巯基改性蒙脱石对中国北方中碱性农田土壤镉钝化效果研究
朱利文,任超,李竞天,田沛宜,肖建辉,李萍
(1. 河南省第一地质矿产调查院有限公司,河南 洛阳 471000;2. 河南省生态环境与勘查地球化学应用工程技术研究中心,河南 洛阳 471000)
镉是一种剧毒重金属,在土壤中有高迁移性、长持久性和生物累积性,对人体健康、动物和植物构成威胁[1],土壤镉污染形势不容乐观,亟待解决。钝化是镉污染土壤修复的主要技术之一,国内外已有大量研究[2-3],但大部分研究针对的是酸性镉污染土壤[4-6],中国北方和南方部分地区多为中碱性土壤[7-9],其镉污染农田的钝化修复材料仍有待研究。蒙脱石是一种2∶1型硅铝酸盐矿物[10],因其特殊层状结构使之比表面积较大,对土壤重金属离子吸附能力较强,被用于钝化修复土壤重金属污染[11-13]。然而,天然蒙脱石表面硅氧结构的亲水性较强、键合能力较弱,导致其吸附过程可逆,吸附效果有限,钝化效果不稳定[14-15],在吸附钝化土壤重金属镉应用上受到限制,因此目前研究热点集中于在使用前对其进行改性,进而增强吸附能力[16-18]。
巯基具有很强的络合能力,黏附性能强,且巯基是一种弱碱、Cd2+是一种弱酸,基于软硬酸碱理论,巯基与Cd2+可形成稳定的结合态,能很好地吸附重金属离子[19-20]。庞婷雯等[21]对天然膨润土进行巯基化、钠化、酸化改性后,开展了Cu2+、Pb2+、Zn2+吸附试验,发现巯基化改性膨润土无论在单一重金属离子的等温吸附环境下,还是在竞争吸附环境下均表现出较好的吸附能力。朱霞萍等[22]采用溶液法对蒙脱石进行巯基改性,通过开展材料表征、动力学和热力学试验发现,改性蒙脱石对Cd的吸附容量提高了近39倍。巯基改性可使钝化材料对重金属离子的吸附能力得到大幅提升,可应用于钝化修复土壤镉污染。目前,已有研究证实了巯基改性蒙脱石对中国南方酸性镉污染土壤存在钝化效果。朱凰榕等[23]将天然蒙脱石进行改性,制成巯基改性蒙脱石,通过盆栽和大田实验表明,巯基改性蒙脱石对南方酸性镉污染土壤(pH 5.03~5.86)有钝化修复效果,土壤Cd水溶态和离子交换态含量均有所减少,有效地抑制了小白菜对Cd的吸收。曾燕君等[24]通过吸附解吸实验也证实材料改性处理能明显提高对Cd的饱和吸附容量,可有效地钝化南方酸性Cd污染土壤。但蒙脱石改性前后对北方中碱性Cd污染农田土壤的钝化研究鲜有报道,考虑到钝化材料对Cd2+的吸附容量受土壤pH值影响较大[22,25],蒙脱石改性前后对北方中碱性镉污染农田土壤的钝化效果有待进一步研究。
本文以蒙脱石为原材料,将巯基基团负载在其表面或层间制备巯基改性蒙脱石,并借助X射线衍射(XRD)、扫描电镜(SEM)、透射电镜(TEM)和傅里叶红外光谱(FTIR)表征巯基改性蒙脱石的特性,同时,开展室内培养试验研究巯基改性蒙脱石在不同添加量的情况下对中国北方中碱性农田土壤Cd的钝化效果,探究蒙脱石改性前后、不同添加量对北方中碱性农田土壤Cd钝化修复效果的影响,最后结合材料表征,分析巯基改性蒙脱石对Cd的钝化机制,以期为修复北方Cd污染农田土壤提供理论依据。
1 实验部分
1.1 实验材料
1.1.1 供试土壤
供试土壤采自河南省洛阳市栾川县赤土店镇镉污染农田,按照梅花五点法采集0~20cm混合土壤样,土壤类型为褐土,质地为粉壤土。经除杂、自然风干、研磨后保存备用。
1.1.2 钝化材料
蒙脱石(M):购自宜阳天冠膨润土有限公司,是由当地矿山开采破碎磨细制得。
巯基改性蒙脱石(GM):以天然蒙脱石矿物为原材料,经破碎、球磨后粒度达到300目后备用;按质量比例(蒙脱石粉∶去离子水=1∶20)混合,充分搅拌均匀后加入9.6%的无水乙醇和1.2%的3-巯丙基三乙氧基硅烷混合剂,常温搅拌6h,用去离子水充分洗净,于80℃下烘干研磨制得,干燥储存,备用。
