Photoperiod Mediates the Effects of Temperature and Light Intensity on the Proliferation of Ulva prolifera
JIANG Jianan, YU Yanyan, CHEN Yili, LI Yahe, and XU Nianjun
Key Laboratory of Marine Biotechnology of Zhejiang Province, School of Marine Sciences, Ningbo University,Ningbo 315211, China
Abstract In order to study the complex effects of photoperiod, temperature, and light intensity on the spore maturation and release number of Ulva prolifera, we cultured thalli segment (2 – 3 mm) under three different photoperiods (L: D = 12:12, 14:10 and 10:14),temperature (15℃ (LT), 25℃ (MT) and 30℃ (HT)) and light intensity (100, 200 and 400 μmol m−2 s−1, noted as LL, ML and HL,respectively) conditions. Then the maturation time, spore release number and chlorophyll fluorescence were analyzed. The results suggested that: 1) The spore maturation time was accelerated by higher temperature or higher light intensity from 62 h to 36 h, and changes in day length accelerated the spore maturation to a certain extent as compared with 12:12 light/dark cycle; 2) Higher light intensity significantly decreased the chlorophyll fluorescence (Fv'/Fm', NPQ, rETRmax and α) of the mature reproductive segment under 30℃ with 12:12 light/dark cycle. But when in the other photoperiods (10:14 and 14:10 conditions), the inhibitory effects of high light intensity were alleviated significantly; 3) The optimum condition for the spore maturation and release was 12:12 light/dark cycle, 25℃, 400 μmol m−2 s−1, with both shorter and longer photoperiod reducing the spore release number; 4) Higher light intensity significantly increased the spore release number under 25℃, but these effects were alleviated by 30℃ treatment. This study is the first attempt to elucidate the coincidence effects of photoperiod, temperature and light intensity on the reproduction of Ulva, which would help to reveal the mechanism of the rapid proliferation of green tide.
Key words light intensity; maturation; photoperiod; reproduction; temperature; Ulva prolifera
1 Introduction
Ulvaspecies were well-known as the green tides algae(Zhaoet al., 2013), and especiallyUlva proliferais the main causative species of green tide in the Yellow Sea(Wanget al., 2018; Sunet al., 2022a, b). In recent years,many studies about the effects of ecological factors, such as temperature, light, salinity, nutrient on the growth,physiological and biochemical characteristics ofU. proliferawere reported (Linet al., 2011; Liuet al., 2012; Cuiet al., 2015; Gaoet al., 2016; Jianget al., 2020a; Fuet al.,2022; Xiaet al., 2022). The daily specific growth rate ofU. proliferaseedlings was up to 80%, higher than otherUlvaspecies (Cuiet al., 2015). WhenU. proliferawas exposed to light intensities lower than 30 μmol m−2s−1,both the photosynthetic rate and growth rate decreased,resulting in reduced buoyancy and sinking, in turn affect their distribution (Xuet al., 2014). Additionally, when the light intensity was higher than the optimum light intensity,the photoinhibition occurred (Liuet al., 2012). Based on the previous studies, the optimal temperature and light intensity for the growth ofU. proliferawas about 20℃and 70 – 100 μmol m−2s−1(Cuiet al., 2015; Wanget al.,2018), but our previous study showed that the growth ofU. proliferaincreased with the increase of light intensity,with the higher growth rate observed under 400 μmol m−2s−1at 20℃ (Jianget al., 2020a), indicating the complex effects of multiple environmental factors on the growth of these species. Additionally, the complex reproductive strategies ofUlvawere also observed (Linet al., 2008;Hanet al., 2015; Cuiet al., 2018), so it is necessary to study the compound effects of multiple environmental factors on the reproduction ofUlvaspecies.
