SOD activity. The enzyme activity of SOD was determined by the nitrogen blue tetrazolium method. 0.1 g of fresh leaves were weighed and 5 mL of PBS with pH=7.8 was added for grinding, centrifuged at 4 000 rpm, and the supernatant was the crude enzyme solution. 100 μL of crude enzyme solution was taken (the control tube was replaced by deionized water) and methionine, azurite azolium, EDTA-2Na, riboflavin and deionized water were added sequentially. One control tube was placed in the dark for zeroing the spectrophotometer, and the rest of the tubes were placed in 4 000 lx light intensity at 25 ℃ for 20 min, and then the absorbance values of each solution were measured at 560 nm. The activity of SOD is described by 50% inhibition of NBT photochemical reduction as one unit of enzyme activity.
Total activity of SOD =(Ack-AE)×V/(Ack×0.5×W×Vt).
whereAckrepresents the absorbance value of the control tube,AErepresents the absorbance of the sample tube,Vrepresents the total volume of the sample solution,Vtrepresents the dosage of measuring andWrepresents the fresh weight of the sample.
2.2.6Determination and analysis of POD activity. The Guaiacol method was used to determine the peroxidase activity. 0.1 g of fresh leaves were weighed and milled to homogenate in 5 mL of pH 6.0 PBS. Then, the enzyme solution was centrifuged in a centrifuge at 4 000 rpm for 20 min, and the supernatant was poured out and diluted to 5 mL using pH 6.0 PBS. 300 μL of the enzyme solution was pipetted into a test tube and 3 mL of the reaction mixture (each milliliter consisted of 1 μL of 30% H2O2and 3 μL of guaiacol) was added to the test tube. The absorbance value of the solution was measured at 470 nm at 30 sec intervals and the magnitude of POD enzyme activity was expressed by the change in absorbance value per minute.
whereArepresents the absorbance value of enzyme solution at 470 nm,Vtrepresents the dosage of measuring,Wrepresents the fresh weight, andVsrepresents the volume of total enzyme solution.
2.2.7Determination of MDA content. The MDA content ofC.oleiferaleaves was determined by the thiobarbituric acid chromogenic method with slight modification. 0.1 g fresh plant leaves were weighed and 5 mL of PBS buffer (pH=7.8) was added to tube, milled into a homogenate and centrifuged at 4 000 rpm for 15 min. The supernatant was aspirated 2 mL (2 mL of deionised water was added to the control) and 0.6% TBA in trichloroacetic acid solution (10%) was added. it was steamed in a boiling water bath for 15 min and placed in a refrigerator to cool down before centrifugation. The absorbance values of the solutions were determined at 532, 600 and 450 nm, respectively by aspirating the supernatant, and the content of MDA in theC.oleiferawas calculated according to the following equation:
Content of MDA (μmol/g)=[6.45×(A532-A600)-0.56A450]×V/(Vt×W)
whereVrepresents the total volume of enzyme solution,Vtrepresents the volume of dosage measuring andWrepresents fresh weight ofC.oleiferaleaves.
2.2.8Determination and analysis of total flavonoids. The determination of total flavonoids was used by the NaNO2-Al(NO3)3colorimetric method, using rutin as the standard solution. For the determination of total flavonoids inC.oleiferaleaves, 0.1 g of fresh leaves from different time periods were weighed and placed in a mortar, 5 mL of 60% ethanol was added, ground, centrifuged and the supernatant was the solution to be measured. Pipetted 0.5 mL of the supernatant solution was added 0.5 mL of 5% NaNO2, 0.5 mL of 10% Al(NO3)3, 4 mL of NaOH, and the solution was allowed to stand for 15 min in the dark. Then the absorbance value was measured at 510 nm. Finally, the total flavonoid content of the samples was calculated according to the standard working curve.
