Effect of overheating-induced minor addition on Zr-based metallic glasses
Fu Yang(杨福), Zhenxing Bo(薄振兴), Yao Huang(黄瑶), Yutian Wang(王雨田),Boyang Sun(孙博阳), Zhen Lu(鲁振), Baoan Sun(孙保安),4,Yanhui Liu(柳延辉),3,†, Weihua Wang(汪卫华),3,4, and Mingxiang Pan(潘明祥),3,4,‡
1Institute of Physics,Chinese Academy of Sciences,Beijing 100190,China
2Center of Materials Science and Optoelectronics Engineering,University of Chinese Academy of Sciences,Beijing 100049,China
3School of Physical Sciences,University of Chinese Academy of Sciences,Beijing 100049,China
4Songshan Lake Materials Laboratory,Dongguan 523808,China
Keywords: metallic glass,thermal properties,melt treatment,overheating,oxygen content
1.Introduction
Metallic glasses (MGs), rapidly quenched from molten alloy liquids, are often considered frozen liquids because the solid MGs inherit to some extent the liquid structure.[1]The disordered atomic structures of MGs result in many unachievable properties with crystalline alloys, such as high strength,[2-6]large elastic limit,[7-9]and thermal-plastic formability.[10-12]Because the atomic structures of MGs are believed to depend on thermal history,[13]many efforts have been devoted to the investigation on the effects of melt treatment, in particular, overheating and quenching temperature,Tq, on thermal stability,[14-30]glass formation ability (GFA),[31-36]magnetic[37-40]and mechanical properties,[27,28,41-44]and corrosion resistance.[45,46]Substantial changes in properties have been experimentally observed.However, the trend of these changes is often inconsistent in reported investigations.For example, in some investigations overheating the molten alloy to a high quenching temperature could lead to higher glass transition temperatureTgand crystallization temperatureTxof the obtained MGs,[14,16-18,21-25,28,47,48]while decreasedTxwith increasingTqwas reported in other investigations when the molten alloy was overheated.[25,26]In addition,it was found that glass transition and supercooled liquid region (SLR) disappear when glass-forming alloys are overheated and quenched from highTq.[25]Nonetheless, the persistence of distinct glass transition and SLR in the MGs quenched from highTqwas also reported.[14-24]The variation of MG properties has been predominately ascribed to the evolution of locally ordered clusters and precipitation of high-temperature phases when the molten alloy is overheated and quenched at differentTq.The MGs obtained from a highTqwere assumed to have a more homogenous structure.However,the investigations by MD simulations suggested that the effect ofTqon the formation of MG is negligible.[49]
For the investigations on the effect of melt treatment,Zrbased MGs were often chosen as model materials because their superior GFA allows changes ofTqin a wide temperature range.[3,50]Additionally, induction melting in a quartz tube was usually used to overheat the molten alloys because the temperature measurement is more convenient during overheating.However, Zr has a large chemical activity with oxygen at high temperatures, and a large amount of oxygen has been detected in the MGs quenched from highTqthrough induction melting.[31,48]Although it is well known that minor additions have a substantial impact on the formation and properties of MGs,[51]the influence of oxygen has not received sufficient attention.As a consequence,it remains elusive whether the property variation of the overheated Zr-based MGs originates from a more homogenous structure or the incorporation of elements such as oxygen.
In this work, we systematically investigate the effect ofTqon the thermal properties, crystallization kinetics, relaxation behaviors, and mechanical properties of Zr50Cu36Al14MG ribbons.We found that with the increase ofTqduring the melt spinning process,the properties of Zr50Cu36Al14MG ribbons undergo significant changes.Through chemical composition analysis, it turns out that the effect ofTqactually roots from the redox reaction between molten alloy and quartz tubes,which renders silicon and oxygen elements incorporated into Zr50Cu36Al14MG ribbons.These results can enable us to comprehensively understand the role of melt treatment on the properties of MGs, and provide some experimental foundation for the control of the structure-performance relationship in glass-forming alloys.