Previous studies showed that the asexual zoospores,sexual reproduction, anisogamy, regeneration from segments, protoplasts and cells, vegetable growth of germ cells and vegetable growth of filaceous microphyte have been reported inU. prolifera(Linet al., 2008; Cuiet al.,2018). The sporulation ofU. lobatawas enhanced by dehydration (Smith, 1947), fragmentation size and abrupt temperature changes (Gaoet al., 2017). The suitable light intensity for sporulation inUlvaspp. ranged between 40 and 75 μmol m−2s−1(Hiraoka and Shimada, 2004). However, the optimal temperature for the reproduction ofUlvaspp. was different, with 25℃ forU. prolifera(Zhaoet al.,2016) and 21℃ forU. mutabilis(Nordby, 1974). Moreover, the spore release was also affected by temperature(Han and Choi, 2005) and circadian rhythm (Lüninget al.,2008). While shorter photoperiod triggered reproduction,longer photoperiod prolonged the vegetative growth phase forLaminaria saccharina(Lüning, 1988) and sporulation was enhanced by the periodic increase (Sousaet al., 2007). Although many studies paid attention to the effects of changes in environmental factors on the growth,reproduction and photosynthesis ofUlvaspp., it is still unclear how the reproduction ofU. proliferais affected by multi-environmental factors, especially the interactive effects of temperature and light, including light intensity and photoperiod.
Considering the circadian rhythm in the seaweed, we choseU. prolifera(adult) as materials and three levels of photoperiod, temperature and light intensity were set and a full factorial experiment under laboratory conditions was performed. Then the maturation time, spore release number and photosynthesis were evaluated, in order to estimate the compound effects of photoperiod, temperature and light intensity as well as their interactions on the reproduction ofU. prolifera.
2 Materials and Methods
2.1 Species and Culture Conditions
Ulva prolifera(ca.3 mm length) obtained from Xiangshan Xuwen Seaweed Development Co., Ltd., Xiangshan County, Zhejiang Province, China (29˚05´065N, 121˚56´153E), which was the same species as green tide species through morphology and genotyped by sequencing the 18S and ITS rRNA genes (Wanget al., 2018). No specific permits were required for the using of this species. Before experiments, they were cultured in sterile artificial seawater (salinity 25) enriched with f/2 medium (without Si)(Guillard and Ryther, 1962) in an illuminated plant incubator (GXZ, Jiangnan Instrument Factory, Ningbo, China)at 25℃ and 80 μmol m−2s−1of photosynthetic active radiation (PAR: 12 L: 12 D) with aerated ambient air (350 mL min−1). GeO2(0.3 mg L−1) was added to remove diatom. Salinity was measured with a visual hand-held refractometer (Index Instruments, Ramsey, UK).
Based on the previous studies, the formation and release of spores ofU. proliferawere affected by segment cutting (Gaoet al., 2010; Heet al., 2019), so the adult thalli were cut into 2 – 3 mm segments (optimal length),and used in the following experiment.
2.2 Experiment and Design
To study the interaction effects of photoperiod, temperature and light intensity on the spore release ofU. prolifera, we set three light/dark cycles (L:D = 10:14, 12:12 and 14:10), three levels of temperature (15, 25 and 30℃,defined as low, mid and high temperature treatments,noted as LT, MT and HT treatments) and three levels of light intensity (100 (low light treatment; LL); 200 (mid light treatment; ML) and 400 μmol m−2s−1(high light treatment; HL). The 2 – 3 mm adult thalli segments (about 900– 1000 segments with approximately 0.08 g fresh weight)were washed 3 times for 15 min with sterile seawater to remove the sporulation inhibitors (Wichard and Oertel,2010), and then transferred to 200 mL sterile seawater(salinity 25) enriched with f/2 medium and aerated (350 mL min−1) with ambient air. Three replicates were used for each treatment, and the subsamples used for the determination of all the parameters were independent of three replicates.
2.3 Maturation Time Determination
The maturation time, which was defined as the time of the algae cell from vegetative state to reproductive state,was measured when the sporangia were formed and mature, through microscope observed and recognized by its yellow/brown color and morphology according to Jianget al. (2022).