2.2.9Analysis of quercitrin, isoquercitrin and quercetin by HPLC. Quercitrin, isoquercitrin and quercetin inC.oleiferaleaves were extracted, measured and analyzed. The leaves were washed and cut and 0.1 g of leaves cut were placed into a 10 mL glass bottle and 1 mL methanol was added. Then ultrasonic extraction was used. The extract was filtered by using a 0.45 μm membrane filter and the filtrate was collected and stored at 4 ℃. The content of quercitrin, isoquercitrin and quercetin were analyzed by using the methodology of Ye[13]. The HPLC system consist of the SHIM-PACK C18CLC ODS reversedphase chromatographic column, solvent A [0.1% formic acid (V/V)] and solvent B (acetonitrile:methanol=11:5 (V/V)). The content of each compound was calculated by the peak area of each compound and the standard compound come from sigma (USA). For every treatment, the three replicateC.oleiferaseedlings were used for extract and analysis.
3 Results and analysis
Soil enzymes are important factors influenced the level of soil microbial diversity. In ecosystems, they provide the necessary power for soil chemical cycling and play a key role in life activities such as nutrient release, fertility and organic matter conversion. Soil quality is also an important indicator of soil microbial diversity[14]. It can be seen from Fig.1 that the infection ofFusariumsp. fungi had an inhibiting influence on the activities of phosphatase and sucrase. Soil acidity was displayed in Fig.1, the soil was acidic and therefore acid phosphatase activity was measured. The pH of the fungal treatment group was lower than the control group and gradually increased (Fig.1). For sucrase, the maximum value was reached in the first week after infection, followed by a gradual decrease and a minimum value in the third week. In the second and third weeks, sucrase activity was significantly lower (P<0.05) in the experimental treatment group compared with the CK group, but in the first weeks. For urease, after inoculation with pathogenic fungi, urease activity reached a maximum value in the second week and a minimum value in the third week, showing an overall trend of increasing and then decreasing. In the second and third weeks, the urease activity of the experimental group was lower than that of the CK group (P<0.05). In the first week, the urease activity was lower in the CK group than in the experimental group. For phosphatase, the phosphatase activity was always lower than the CK group from the beginning of inoculation with theFusariumsp. fungi. In the second week after inoculation, it reached a minimum value. In the third week, the phosphatase activity of the experimental group was significantly lower than that of the CK group (P<0.05). With the passage of time, there was a slow increasing trend in phosphatase activity after pathogenic fungi infection in the CK group, and the largest difference in enzyme activity was observed in the third week.
The content of soil nutrient elements represents soil fertility and indirectly influences plant growth. As showed in Fig.2, ammonium nitrogen in the soil showed an overall decreasing trend in the CK group and experimental group. Starting from the inoculation ofFusariumsp., it reached the maximum value in the second week and then gradually decreased. The ammonium nitrogen content of the experimental group was lower than that of the CK group except for the second and third weeks. In the first week, the CK group was significantly higher than the experimental group, whereas in the second week the experimental group was significantly higher than the CK group (P<0.05). For phosphorus, the experimental group showed a decreasing trend, reaching a maximum value in the first week and a minimum value in the third week. The CK group, on the other hand, showed a gradual increasing trend from the first week to the third week. The phosphorus content of the experimental group was significantly higher than that of the CK group in the first and second weeks (P<0.05).
But in the third week there was a reverse, the phosphorus content of the experimental group was lower than that of the CK group but not to a significant level (Fig.2). For available potassium, the experimental group had lower levels than the CK group, except for the third week. In the CK group, a minimum was observed in the third week. The experimental group, on the other hand, a trend of increasing and then decreasing was observed, reaching a maximum in the third week. In the first and second weeks, the available potassium content of the experimental group was lower than that of the CK group but did not reach a significant level, and in the third week the content of the CK group was significantly higher than that of the experimental group (P<0.05) (Fig.2). There was no significant difference of the content of organic matter in the CK and experimental groups. In the first and third weeks, the experimental group was lower than the control group and the experimental group was higher than the CK group in the second week (Fig.2).