2.Experiment
Master alloys with the nominal compositions (at.%) of Zr50Cu36Al14and Au50Cu25.5Ag7.5Si17were prepared by arcmelting a mixture of pure metals in a Ti-gettered argon atmosphere.The purities of the raw materials are Zr 99.9%,Cu 99.99%, Al 99.999%, Au 99.999%, Ag 99.999%, and Si 99.999% (all in wt.%), respectively.In order to achieve homogeneity in composition, each ingot was remelted more than six times.Melt spinning was used to prepare MG ribbons.The preparation was carried out under the protection of a high-purity argon atmosphere after the vacuum reached 8×10-4Pa.The ingots were heated by induction heating in quartz tubes to a specificTqand then immediately quenched the melt at thatTq.The MG ribbons were obtained by quenching the molten liquid at different temperatures.We used a new quartz tube for every MG ribbon.The molten liquid temperature was measured by a radiation thermometer with an accuracy of±20 K.The quartz-tube nozzle hole diameter,the copper wheel rotation speed,and ejection pressure difference were set to 0.4 mm,2400 rpm,and 0.2 MPa,respectively.The average oxygen contents were measured by a high-temperature thermal decomposition analyzer(Yanrui NO-330)with an accuracy of 1 wt.ppm for oxygen.The compositions of the MG ribbons were analyzed by employing inductively coupled plasma-atomic emission spectroscopy(ICP-AES).
The x-ray diffraction(XRD)analysis was carried out by using a Bruker D8 Advance diffractometer with a CuKαsource.A high-resolution transmission electron microscope(HRTEM) was also used to characterize the structure of MG ribbons obtained from differentTq.The HRTEM specimens were prepared by ion milling with 3 kV Ar ions.HRTEM observation was carried out by a transmission electron microscope (TEM, JEM-2100PLUS, JEOL, 200 kV) with a field emission gun.
A Perkin-Elmer differential scanning calorimetry(DSC)8000 was employed to examine the thermodynamic properties of the MG ribbons at a heating rate of 20 K·min-1with 20 mL·min-1flowing pure argon to prevent possible surface oxidation.The melting points and liquidus temperatures were measured by a differential thermal analyzer (Netzsch DSC 404C).The dynamic mechanical analysis (DMA) measurements were performed on a TA DMA Q800 in a temperatureramp mode under the frequency of 1 Hz and an amplitude of 10 µm.The stress relaxation measurement was performed through DMA at a temperature of 0.8Tgand a tensile strain of 0.7%.
Nanoindentation measurements were carried out on Hysitron TI980 using a calibrated diamond Berkovich indenter with a nominal tip radius of curvature of 50 nm under the load-controlled mode.The tests were conducted by using partial unload mode,which included 33 loading-unloading cycles from 500 µN to 10000 µN at room temperature.Each cycle comprised of loading, holding, and unloading.The loading time,hold time,and unloading time were all one second.The unloading fraction parameter which controlled the percentage of unloading for each unloading segment was set to 0.5.
3.Results and discussion
Figure 1(a) shows XRD patterns of Zr50Cu36Al14MG ribbons obtained from differentTq.It can be seen that there only exist broad diffraction peaks without any sharp crystal diffraction peaks within the resolution of XRD, indicating the amorphous structure of the MGs.In order to exclude the possibility of nanocrystalline precipitation in the MG ribbons,we also characterized the structure of the MGs through HRTEM and selected area electron diffraction (SAED).Figures 1(b)-1(f) show the HRTEM images and SAED patterns of Zr50Cu36Al14MG ribbons prepared from 1.1Tlto 1.5Tlin turn.The maze-like patterns and diffraction halos confirm that the alloys are of disordered structure without the formation of nanocrystals.