2.4 Chlorophyll Fluorescence Measurements
A PSI fluorometer (AquaPen-C, Photon System Instruments, Czech Republic) was used to determine the effective quantum yield of PSII (Fv'/Fm'), the non-photochemical quenching (NPQ) and the rapid light curves(RLC) when the reproductive segment was matured. NPQ was measured after dark adaptation for 15 min, andFv'/Fm' were measured with the cultivated light intensity as the actinic light. RLC was fitted as rETR =I/(aI2+ bI+ c)(Eilers and Peeters, 1988), whereIis the photon flux density of activity light intensity (μmol m−2s−1), a, b and c are the adjustment parameters. The initial slop (α), the maximum electron transport rate (rETRmax), and the light saturation parameter (Ek) were expressed as a function of the parameters a, b, and c as follows:α=1/c; rETRmax=1/(b+2(a×c)1/2);Ek=c/(b+2(a×c)1/2).
2.5 Spore Release Number Measurement
Twelve mature fragments were chosen, and their surface area were measured, and then they were put into 24 well cell culture plate respectively. The plate included 0.5 mL sterile seawater (salinity 25) enriched with f/2 medium, then were cultured under the corresponding treatments. After 0.5 – 12 h, the spore was released. When the discharged areas were over 50%, it was defined as‘spore release’ (Danet al., 2002). We used the microscope to measure the spore release number through Lugol’s iodine solution. The spore release number was determined in each group. In details, the liquid with released spores was mixed fully by using an oscillator, then 50 μL liquid was added to a tube containing a Lugol’s iodine solution (about 2 μL), and mixed well. Both the plankton counting chamber and optical microscope(Olympus Corporation, Tokyo, Japan) were used to determine the spore number. Quantity of spore release (QSr)was calculated: QSr (cm2) = (S0·V0)/ (V1·St·2), where QSr is the cell dispersion (cm2) of alga per unit area,S0represents the number of cells in a single square in the counting plate under the microscope;V1is the amount of added liquid;V0is the total amount of liquid;Stis the surface area of the single layer of algal segment.
2.6 Statistical Analysis
Three replicates for each treatment were used in all the treatments. The data are shown as means ± SD. The normal distribution of all data and the homogeneity of variance were confirmed by Shapiro-Wilk’s and Levene’s tests (P> 0.05), respectively. The effects of photoperiod,temperature, light intensity and their interactions were analyzed by three-way analysis of variance. A turkey post hoc test (Turkey HSD) was performed to show difference between photoperiod, temperature and light intensity treatments. The significant levels were set asP< 0.05.
3 Result
3.1 Effects of Photoperiod, Temperature and Light Intensity on the Chlorophyll Fluorescence Parameters
There were significant effects of photoperiod, temperature and light intensity on the effective quantum yield(Fv'/Fm'), especially under 10:14 and14:10 light/dark cycles (Figs.1i – iii). In details, under 10:14 light/dark cycle,compared to LT,Fv'/Fm' was decreased significantly by MT and HT conditions under high light intensity (HL;400 μmol m−2s−1) (MT,F1,4= 16.07,P= 0.02; HT,F1,4=1201.58,P< 0.01), similar trend was observed under ML condition with 12:12 light/dark cycle (Figs.1i, ii) (MT,F1,4= 23.06,P= 0.01; HT,F1,4= 1188.1,P< 0.01). A higherFv'/Fm' was observed at MTML with 10:14, MTHL with 12:12 and MTLL with 14:10 light/dark cycles. How- ever,no significant effects of temperature were observed under LL with 10:14, HL with 14:10 light/dark cycles conditions (10:14, LL,F2,6= 0.05,P= 0.95; 14:10, HL,F2,6=3.94,P= 0.08) (Figs.1i, iii). High light intensity significantly decreased theFv'/Fm', especially at HT under all the three light/cycles (10:14,F1,4= 663.81,P< 0.01; 12:12,F1,4= 707.56,P< 0.01; 14:10,F1,4= 151.25,P< 0.01). The temperature, light intensity and photoperiod showed double or triple interactive effects on theFv'/Fm' and NPQ(Table 1).