Changes in POD activity ofC.oleiferaleaves in the first, second, and third weeks are shown in Fig.3. The changes in POD activity of the tissues ofC.oleiferaseedlings in the CK and experimental groups showed a decreasing and then increasing trend and the maximum of POD activity in the first week and the lowest enzyme activity of POD in the second week were observed. The enzyme activities in the CK group were significantly higher than that of the experimental group and were 198%, 397%, and 125% higher than that of the CK group, respectively. From the first week to the second week, the decrease of 39% and 70% of the CK group and the experimental group were separately observed. The above results showed that inoculation ofC.oleiferaseedlings withFusariumsp. fungi could decrease the POD enzyme activity inC.oleifera.
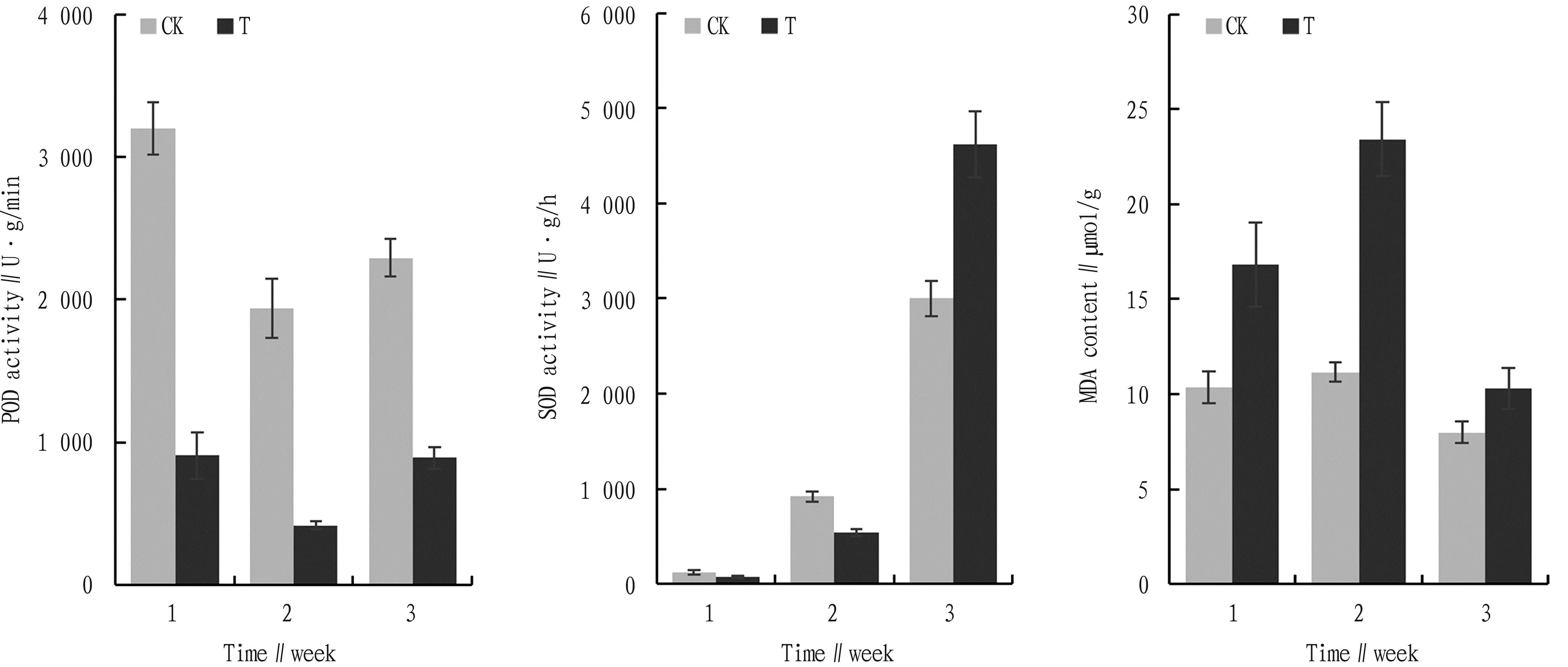
Fig.3 Effect of Fusarium sp. infection on POD and SOD activity and MDA content
The SOD activity was shown in Fig.3. In natural conditions, the SOD enzyme activity of both CK and experimental groups showed a trend of increasing and then decreasing from the first to the third week. Both reached the maximum value in the third week, with the greatest increase from the second to the third week.