Figure 2(a)shows the DSC curves for Zr50Cu36Al14MG ribbons prepared from variousTq.As can be seen from the DSC curves, the calorimetricTggradually shifts towards a higher value whenTqincreases from 1.1Tlto 1.4Tl.However, when the MG ribbon is quenched from 1.5Tl, neither glass transition nor SLR could be observed.This phenomenon is consistent with the previous report,[25]and the disappearance of glass transition and SRL was ascribed to the change of atomic configurations from densely-coordinated to looselycoordinated atomic packing.Whereas, it suggested that the phenomenon could be observed only whenTqis 700 K aboveTl.[25]In our case,the phenomenon takes place at a lowerTq,i.e.,theTqis only 400-500 K higher thanTl.According to previous investigations, distinct glass transition and SLR should be observable in our case.[14-24]
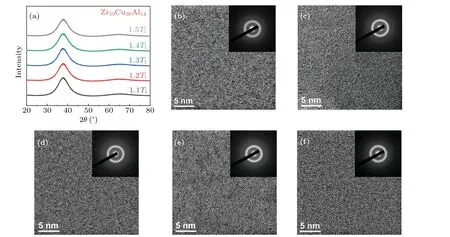
Fig.1.Structural characterization.(a) XRD patterns of Zr50Cu36Al14 melt-spun MG ribbons prepared by melt spinning from different quenching temperatures(Tq)ranging from 1.1Tl to 1.5Tl.HRTEM and SAED images for the Zr50Cu36Al14 melt-spun MG ribbons quenched from(b)1.1Tl,(c)1.2Tl,(d)1.3Tl,(e)1.4Tl,and(f)1.5Tl.
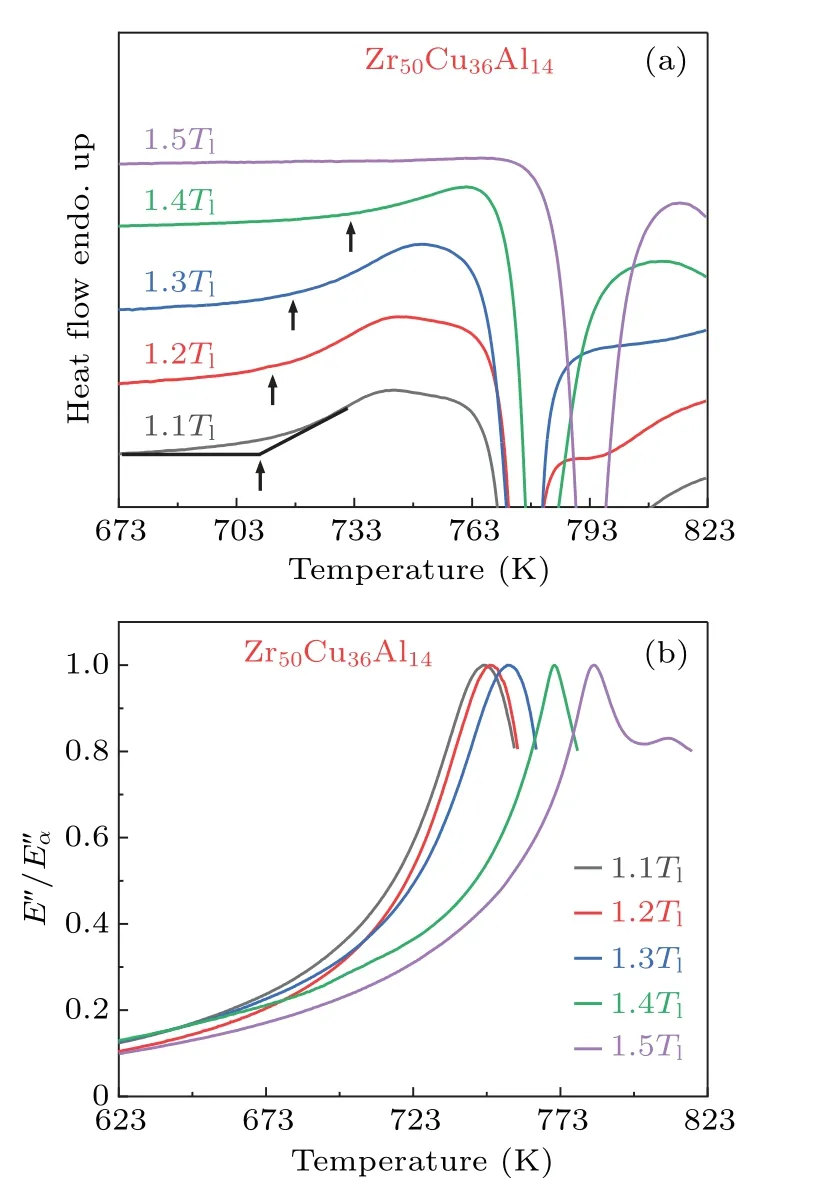
Fig.2.The variation of glass transition.(a)Glass transition characterized by DSC for Zr50Cu36Al14 melt-spun MG ribbons prepared from different Tq.The heating rate is 20 K/min. Tg is marked out with the arrows and defined from the onset of the transition as indicated by the intersection of the black lines.(b)The normalized loss modulus E′′/E′′α at frequency f =1 Hz and heating rate of 3 K/min.The peak temperature is taken as the kinetic glass transition temperature Tα.