1.2 实验方法
镉污染土壤制备:将99.95%的氯化镉(半五水合物)作为镉源配制成镉溶液,加入供试土壤中,充分混匀,并采用称重法调节土壤含水量保持在田间持水量的70%,在25±2℃的恒温培养箱中培养7天后,取出自然风干,作为镉污染的土壤用于试验,按每份100g土样装入5号自封袋中储存备用。
室内培养试验:共设置7个处理。空白对照处理CK;添加1%、3%、5%(占供试土壤质量百分比,添加到每份100g污染土壤中)的蒙脱石粉处理M1、M3、M5;添加1%、3%、5%的巯基改性蒙脱石粉处理GM1、GM3、GM5;每个处理设置3个重复。按比例将钝化材料添加到Cd污染土壤中,于5号自封袋内充分混匀。经上述处理的土壤样品分别准确移至相应的玻璃培养皿内(模拟实验的土壤深度15mm),每隔2天用去离子水给土壤补充水分,采用称重法控制土壤含水量保持在田间持水量的70%,盖上培养皿盖,置于25±2℃的恒温培养箱内培养,与此同时用塑料勺对土壤样品进行搅拌混合,确保多次充分混合。分别在7、15、30、50、70天分5次取样,每次称取20g土样在自然状态下风干过20目筛,测定土样的pH和全Cd、有效态Cd及Cd各赋存形态含量。
1.3 测试项目与测试方法
测试项目和具体检测分析方法及依据见表1。包括:对供试土壤样品进行pH、阳离子交换量(CEC)、有机质、营养元素和Cd含量分析,对室内培养试验得到的土壤样品进行pH、全Cd、有效态Cd和Cd各赋存形态含量分析。对钝化材料进行pH、CEC、重金属全量、BET、XRD、SEM、TEM和FTIR分析。具体检测分析方法及依据见表1。所有样品检测均做两次平行实验,测定结果取平均值,平行样测量值的相对偏差小于10%。

表1 样品的检测分析方法及依据Table 1 Detection and analysis methods and their basis of samples.
2 结果与讨论
2.1 供试土壤的基本性质
本研究目的是钝化材料对镉污染农田土壤镉钝化(降低其有效性)的影响,并为研发镉污染农田土壤钝化材料提供依据。为使研发的材料性能更贴近实际,实地采集了镉污染土壤,对其理化性质、营养元素和重金属Cd元素进行分析检测。
土壤pH值为7.96,全镉含量为1.33mg/kg,CEC为12.0cmol(+)/kg,有机质含量为15.4g/kg,全氮、全磷和全钾含量分别为0.99、1.74、21.7g/kg,碱解氮、有效磷和速效钾含量分别为107、14.8、84.2mg/kg。
2.2 钝化材料的基本性质和材料表征
2.2.1 基本性质
蒙脱石pH值为10.64,CEC为41.6cmol(+)/kg,重金属Cd、Hg、As、Pb、Cr、Cu、Ni、Zn含量为0.07、0.04、9.60、41.9、24.1、8.00、5.53、79.9mg/kg,考虑到田间实际应用,根据《耕地污染治理效果评价准则》(NY/T 3343—2018),蒙脱石作为钝化材料投入品,满足不对耕地或地下水造成二次污染的要求。
蒙脱石的比表面积为17.14m2/g,平均孔径7.28nm。巯基改性蒙脱石的比表面积为10.74m2/g,平均孔径27.90nm。
2.2.2 X射线衍射表征晶体结构
通过XRD来分析晶体结构信息变化,如图1所示,蒙脱石主要含有SiO2、KAlSi2O3等矿物,巯基改性蒙脱石与蒙脱石相比,两者XRD图谱基本一致,说明这种改性方法没有破坏原始矿物结构,巯基可能被负载在材料层间,也可能包裹在材料外面,进而完成改性。
图1 蒙脱石和巯基改性蒙脱石的X射线衍射图谱Fig. 1 X-ray diffraction patterns of montmorillonite and thiolmodified montmorillonite.