In the first and second weeks, the activity of SOD in the CK group was higher than that in the experimental group. However, in the third week, the activity of SOD in the experimental group was higher than that in the CK group, which may be related to the colonization of the fungus intoC.oleiferatissues. Except for the second week,Fusariumsp. infection helped to increase SOD activity in the tissues ofC.oleiferaseedlings.Fusariumsp. infection significantly increased SOD activity and it shows thatC.oleiferacan rely on increasing SOD activity to eliminate the effects of ROS when subjected toFusariumsp. infection.
MDA is one of the most important products of membrane lipid peroxidation and the level of its content can reflect the degree of peroxidation of plant cell membranes, thus it can indirectly reflect the plant’s ability to resist adversity. The changes of MDA content in the tissues ofC.oleiferaseedlings at different periods are shown in Fig.3, where the content of MDA withFusariumsp. infection firstly increased and then decreased. The MDA content of both CK and experimental groups reached the maximum value in the second week and the experimental group was significantly higher than the CK group except the third week (P<0.05). From first week to third week, the difference in MDA content between the CK group and the experimental group became smaller and smaller, which can be concluded thatC.oleiferahas a strong adaptation ability to Fusarum sp. infection.
Flavonoids are widely synthesized by plants and have a variety of biological activities. Quercetin and its glycosides are the three flavonoids metabolized byC.oleiferaand have some antifungal activity. The content of total flavonoids inC.oleiferawas shown in Fig.4. In the first and second weeks, the content of total flavonoids was higher in the experimental group than in the CK group. Then the content of total flavonoids decreased and was lower in the experimental group than in the CK group. For quercetin, quercetin and isoquercetin, the trends were similar for all three compounds, with content of the experimental group significantly higher than the CK group in the first week (P<0.05). Thereafter, the content of the experimental group was lower than the CK group (P<0.05). Because of the fast infection rate ofFusariumsp.,C.oleiferaresponded to the invasion ofFusariumsp. by synthesising a large number of flavonoids at the early stage of infection to counteract the invasion ofFusariumsp.