To confirm the disappearance of glass transition,we conducted DMA measurements.In contrast to other thermodynamic methods such as DSC and thermal mechanical analysis,DMA is more sensitive in detecting the occurrence of various atomic motions and transformations, and the glass transition can be revealed as a dynamic process at the dynamical glass transition temperatureTα.Figure 2(b)illustrates the temperature dependence of the normalized loss modulus,E′′/E′′α, for Zr50Cu36Al14MG ribbons.One can see thatTαincreases from 748 K to 784 K whenTqvaries from 1.1Tlto 1.5Tl.This indicates that higher energy is required to activate the cooperative rearrangement of atoms in the MGs quenched from higher melt temperatures.The measurement results by DMA are consistent with those by DSC.In both cases, the glass transition temperature shifts to a higher value whenTqis increased.Furthermore,we prepared Zr50Cu36Al14MG ribbons at the sameTqbut at different spinning speeds of the copper wheel.As shown in Fig.S1,Tαremains unchanged.This suggests that the cooling rate has a negligible contribution to the increase ofTα.It is worth mentioning that the MG ribbon obtained from the highTqof 1.5Tlstill shows a distinct dynamic glass transition.
In general, the enhanced kinetic stability of MGs can be interpreted from the perspective of potential energy landscape (PEL).[52-55]Hence, we probed the distribution of relaxation energy barriers for the Zr50Cu36Al14MG ribbons.Figure 3(a) shows the isothermal stress relaxation responses of Zr50Cu36Al14MG ribbons.Figure 3(b)presents the distributions of relaxation energy barriers derived from the stress relaxation curves according to the activation energy spectrum model.[56,57]It can be seen that for the MG with a higherTq,the energy barrier distribution shifts towards a higher value.This suggests that the MGs quenched from higher temperatures are located in the deeper basins in PEL.Figure 3(c)shows the apparent relaxation time,τ, obtained by fitting the stress relaxation curves with the KWW equation.[58,59]As can be seen,τincreases from 1209±28 s to 2812±45 s whenTqincreases from 1.1Tlto 1.5Tl.This indicates that the atomic mobility in Zr50Cu36Al14MG ribbons reduces with the rise ofTq.