The significant effects of photoperiod, temperature and light intensity on the non-photochemical quenching (NPQ)were also observed, especially under short (10:14) and long photoperiod (14:10) conditions (Figs.1iv – v). In detail, under LL condition with 10:14 light/dark cycle, compared to LT, NPQ was decreased significantly by higher temperature (MT,F1,4= 14.17,P= 0.02; HT,F1,4= 25.66,P= 0.01) (Fig.1iv), similar trend was observed under HL with 14:10 light/dark cycle conditions (MT,F1,4= 78.8,P< 0.02; HT,F1,4= 70.82,P< 0.01) (Fig.1v). These thalli cultivated under higher temperature and 10:14 light/dark cycle, high light increased the NPQ of this species, and similar trends were observed under all the three light intensities with 14:10 light/dark cycles. The temperature,light intensity and photoperiod showed double or triple interactive effect on theFv'/Fm' and NPQ (Table 1).

Fig.1 Effective quantum yield (Fv'/Fm') (i – iii) and non-photochemical quenching (NPQ) (iv – vi) of U. prolifera segment cultivated at 15, 25 and 30℃ under three light intensity levels. Different uppercase letters indicate significant differences between different light intensity levels for the same temperature, while different lowercase letters represent significant differences between temperature treatments at the same light intensity level (P < 0.05). ‘LL’, ‘ML’ and ‘HL’ mean low (100 μmol m−2 s−1), mid (200 μmol m−2 s−1) and high light (400 μmol m−2 s−1), respectively.
The fitting variables (rETRmax,αandEk) derived from the rapid light curves ofU. proliferacultured at different conditions are shown in Fig.2. Significant effects of the temperature, light intensity and photoperiod, as well as double or triple interactive effects on the rETRmaxwere observed (Table 1). In detail, under HL condition with 10:14 light/dark cycle, compared to LT treatment, rETRmaxwas significantly decreased by higher temperature(MT, F1,4= 14.17,P= 0.02; HT, F1,4= 25.66,P= 0.01),similar trends were observed under ML with 12:12, LL and HL with 14:10 conditions (12:12, ML, MT, F1,4=173.64,P< 0.01; HT,F1,4= 224.11,P< 0.01; 14:10, LL,MT,F1,4= 11.26,P= 0.03; HT,F1,4= 60.62,P< 0.01; HL,MT,F1,4= 10.53,P= 0.03; HT,F1,4= 1378.81,P< 0.01)(Fig.2i – iii). When the thalli were cultured under LT and 10:14 light/dark cycles condition, the rETRmaxincreased with the light intensity increasing, but higher rETRmaxwas observed at MTLL and HTLL treatments. However,when the photoperiod increased, higher rETRmaxwas observed under ML with 12:12 and LL with 14:10 light/dark cycles conditions (12:12,F1,4= 59.58,P< 0.01; 14:10,F1,4= 70.18,P< 0.01). Additionally, the effects of these factors (temperature, light intensity and photoperiod) on α were similar to those on rETRmax(Fig.2iv – vi) (Table 1).
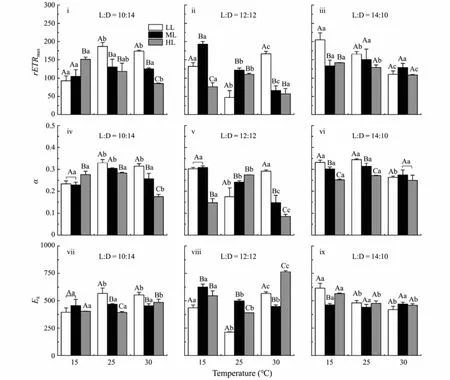
Fig.2 Maximum electron transport rate (rETRmax) (i – iii), initial slop (α) (iv – vi) and light saturation parameter (Ek) (vii – ix)of U. prolifera segment cultivated under different conditions. Different uppercase letters indicate significant differences between different light intensity levels for the same temperature, while different lowercase letters represent significant differences between temperature treatments at the same light intensity level (P < 0.05).