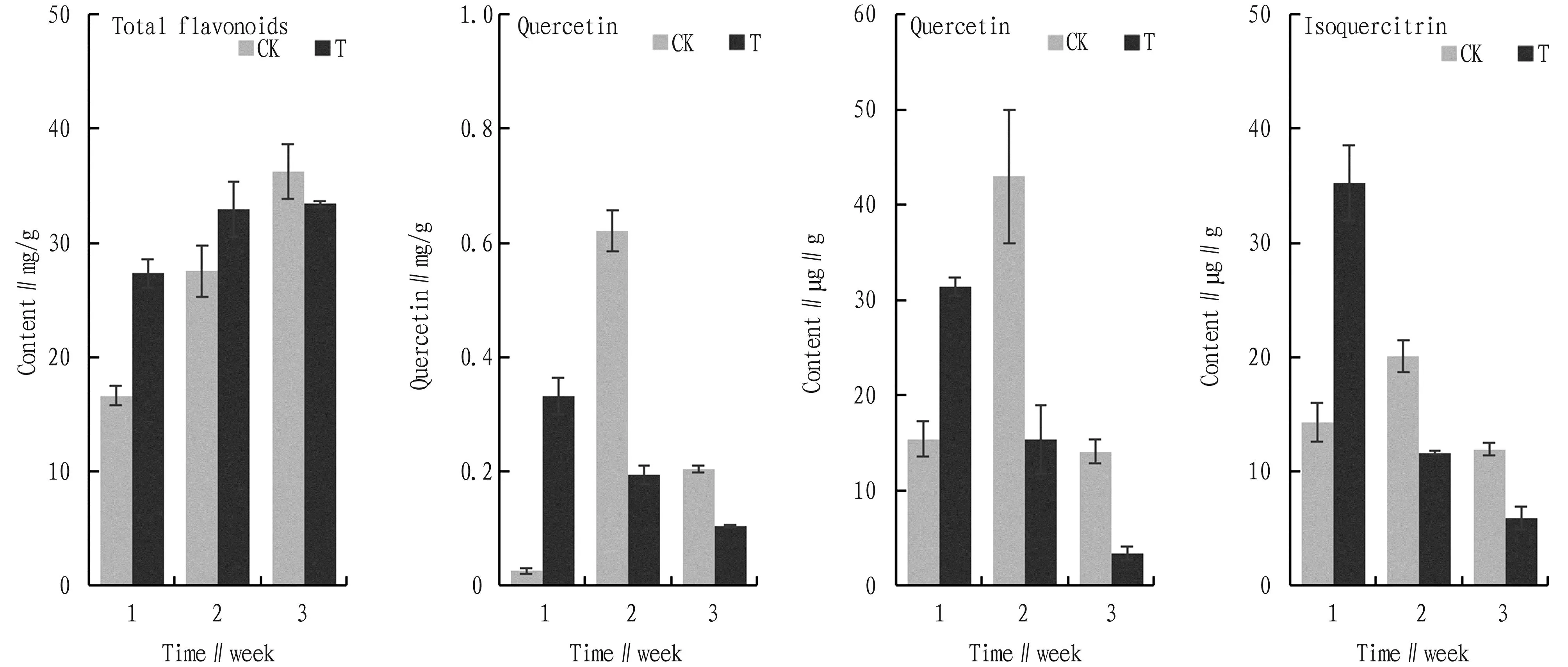
Fig.4 Effects of Fusarium sp. infection to primary metabolites of Camellia oleifera total flavonoids, quercetin, quercitrin and isoquercitrin
4 Discussion
Plants are subjected to a wide range of environmental stresses during their growth stages, including abiotic and biotic stresses. Abiotic stresses, including drought stress and salt stress, have been extensively studied, but biotic stresses, especially pathogenic fungi stresses, have been less widely studied. Pathogenic fungi stress also is a kind of environmental stress. Pathogenic fungi stresses are very common in crop growth. For example, tomato wilt caused byRalstoniasolanacearum[2, 9]was found to be the most destructive disease[15-16]. It can cause a severe impact to the soil environment. Air, water and soil are considered to be the transmission medium for the most pathogenic fungi[17-21]. Therefore, when monocultures are grown for a long period of time, the problem of slow growth was found. The relative abundance of potential pathogens in the soil increases can lead to problems such as pathogenic infections[22-24].C.oleiferais one of the most pathogen tolerant plants and understanding how it responds to pathogen stress is an important step in hopefully solving the problem of crop diseases caused by pathogen infection. The rhizosphere is an interesting region where plant and microbial activity are most closely linked. Plants are able to release products such as sugars and amino acids in this region, and microorganisms are able to utilize these carbon sources to perform essential metabolic activities for life. And some metabolites of microorganisms can also be utilized by the host plant. The frequent plant and microbial activities make the ecological environment of this region significantly different from that of other regions, and thus the strong fungal resistance ofC.oleiferamay be closely related to its rhizosphere microbial structure and environment.