In addition, the ease of glass transition which involves cooperative rearrangement of atoms[60]can be quantified by the apparent activation energy (Eg).Figure 3(d) shows the DSC traces with the heating rates ranging from 20 K/min to 140 K/min for the Zr50Cu36Al4MG ribbon quenched at 1.3Tl.It can be seen thatTgand the temperature of the first crystallization peak(Txp)shift to higher temperatures with the increase of the heating rate, which arises from the involvement of thermal activation in these kinetic processes.The activation energy for glass transition and crystallization can be obtained through the Kissinger equation[57,61]
whereΦis the heating rate,Cis a constant,Ris the ideal gas constant, andE*is the apparent activation energy.As shown in Fig.3(e), the activation energy increases remarkably with the increase of quenching temperatures.For example,Egis enhanced from 660±48 kJ·mol-1to 991±52 kJ·mol-1whenTqincreases from 1.1Tlto 1.5Tl.It should be noted that theEgvalue for the MG prepared from 1.1Tlis in line with that reported in the literature.[62]We also estimated the fragility(m)of the Zr50Cu36Al4MG ribbons quenched from different temperatures(Fig.3(f)).As can be seen, with the increase ofTq,the MGs become more and more fragile.

Fig.3.The evolution of atom motion in solid glass and supercooled liquid.(a) The stress relaxation curves of Zr50Cu36Al14 MG ribbons prepared from different Tq.In the stress relaxation experiments,a tensile strain ε =0.7%is applied to the MG ribbons at 0.8Tg (619 K).Solid lines are fittings to the data.(b) Normalized activation energy spectra P(E).(c) Variation of relaxation time τ as a function of Tq.(d) DSC traces for the Zr50Cu36Al14 MG ribbons quenched from 1.3Tl.The heating rates range from 20 K/min to 140 K/min. Tg is marked out with the arrows.(e)Activation energies of glass transition and crystallization as a function of Tq for Zr50Cu36Al14 MG ribbons.The temperature of the first crystallization peak is taken as Txp.(f)Dependence of fragility m of Zr50Cu36Al14 MG ribbons on Tq.
We also investigated the influence ofTqon the crystallization kinetics of the Zr50Cu36Al4MG.The MG ribbons prepared from 1.1Tl, 1.4Tl, and 1.5Tlwere heated to their respectiveTx, and then the evolution of crystallization phases with isothermal time was investigated(Fig.4).We found that the MG ribbons quenched from 1.4Tland 1.5Tlcan maintain the amorphous structure if they are heated to their correspondingTxand immediately cooled down to room temperature(see Figs.4(b) and 4(c)).In contrast, the diffraction hump is distorted for the MG ribbon quenched at 1.1Tl, suggesting the formation of nanocrystals in the alloy.After isothermal annealing for 2 min atTx, sharp diffraction peaks emerge in all three alloys,indicating the precipitation of crystalline phases.For the MG prepared from 1.1Tl, the crystalline phases are identified to be CuZr2, Al3Zr, and AlCu2Zr after isothermal annealing for 2 min.Prolonged annealing leads to the formation of AlZr3and AlCu4phases.However, for the MGs prepared from 1.4Tland 1.5Tl, the crystalline phases do not change with annealing time.The crystalline phases are identified to be mainly Cu10Zr7, AlZr2, and Al3Zr2, respectively.These observations manifest that the variation ofTqcan lead to different crystallization processes, which probably depend on the evolution of the structures,types,and quantity of local atomic clusters with the increase of melt temperatures.

Fig.4.The crystallization and phase formation.XRD patterns for Zr50Cu36Al14 melt-spun MG ribbons prepared from(a)1.1Tl,(b)1.4Tl,and(c)1.5Tl.

Fig.5.The modulus and hardness characterization.(a) The average modulus E and hardness depend on Tq for a series of Zr50Cu36Al14 melt-spun MG ribbons determined by nanoindentation measurements.The error bars were obtained by standard deviation from the statistical average value of six test sites in each alloy.(b) Variation of Tα as a function of E.