As forEk, the highest value was observed at HTHL treatment with 12:12 light/dark cycle, while the lowestEkwas observed at MT condition (Fig.2viii). Interactive effects of temperature, light intensity and photoperiod onEkwere observed (Table 1).
3.2 Effects of Photoperiod, Temperature and Light Intensity on the Maturation Time
The maturation time was significantly affected by photoperiod, temperature and light intensity (Figs.3i – iii; Table 2). In detail, under 10:14 light/dark cycle, low temperature (15℃; LT) significantly increased the maturation time under LL (100 μmol m−2s−1) and ML (200 μmol m−2s−1) conditions (LL, LT/MT,F1,4= 791.53,P< 0.01; LT/HT,F1,4= 3276.8,P< 0.01. ML, LT/MT,F1,4= 924.8,P< 0.01;LT/HT,F1,4= 952.2,P< 0.01) (Fig.3i). Under 12:12 light/dark cycle, high temperature (30℃; HT) significantly promoted the reproduction of this species (LL, HT/LT,F1,4= 237.58,P< 0.01; HT/MT,F1,4= 228.52,P< 0.01.ML, HT/LT,F1,4= 720.14,P< 0.01; HT/MT,F1,4= 75.82,P< 0.01) (Fig.3ii), no significant difference was observed between LT and MT treatments (LL,F1,4= 1,P= 0.37; ML,F1,4= 0.09, P = 0.78) (Fig.3ii). Under 14:10 light/dark cycle,higher light intensity (200, 400 μmol m−2s−1) significantly inhibited the spore mature at LT and MT conditions (LT,ML/LL,F1,4= 1300.5,P< 0.01; HL/LL,F1,4= 702.25,P<0.01; MT, ML/LL,F1,4= 72,P< 0.01; HL/LL,F1,4= 9.8,P= 0.04) (Fig.3iii), but no significant difference was observed at HT treatments (ML/LL,F1,4= 1,P= 0.37; HL/LL,F1,4= 0.5,P= 0.52) (Fig.3iii). These thalli cultivated under 10:14 light/dark cycle, their maturation time was significantly longer than 14:10 light/dark cycle (LTLL,F1,4= 3276.8,P< 0.01; LTML,F1,4= 72,P< 0.01; LTHL,F1,4= 961,P< 0.01; MTLL,F1,4= 177.94,P< 0.01; MTML,F1,4= 25.94,P< 0.01; MTHL,F1,4= 72,P< 0.01; HTLL,F1,4= 220.5,P< 0.01; HTML,F1,4= 24.14,P< 0.01;HTHL,F1,4= 64,P< 0.01). Interactive effects of temperature, light intensity and photoperiod on the release were observed (Table 2).

Table 2 Results of two-way analysis of variance for the effects of temperature and light intensity and photoperiod on maturation time of U. prolifera under different treatments

Fig.3 Maturation time of U. prolifera segment cultivated at 15, 25 and 30℃ under three light intensity levels. Different uppercase letters indicate significant differences between different light intensity levels for the same temperature, while different lowercase letters represent significant differences between temperature treatments at the same light intensity level (P< 0.05).
3.3 Effects of Photoperiod, Temperature and Light Intensity on Spore Release
There were significant effects of photoperiod, temperature and light intensity on the release of spores, especially under the middle and long photoperiod conditions(Table 3) (Figs.4i – iii). In detail, the highest spore release number was observed under MT with 12:12 light/dark cycles (Fig.4ii). But when the thalli were cultured under HL condition with 10:14 and 14:10 light/dark cycles, no significant effects of temperature were observed (Figs.4i,iii) (10:14, HL,F2,6= 1.04,P= 0.41; 14:10, HL,F2,6= 3.43,P= 0.1). Compared to 12:12 light/dark cycle, the spore release number was reduced by the changes in photoperiod (Figs.4i – iii). Higher light intensity significantly increased spore release number, especially at HT under all three light/cycle conditions (10:14, HL,F1,4= 11.66,P=0.03; 12:12, ML,F1,4= 93.03,P< 0.01; HL,F1,4= 11.82,P= 0.03; 14:10, ML,F1,4= 224.14,P< 0.01; HL,F1,4= 66.5,P< 0.01). Double or triple interactive effects of temperature, light intensity and photoperiod on spore release number were observed (Table 3).