2.2.3 扫描电镜表征微观结构
由蒙脱石、巯基改性蒙脱石的SEM图像(图2)可看出,蒙脱石(图2中a,b)为致密的片层状结构,表面结构平坦规整,层状结构间的通道和聚集颗粒间的空隙为重金属离子的吸附提供空间。巯基改性后的材料(图2中c,d)表面结构卷曲、松散,这可能是由于硅烷的黏度较大,导致小颗粒团聚。聚集形态不规则,颗粒间吸附空位明显增多,表面粗糙疏松,说明巯基在蒙脱石内部负载并不均匀,对原蒙脱石也有表面改性的作用[26]。
2.2.4 透射电镜表征颗粒形貌和元素分布
由图3可以看出,改性前的蒙脱石(图3中a,b)为致密的片层状结构,内部出现小部分且少量的团聚现象,其内部元素分布相对均匀。经过巯基改性后的蒙脱石(图3中c,d)与未改性前相比粒度明显增大,与SEM分析结果一致,改性前蒙脱石呈片状,改性后片状消失,但改性前后元素并未发生改变且分布均相对均匀。
2.2.5 傅里叶红外光谱表征官能团信息
改性前后的FTIR图谱(图4)存在明显差异,改性前的天然蒙脱石FTIR图谱显示出,3634cm-1附近是结构层内的-OH伸缩振动峰,3434cm-1附近是吸附的层间水形成的-OH伸缩振动峰,1643cm-1附近是C=O伸缩振动峰,1424cm-1附近是C-O反对称伸缩振动峰,1037cm-1附近是Si-O-Si反对称伸缩振动峰,796cm-1附近是Si-O-Si对称伸缩振动峰。巯基改性蒙脱石在上述位置均出现特征吸收峰,且在3419cm-1附近的-OH伸缩振动峰和1640cm-1附近的C=O伸缩振动峰强度均明显增强,表明负载巯基后没有改变蒙脱石固有的官能团,但增强了-OH和C=O化学键的活性。另外,在2000~3000cm-1还增加了两个吸收峰,2932cm-1附近是饱和C-H对称伸缩振动,2027cm-1附近是S-H伸缩振动。
根据SEM、TEM分析及BET结果,改性后蒙脱石粒度增大,平均孔径由7.28nm增加至27.90nm,层间距减小,比表面积由17.14m2/g减少至10.74m2/g,而巯基位于另一端,也可以有效地嫁接于蒙脱石表面或层间,巯基在蒙脱石上成功实现负载,形成了稳定的巯基改性蒙脱石。结合FTIR分析图谱,发现改性后的蒙脱石不仅新增了C-H、S-H共价键,而且增强了-OH和C=O化学键的活性。
2.3 钝化材料对土壤pH的影响
土壤重金属钝化修复是通过施加钝化材料与土壤重金属发生吸附、离子交换、络合、沉淀、固胶体、氧化还原等作用来降低土壤重金属的活性[27-28],其中碱性物质,如硅酸钠、石灰、碳酸盐类等,钝化机理是通过提高土壤的酸碱度,增加其吸附重金属离子的能力,促进重金属在土壤中的沉淀反应,减少其迁移性[29];黏土矿物,如蒙脱石、海泡石、高岭土等,因其特殊的晶体结构,具有较大的孔容和比表面积,层间离子可交换[12],能与重金属发生吸附、离子交换、共沉淀和配位反应[13],进而降低土壤中重金属离子浓度与活性。针对中国北方广泛分布的中碱性土壤[8],考虑到钝化材料的施加会影响土壤pH,过高的pH易导致土壤板结、保水保肥能力降低[9],故针对其重金属污染问题,不适合施加pH调节型碱性钝化材料,环境友好且有一定钝化效果的黏土矿物或改性黏土矿物更适用于北方中碱性农田土壤。在本研究中,添加不同剂量的蒙脱石、改性蒙脱石后,土壤pH分别提高了0.12~0.78、0.30~0.85,提升幅度不大(表2)。这可能是由于蒙脱石自身呈碱性且含有羟基等基团[30],使土壤pH值升高。与蒙脱石相比,巯基改性蒙脱石对土壤pH也有轻微升高,可能是由于改性材料是3-巯丙基三乙氧基硅烷,它的酸度系数(pKa)比较高,可达到10.39,因此巯基改性蒙脱石加入土壤后确实可在一定程度上提升土壤的pH值,但影响不大。

表2 不同钝化处理对土壤pH值的影响(70天)Table 2 Effects of different passivation treatments on soil pH(70d).