We utilized nanoindenter to measure the modulusEand hardness.TheEand hardness dependence onTqis illustrated in Fig.5(a)for Zr50Cu36Al14MG ribbons.With the increase ofTqfrom 1.1Tlto 1.5Tl,Eincreases from 100.67±0.76 GPa to 116.84±0.85 GPa.The hardness also increases,i.e.,from 6.05±0.07 GPa to 7.38±0.10 GPa.Figure 5(b)showsTαas a function ofEfor Zr50Cu36Al14MG ribbons quenched from different temperatures.It can be seen thatTαincreases monotonically withE.Those results are in line with reported results thatTgand hardness are directly proportional toE.[54]One can see that the MG ribbons obtained from higherTqshow higherTα,hardness,andE.
The variation in the properties of MGs withTqhas been reported in previous literature.In the majority of past studies, the structural origin of property changes is generally interpreted as the gradual dissipations of local atomic clusters or the nucleation cores at high melt temperatures so that less ordered clusters are retained in MGs after quenching.[14,21,22,24,25]Nevertheless,it has been reported that the elevation ofTqwill lead to the increase of oxygen content in the alloy.[31,48]To understand theTq-dependence of property changes, we carefully analyzed the chemical compositions of the MG ribbons quenched from different melt temperatures.Figure 6(a)shows the dependence of the analytical oxygen content onTqfor a series of Zr50Cu36Al14MG ribbons.It can be seen that the oxygen content in MG ribbons increases monotonically with the increase ofTq.This invokes us to scrutinize the correlation between the property changes and the variation of oxygen content.We gathered data on the change ofTgand oxygen content in Zr-based MGs fabricated at differentTqfrom the literature,and found that the greater the extent of variation in oxygen content,the more pronounced the magnitude of change inTg(see Fig.S2).[25]It is known that the constituents in Zr-based alloy have a chemical affinity with oxygen, in particular, at high temperatures.[31]For example,the heat of formation for ZrO2is about 1100 kJ·mol-1,larger than that of SiO2(908 kJ·mol-1), which is the main component of quartz tubes.Thermodynamically,O can be absorbed by Zr through the redox reaction at high temperatures.[31]Moreover, we found that the content of Zr does slightly decrease with the increase ofTq, and there exist 0.76 at.% and 1.24 at.%Si in the MG ribbons quenched from 1.4Tland 1.5Tl,respectively(as shown in Table 2).This indicates that the incorporation of oxygen in the Zr-Cu-Al alloys is associated with the reaction of molten alloy with SiO2.This is supported by the chemical analysis of a Fe-based MG, as shown in Table S4.We found that the variation of oxygen content is ignorable between Fe78Si9B13ingot and the ribbon quenched from a higher temperature(1576 K).1.1Tl49.71 35.79 13.87 - 0.52 1035 1.2Tl49.10 36.26 13.92 - 0.53 1941 1.3Tl48.73 36.34 13.91 - 0.53 4961 1.4Tl47.88 35.71 13.71 0.76 0.52 14227 1.5Tl47.26 34.91 13.66 1.24 0.51 24457
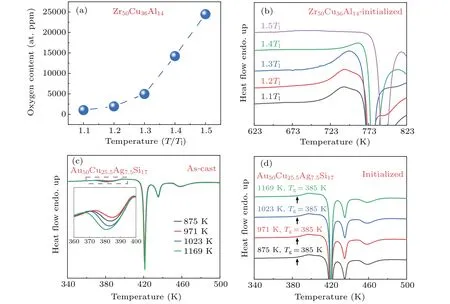
Fig.6.Confirming oxygen as the dominant factor for the change of thermal properties.(a) The Tq-dependence of oxygen content for the Zr50Cu36Al14 melt-spun MG ribbons.(b)DSC curves of Zr50Cu36Al14 melt-spun MG ribbons after initialized by heating to the supercooled liquid region.(c)DSC curves of Au50Cu25.5Ag7.5Si17 melt-spun MG ribbons prepared by melt spinning from different Tq of 875 K,971 K,1023 K, and 1169 K.The inset in (c) is the enlargement of the DSC curves related to the relaxation enthalpy regions.(d) DSC traces of Au50Cu25.5Ag7.5Si17 melt-spun MG ribbons initialized by heating to 405 K.