Fusariumsp. is classified as one of the pathogenic fungi around the world and the soil borne pathogenic fungus can seriously harm the healthy growth of crops. Plant diseases caused byFusariumsp. is one of the major causes of yield loss in many crops around the world. As the world population’s demand for food gradually increases, crop diseases have also become more widely prevalent and the seriousness of the damage they cause to crops is being constantly emphasized. In recent years, knowledge of microorganisms has led to new insights into the control ofFusariumsp. For example, inhibiting the growth ofFusariumsp. by using microorganisms that are antagonistic toFusariumsp. can be a new approach. The screening of such microorganisms is usually done by isolating them from plant tissues or from the rhizosphere. The microbial community structure in the rhizosphere which is influenced by plant secretion is different from other soil environments. Microbial structure and composition vary from plant to plant. Root secretions produced by plants attract and recruit specific microbial species to the rhizosphere. These microorganisms can have an effect on plant growth and resistance. For instance, maize flavones enrich the rhizosphere Oxalobacteraceae to improve maize performance under nitrogen deprivation[25]. The rhizosphere microorganisms are also a source from which plants acquire disease resistance. The activities of specific microorganisms cause changes in the physicochemical properties of the rhizosphere soils[25, 27]. Infection by soil borne pathogenic fungi can cause changes in soil microbial structure and soil nutrients, and these changes in the soil environment caused by pathogenic fungi infection can lead to changes in plant metabolic activity, ultimately affecting plant growth and metabolic activity.
This study analyzed the effects ofFusariumsp. infection on the physicochemical properties and soil enzyme activities ofC.oleiferarhizosphere soil. Various environmental stresses and pollution can cause decrease of soil enzyme activities and soil quality[28-30]. Some studies have found that heavy metal contamination leads to a decrease in soil quality and enzyme activity. Soil enzymes have been defined as suitable indicators for evaluating soil quality, which are sensitive to changes in the structure of soil microbial communities and have an important relationship with plant nutrient absorption[31]. Our study found that infection of pathogenic fungi significantly affected the rhizosphere soil enzyme activities ofC.oleifera. In particular, the activity of soil phosphatase was most influenced by the pathogenic fungi, and the activity of sucrase and urease and soil nutrient elements were decrease. This is due to the invasion ofFusariumsp. into theC.oleiferarhizosphere and the changes in soil enzyme activities are closely related to microbial activities. In addition, the decrease of soil pH may also be a reason for the change in soil enzyme activities. Soil enzymes play a key role in plant nutrient intake, transforming the form of nutrients, exist to facilitate plant intake. Therefore, there is a direct relationship between the level of soil enzyme activity and soil nutrient elements content. In this study, we found thatFusariumsp. infection lead to a decrease in soil nutrient elements content, including ammonium nitrogen, available phosphorus, available potassium, and organic matter. The reduction of soil nutrient elements content and soil enzyme activity can affect plant growth. In the adverse environment, plants are able to maintain normal physiological activities by regulating the antioxidant enzyme system in the body in order to protect the plant health. Thus, we investigated the changes of POD and SOD activities and MDA content inC.oleiferatissue when it was infected byFusariumsp. fungi. Reactive oxygen species (ROS) produced by cellular metabolism are ability to cause severe effects on the organisms and ultimately result in biological damage when plants are subjected to adversity stress.