2.4 钝化材料对土壤中镉的钝化效果
2.4.1 钝化材料对土壤有效态镉含量的影响
重金属元素的有效态含量是影响其在土壤的生物有效性和移动性的主要因素,影响重金属被植物吸收累积程度,能很好地表征其污染特征以及对植物毒害程度[31-32]。蒙脱石、改性蒙脱石在1%、3%、5%添加量下土壤有效态Cd含量的变化见图5。两者均能降低土壤有效态Cd含量,但改性蒙脱石对有效态Cd含量的降低效果显著优于蒙脱石。
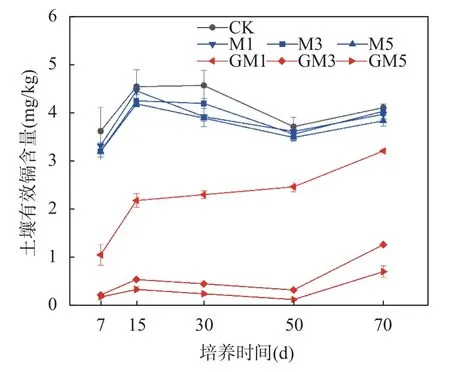
图5 钝化材料1%、3%、5%添加量下土壤有效态镉含量的变化Fig. 5 Changes of soil available cadmium content under 1%,3% and 5% addition of passivation materials.
蒙脱石本身具备一定的离子交换性及吸附性,对土壤有效态Cd含量的降低幅度仅为1.80%~6.71%,效果不显著(p<0.05),这与化党领等[33]、任露陆等[34]的研究结果一致。Gupta等[35]研究表明,有效态Cd含量的降低主要是由于蒙脱石自身的负电荷对Cd2+的静电吸附作用,其次是由于蒙脱石表面或层间的-OH、OH2+等基团与Cd2+、CdOH+发生的离子交换行为,而且其-OH官能团能与Cd发生配位吸附,形成Cd(OH)2沉淀。改性后制得的巯基改性蒙脱石在吸附土壤有效态Cd时表现出高效性能,对土壤有效态Cd含量的降低幅度达到21.92%~82.90%,效果显著。添加改性蒙脱石70天后,土壤有效态Cd含量略有提升,而且改性蒙脱石与蒙脱石均出现同样的趋势,该变化可能与培养过程中土壤性质的变化有关,并不是改性蒙脱石特有。不同添加量的钝化材料对有效态Cd的降低效果也有所差别,其中,蒙脱石的不同添加量对土壤镉的钝化效果差异不大,1%、3%、5%的添加量对土壤有效态Cd分别降低了3.37%、1.80%、6.71%。改性蒙脱石1%、3%、5%的添加量对土壤有效态Cd分别降低了21.92%、69.11%、82.90%。由此可见,改性蒙脱石对土壤镉的钝化效果显著,钝化效果与添加量成正比,施用5%改性蒙脱石对土壤镉的钝化效果最好,土壤有效态Cd含量降低幅度高达82.90%。
卿艳红等[12]研究发现,巯基改性的蒙脱石既具备蒙脱石自身的尺寸稳定性、吸附性和阻隔性,又具有硅烷分子多种功能基团的反应活性。结合材料表征结果,XRD分析结果显示出改性后蒙脱石的结构并未发生改变,对有效镉仍具备静电吸附、离子交换吸附、羟基配位吸附能力。FTIR分析结果显示,改性后蒙脱石对土壤中有效态Cd含量的大幅降低主要来源于两方面:一方面是由于改性后新增了C-H、S-H共价键,能与Cd2+发生巯基配位吸附作用,其作用力明显强于静电吸附、离子交换吸附和羟基配位吸附作用力,这与以往[24,36]的研究结果一致;另一方面是由于改性后增强了-OH和C=O化学键的活性,增强了蒙脱石原有的羟基配位吸附作用,反应示意图见图6。
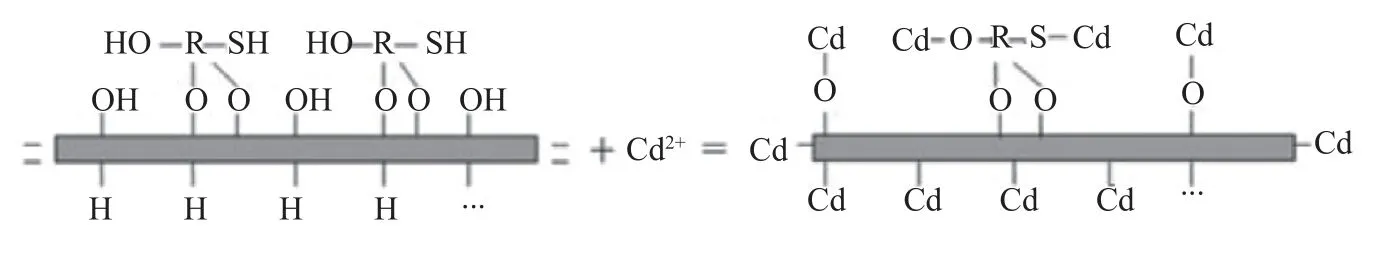
图6 巯基改性蒙脱石与镉的反应示意图Fig. 6 Reaction diagram of mercapto thiol-modified montmorillonite with Cd.
2.4.2 钝化材料对土壤镉赋存形态的影响
本研究钝化修复Cd污染土壤的主要原理是基于对土壤Cd赋存形态的转化,故分析改性前后的蒙脱石对土壤Cd赋存形态的影响。考虑到待测土壤样品的pH值为7.91~9.26,偏碱性,采用Tessier修正顺序七步提取法对重金属活性态进行提取[37]。原状土的Cd以离子交换态和碳酸盐结合态为主要赋存形态,蒙脱石改性前后均对土壤Cd赋存形态有所影响,培养70天后土壤镉的赋存形态变化如图7所示。

图7 添加钝化材料培养70天后土壤镉赋存形态变化Fig. 7 The change of cadmium speciation in soil after 70 days of incubation with passivation materials.
添加蒙脱石对土壤Cd赋存形态的影响不大,变化幅度小于5%。培养70天后(图7a),水溶态、强有机结合态和硅酸盐残余态Cd含量基本无变化,其他形态Cd含量发生一定程度的变化,离子交换态Cd所占比例降低1.17%~4.87%,碳酸盐结合态、腐植酸结合态、铁锰氧化物结合态Cd所占比例分别升高0.97%~3.53%、0.74%~1.06%、0.19%~0.51%。添加蒙脱石后土壤Cd主要由离子交换态转化为碳酸盐结合态,仍以这两种赋存形态为主。