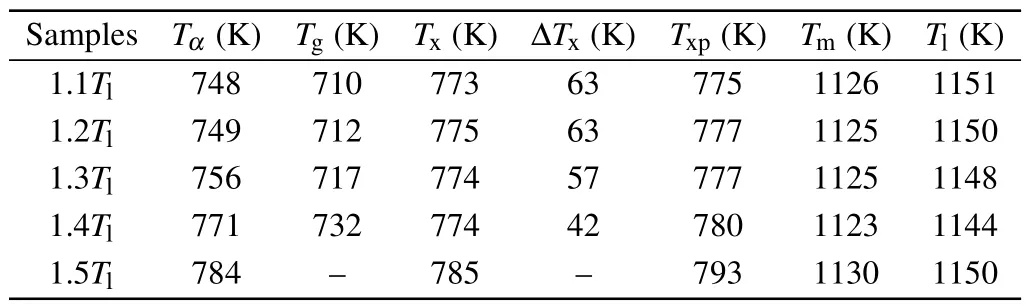
Table 1.Calorimetric glass transition temperature (Tg), kinetic glass transition temperature(Tα),onset crystallization temperature(Tx),the temperature of the first crystallization peak (Txp), the width of the supercooled liquid region(ΔTx),melting point(Tm)and liquidus temperature(Tl)of Zr50Cu36Al14 MG ribbons prepared from different temperatures.

Table 2.The compositions of Zr50Cu36Al14 melt-spun MG ribbons determined by chemical analysis.
In order to further confirm that the property changes of the Zr50Cu36Al14MG ribbons prepared from differentTqresult from the redox reaction between molten alloy and quartz tubes, we prepared Au50Cu25.5Ag7.5Si17MG ribbons which are chemically inert.[63]As in the case of Zr50Cu36Al14MG,the Au50Cu25.5Ag7.5Si17MG ribbons were prepared from variousTqranging from 875 K to 1169 K,which are 220-520 K higher thanTl(Tl=655 K)(as shown in Figs.6(c)and 6(d)).To eliminate the history-dependence and better compare the thermal properties of different samples, the as-quenched MG ribbons were initialized.After initialization, DSC measurements on the Au-based MG indicate that theTgandTxremain essentially unchanged whenTqis varied.[64]In comparison,theTgandTxof Zr50Cu36Al14MG after initialization still maintain the difference with the elevation ofTq(Fig.6(b)).These results clearly demonstrate that the effect of overheating temperature on the variation ofTgandTxis negligible in Zr50Cu36Al14MG ribbons.Therefore,it is considered that the property changes for Zr50Cu36Al14MGs with the variation ofTqhighly possibly arise from the reaction of the molten alloy with quartz tubes,rather than the dissipation of locally ordered clusters or high-temperature crystalline phases.