SOD and POD constitute the main barrier of the plant ROS clearance system, effectively inhibiting the harm of ROS to biological organisms and the level of their enzyme activities reflects the plant’s resistance to environmental stress[32]. When plant is subjected to environmental stress, protective enzymes are activated. In order to maintain normal plant growth and reduce ROS damage, plants can then use these enzymes to eliminate excess free radicals by increasing or decreasing antioxidant enzyme activities. Some studies have shown that Ceratocystis fimbriata leads to an increase and then a decrease in SOD enzyme activity, an increase and then a decrease in POD and finally a flattening out and a gradual increase in MDA content in the sweetpotato. This study showed thatC.oleiferainfected byFusariumsp. induced a defense response, which would maintain normal growth and metabolism ofC.oleiferaby increasing SOD enzyme activity, and then POD enzyme activity decreased. MDA is one of the most important products of membrane lipid peroxidation, and its content can reflect the degree of peroxidation of plant cell membranes, which can indirectly reflect the plant’s ability to resist adversity. And the higher content indicates that the more serious the membrane lipid peroxidation in the plants. The accumulation of heavy metal cadmium cause the increase of MDA content inP.striotes, which indicates that the accumulation of cadmium can induce more serious the plant membrane lipid peroxidation[33]. Our results showed that the MDA content ofC.oleiferaincreased in a short period when it was infected byFusariumsp. fungi, which indicates that the antioxidant system ofC.oleiferawas not able to effectively solve the damage caused by cellular lipid peroxidation of membranes, but the MDA content ofC.oleiferashowed a decrease over time which suggests that in the long term, the antioxidant system ofC.oleiferais able to effectively alleviate the peroxidation of membranes to maintain the normal physiological activities and metabolic balance.
Flavonoids are one of the important secondary metabolites secreted by plants and have biological activities such as antioxidant and antimicrobial. The release of flavonoids by secreted plants into the plant rhizosphere region recruits specific microbiomes that promote plant growth under adversity and also help the plant to resist invasion by pathogenic fungi. In addition, antimicrobial activity of flavonoids also can help plants to resist pathogenic fungi. The flavonoids ofC.oleiferais rich, and quercetin, quercitrin and isoquercetin are the main secondary metabolites ofC.oleifera.Fusariumsp. infection intoC.oleiferacan lead to an increase in the content of total flavonoids, andC.oleiferamay be able to increase its resistance toFusariumsp. through the synthesis of flavonoids, which makesC.oleiferaa strong antifungi agent. The antioxidant properties of total flavonoids and the antioxidant enzyme system together form the antioxidant system ofC.oleifera, which helpsC.oleiferato be able to maintain normal growth in such environmental stresses. Although flavonoids have some antimicrobial capacity, this is not the only reason whyC.oleiferahas strong disease resistance. This should be further researched. In conclusion, our study found thatFusariumsp. infection can lead to a decrease in enzyme activity and nutrient elements content ofC.oleiferarhizospheres soil, andC.oleiferaresists this environmental stress by regulating peroxidase activity and the content of its secondary metabolite.
5 Conclusions
In this study, we found thatFusariumsp. infection was a key factor of altering the physiological metabolism ofC.oleiferaand soil enzyme activities. Soil enzyme activities and the content of soil nutrient elements were differently changed under the infection ofFusariumsp. stress, compared with the control group. In addition, the different changes of the main secondary metabolites and antioxidant enzyme activities ofC.oleiferaalso were found. These changes may have a potential effect on the growth ofC.oleiferaand helpC.oleiferato overcome external stresses underFusariumsp. infection and other environmental stresses. Our study identified the response ofFusariumsp. stress on secondary metabolites and soil enzyme activities ofC.oleifera. In future, the response mechanism ofC.oleiferatoFusariumsp. stress should be further researched. The problem of crop exposure to pathogenic fungi must be taken into concern and the problem of pathogen infiltration must be addressed from a new perspective.
 Asian Agricultural Research2024年2期
Asian Agricultural Research2024年2期
- Asian Agricultural Research的其它文章
- Food Security Problems and Solutions in China Based on the Strategy of Sustainable Agricultural Development
- Discussion on Dust Removal (Suppression) System of Crushing Station in Open-Pit Coal Mine
- Promoting High-quality Development of Grain and Oil in Ethnic Areas of the Yangtze River Economic Belt from the Perspective of Agricultural Power
- A Comparative Study on Factors Influencing Food Security in China and India
- Animal Safety Test of Bacillus thuringiensis BT Protein
- Study on Germplasm Resources of Peach Cultivars