添加改性蒙脱石70天后,土壤Cd赋存形态发生显著改变(图7a),与空白对照组相比,水溶态Cd含量略有提升,但仍保持在低位稳定状态,离子交换态Cd所占比例大幅降低(12.76%~40.84%),碳酸盐结合态、腐植酸结合态、铁锰氧化物结合态Cd所占比例分别提高了2.86%~8.45%、2.76%~9.34%、6.67%~34.75%,而强有机结合态、硅酸盐残余态Cd含量略有下降。添加巯基改性蒙脱石后,主要促使土壤Cd形态从离子交换态转化为铁锰氧化物结合态。不同添加量的改性蒙脱石对土壤Cd赋存形态分布差异显著(1%、3%和5%添加量对土壤Cd赋存形态的影响分别见图7中b、c和d),随着改性蒙脱石添加量的增加,离子交换态Cd含量大幅降低,铁锰氧化物结合态Cd含量大幅增加,碳酸盐结合态Cd含量也有提升,其他形态(水溶态、腐植酸结合态、强有机结合态、硅酸盐残余态)Cd含量变化不大,这可能是由于土壤添加改性蒙脱石后并没有改变土壤有机质含量和性质[38],因此,其他形态发生变化过程中,没有影响强有机结合态Cd含量。通过对比改性前后的蒙脱石对土壤Cd赋存形态的影响差异,对于改性蒙脱石而言,巯基的增加可与土壤Cd发生巯基配位吸附[39],促使离子交换态转变为铁锰氧化物结合态,另外羟基的活性增强可与土壤Cd发生羟基配位吸附,促使离子交换态转化为碳酸盐结合态,两者均可使土壤Cd的活性降低。
3 结论
用3-巯丙基三乙氧基硅烷对天然蒙脱石进行改性后能够实现巯基在蒙脱石上成功负载,形成稳定的巯基改性蒙脱石。改性后的蒙脱石不仅新增了C-H、S-H共价键,而且增强了-OH和C=O化学键的活性,并通过与Cd2+发生巯基及羟基的配位吸附作用,提高蒙脱石对土壤镉的吸附能力。当施加5%巯基改性蒙脱石时,土壤有效镉的降低幅度可在对照组6.71%的基础上,提高到82.90%。
施加巯基改性蒙脱石后,土壤Cd赋存形态发生显著改变,离子交换态大幅减少,新增的巯基配位吸附作用使其转化为铁锰氧化物结合态,增强的羟基配位吸附作用使其转化为碳酸盐结合态。巯基改性蒙脱石对中国北方镉污染农田土壤的钝化效果显著,可作为北方镉污染农田土壤安全利用的钝化材料。
BRIEF REPORT
Significance:Heavy metal pollution in farmland soil is the main factor affecting the environmental quality and safety of agricultural products in China. Passivation material is a key material for repairing heavy metal contaminated soil in farmland. Research and development of efficient soil heavy metal passivation materials is very important for repairing heavy metal contaminated farmland and ensuring the food safety of agricultural products.Passivation is one of the main technologies for the remediation of Cd-contaminated soil. There have been a lot of studies at home and abroad[2-3], but most of the studies are aimed at acidic Cd-contaminated soil[4-6]. Most of the northern and southern parts of China are alkaline soil[7-9], and the passivation remediation materials for Cdcontaminated farmland still need to be studied.