In the present system, the atomic radii of components are Zr=2.06 °A, Cu=1.45 °A, Al=1.18 °A, Si=1.11 °A,and O = 0.48 °A.Compared with the original constituents,Si and O are of smaller atom sizes.Therefore, the incorporation of Si and O elements will increase the degree of atomic size difference between the alloying elements.At the same time, smaller atoms are prone to occupy interstitial spaces in the alloy, improving the local packing efficiency.Furthermore, the Si and O elements possess large mixing heat with other constituent elements.For example, the mixing heat values of Zr-Si, Cu-Si, Al-Si, Zr-Cu, Zr-Al, Al-Cu are-84 kJ/mol,-19 kJ/mol,-19 kJ/mol,-23 kJ/mol,-44 kJ/mol,and-1 kJ/mol,respectively.[65]These large negative values of mixing heat enhance the atomic interactions and promote chemical short-range order, which can render long-range atomic diffusion difficult.It has been reported that the addition of oxygen can result in the formation of Zr-O octahedral structure with an O atom surrounded by six Zr atoms.[66,67]The bond energy of Zr-Zr, Cu-Cu, Al-Al, and Zr-O is 298.2±0.1 kJ·mol-1, 182±2.5 kJ·mol-1, 264.3±0.5 kJ·mol-1, and 766.1±10.6 kJ·mol-1, respectively.[68]It can be seen that the Zr-O atomic pair possesses the highest bond energy, indicating that the atomic bonding in the Zr-O local clusters is stronger than that in the other clusters.In a word, the addition of Si and O elements will facilitate the formation of local atomic clusters with stronger interatomic interaction.To activate the diffusion of these clusters, higher energy is required.With the increase ofTq, more Si and O elements are reacted into the MG ribbons, so the atomic cooperative rearrangements are delayed to higher temperatures,leading to an elevatedTg(orTα) upon heating, a higherE,and hardness.In addition,the gradual increase of local atomic clusters, especially for Zr-O local clusters, will lead to the reduction of preferentially activated atoms involved in glass transition.This is reflected in the signal intensity of glass transition in DSC curves.This may be the reason why glass transition and SLR disappeared in the DSC curves for the MGs quenched from 1.5Tl.On top of that, the structure, type, and quantity of local atomic clusters will evolve with the variation of Si and O contents,which causes the variation in crystallization kinetics and crystalline products with the elevation ofTqfor Zr50Cu36Al14MG ribbons.
4.Conclusions
In summary,our results indicate that the variation of thermal properties of the Zr-based MG ribbons obtained by overheating arises from the incorporation of oxygen and silicon due to the reaction of the molten alloy with quartz tubes.We found thatTgmonotonically increases with the elevation ofTq.For the MGs quenched from 1.5Tl,the SLR disappears and the intensity of glass transition weakens upon heating, at which the MGs should have a distinct glass transition and SLR according to reported literature.Through chemical analysis,we confirmed that the variation of thermal properties caused by overheating mainly originates from the incorporation of oxygen and silicon elements.The oxygen and silicon are incorporated by the redox reaction between Zr elements and SiO2tubes during melting.Due to the smaller atom size and large negative mix heating values with other constituents,the incorporation of both Si and O can enhance the interatomic interaction,and even facilitate the formation of local atomic clusters with strong interatomic bonding.This slows down the motion of atoms and hinders the cooperative rearrangement of atoms during the glass transition process, and thus enhances the kinetic stability of the MGs.Since induction melting followed by injection casting is one of the most commonly used techniques for manufacturing BMGs including Zr-based BMGs,our results suggest that in some cases the reaction of quartz tubes with molten alloy should be taken into account, especially when correlating properties with MG composition.
Acknowledgements
The authors thank Zijian Wang, Rui Zhao, and Huaping Zhang for helpful discussions.The work was financially supported by the National Key Research and Development Program of China (Grant Nos.2018YFA0703600,2021YFA0716302,and 2021YFA0718703),the National Natural Science Foundation of China (Grant Nos.51825104 and 52192602), and China Postdoctoral Science Foundation(Grant No.2022T150691).
- Chinese Physics B的其它文章
- Does the Hartman effect exist in triangular barriers
- Quantum geometric tensor and the topological characterization of the extended Su–Schrieffer–Heeger model
- A lightweight symmetric image encryption cryptosystem in wavelet domain based on an improved sine map
- Effects of drive imbalance on the particle emission from a Bose–Einstein condensate in a one-dimensional lattice
- A new quantum key distribution resource allocation and routing optimization scheme
- Coexistence behavior of asymmetric attractors in hyperbolic-type memristive Hopfield neural network and its application in image encryption