The passivation effect of montmorillonite before and after modification on the alkaline Cd contaminated farmland soil in northern China needs to be further studied. Montmorillonite is a 2∶1 type aluminosilicate mineral[10]. Due to its special layered structure, it has a large specific surface area and strong adsorption capacity for heavy metal ions in soil, and has been used to passivate and repair heavy metal pollution in soil[11-13]. However, due to the strong hydrophilicity and weak bonding ability of the silicon-oxygen structure on the surface of natural montmorillonite, the adsorption process is reversible, the adsorption effect is limited, and the passivation effect is unstable[14-15], which is limited in the application of adsorption and passivation of soil heavy metal Cd. Therefore,the current research focuses on the modification of natural montmorillonite before use, thereby enhancing the adsorption capacity[16-18]. The thiol group has a strong complexing ability, strong adhesion, and is a weak base. Cd2+is a weak acid, based on the theory of soft and hard acid-base, thiol group and Cd2+can form a stable binding state,and can be a good adsorption of heavy metal ions[19-20]. At present, studies have confirmed that thiol-modified montmorillonite has a passivation effect on acidic Cd-contaminated soil in the south. However, considering that the adsorption capacity of passivation materials for Cd2+is greatly affected by soil pH[22,25], and the passivation of montmorillonite before and after modification on alkaline Cd-contaminated farmland, soil in the north is rarely reported. Therefore, this study was carried out to analyze the changes in material characterization before and after montmorillonite modification, the passivation effect of montmorillonite before and after modification, and different amounts of montmorillonite on Cd in alkaline farmland in the north.
Methods:Montmorillonite was used as the raw material, and the thiol group was loaded on the surface or interlayer of montmorillonite to prepare thiol-modified montmorillonite. The characteristics of thiol-modified montmorillonite were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and Fourier transform infrared spectroscopy (FTIR). At the same time, indoor soil experiments were carried out to study the passivation effect of thiol-modified montmorillonite on Cd in northern alkaline farmland soil with different addition amounts, and explore the effects of montmorillonite modification and different addition amounts on the passivation and repair effect of Cd in northern alkaline farmland soil. Finally, combined with material characterization, the passivation mechanism of thiol-modified montmorillonite on Cd was analyzed in order to provide a theoretical basis for the remediation of Cd-contaminated farmland soil in northern China.
A total of 7 treatments were set up. Blank control treatment CK; montmorillonite powder treatments M1, M3 and M5 were added with 1%, 3% and 5% (percentage of soil quality, added to 100g contaminated soil per share).GM1, GM3 and GM5 were treated with 1%, 3% and 5% thiol-modified montmorillonite powder. Each treatment was set up with 3 replicates. The passivation material was added to the Cd-contaminated soil in proportion and fully mixed in the No.5 self-sealing bag. The soil samples treated above were accurately moved to the corresponding glass culture dish (the soil depth of the simulation experiment was 15mm), and the soil was replenished with deionized water every 2 days. The soil moisture content was controlled by the weighing method to maintain 70% of the field water holding capacity, and the culture dish was covered and placed in a constant temperature incubator at 25±2℃. At the same time, the soil samples were stirred and mixed with a plastic spoon to ensure multiple full mixing. Samples were taken 5 times at 7, 15, 30, 50 and 70 days, and 20g soil samples were weighed and dried in natural state through 20 mesh sieves. The pH, total Cd content, available Cd content and Cd content of each form were determined. The specific detection and analysis methods and basis are shown in Table 1.
Data and Results:The crystal structure, microstructure, particle morphology and functional group information were analyzed by material characterization techniques. The change of crystal structure was analyzed by XRD. The XRD patterns of montmorillonite and thiol-modified montmorillonite (Fig.1) were basically the same, indicating that this modification method did not destroy the original mineral structure. The thiol group may be loaded between the layers of the material, or it may be wrapped outside the material to complete the modification. According to the results of SEM (Fig.2), TEM (Fig.3) and BET, the particle size of montmorillonite increased after modification, the surface structure changed from flat and regular to curly and loose, and the interlayer spacing decreased. The thiol group had been effectively grafted onto the surface or interlayer of montmorillonite, forming a stable thiol-modified montmorillonite. Combined with FTIR analysis (Fig.4), it was found that the modified montmorillonite not only added C-H and S-H covalent bonds, but also enhanced the activity of -OH and C=O chemical bonds.
The passivation effect of thiol-modified montmorillonite on Cd in soil was significant. After the addition of thiol-modified montmorillonite to the test soil, the ion-exchanged Cd in the soil was converted into an ironmanganese oxide-bound state due to the thiol coordination adsorption, and the enhanced hydroxyl coordination adsorption converted it into a carbonate-bound state. As a result, the occurrence form of Cd in the soil was significantly changed, the ion exchange state was greatly reduced, and the available Cd in the soil absorbed by the crop roots was significantly reduced. After adding 1%, 3% and 5% thiol-modified montmorillonite, the available Cd in soil decreased by 21.92%, 69.11% and 82.90%, respectively (Fig.5). As the control group, the addition of 1%,3%, and 5% montmorillonite only decreased by 3.37%, 1.80%, and 6.71%, respectively. The passivation effect of montmorillonite on soil Cd was significantly improved after thiol modification, and the decrease of available Cd in soil tended to increase with the increase of thiol-modified montmorillonite. When 5% thiol-modified montmorillonite was applied, the decrease of available Cd in soil could be increased to 82.90% on the basis of 6.71% in the control group. The passivation effect of thiol-modified montmorillonite on Cd-contaminated farmland soil in northern China is significant, and it can be used as a passivation material for safe utilization of Cdcontaminated farmland soil in Northern China.