Table 3 Results of two-way analysis of variance for the effects of temperature and light intensity and photoperiod on the spore release of U. prolifera under different treatments

Fig.4 Spore release number of U. prolifera at 15, 25 and 30℃ under three light tensity levels. Different uppercase letters indicate significant differences between different light tensity levels for the same temperature, while different lowercase letters represent significant differences between temperature treatments at the same light intensity level (P < 0.05).
4 Discussion
Previous study showed that the maturation period forU. proliferasporophytes could extend up to almost 40 days (Cuiet al., 2018) andUlvaspecies always exhibited vegetative growth for the rapidly expanding in initial blooms (Zhaoet al., 2019), but it was also difficult to maintainUlvaspecies in the vegetative state (Castelaret al., 2014). Previous study showed that light intensity higher than 16 μmol m−2s−1was suitable for the maturation and release of reproductive cells ofU. prolifera(Danet al.,2002), which was lower than the required light intensity for the optimal growth of this species (Cuiet al., 2015).In the natural sea, after the green tide disappeared, and the temperature and light intensity were lower thanUlvabloom period, they produced many spore cells and deposited on the sea bed to form a ‘seed bank’ (Huoet al.,2014). All these indicated that both the temperature and light not only affected the growth, but also affected the maturation and reproduction ofUlvaspp.
The previous study showed that the optimal temperature and light intensity for the spore release ofU. proliferawas 20 – 30℃, 180 – 300 μmol m−2s−1with the 12:12 light/dark cycle, respectively (Hanet al., 2015). In our experiments, the suitable condition for the spore maturation and release was about 25℃, 400 μmol m−2s−1with 12:12 light/dark cycle. The difference between our experiment and Hanet al. (2015) study can be explained by the growth light/temperature history (Haederet al., 2014;Gaoet al., 2017), salinity conditions (Sousaet al., 2007;Wanget al., 2012) and the fragment size of thalli (Gaoet al., 2017). In detail, the thalli used in this study was grown in the laboratory from seed to adult, the light intensity was constant and higher than the average light intensity in natural sea, but the sample used in Hanet al.(2015) was collected from Rudong sea area of Jiangsu in May. Additionally, the sporulation and spore release ofU.proliferawere affected by salinity. Previous studies showed that higher salinity (30 – 35) decreased the growth by inhibiting the nitrate reductase activity and increasing oxidative stress (Wanget al., 2012), promoting the transformation from the vegetative stage to the reproductive stage (Linet al., 2011), and increasing spore release by affecting the turgor pressure and diameter of the exit pore of sporangia (Hanet al., 2008). What’s more, the reproduction was also affected by fragment size, when the fragment diameter decreased from 10 to 0.9 mm, the reproductive rate ofU. pertusaincreased (Hiraoka and Enomo- to, 1998) and the spore release number ofU.rigidaincreased with the fragment diameter decreasing(Gaoet al., 2017).
The results of this study showed that spore formation was significantly affected by temperature, which was mediated by photoperiod and light intensity. When the temperature increased from 10 to 20℃, the reproductive period ofU. fenestratadecreased from 30 to 5 days (Kalita and Titlyanov, 2011). In this study, regardless of the light intensity and photoperiod, compared to 15℃, the maturation time was significantly decreased by 30℃, with the maturation time of 30 – 40 h, that’s means the higher temperature promoted the maturation of this species. It is well known that growth was significantly inhibited by high temperature, and the inhibitory effects can be offset by the high light intensity (Jianget al., 2020a). So, we speculate thalli accelerates mature to deal with the environmental stress. Moreover, temperatures promoted growth and reproduction by increasing enzyme activity, and increased nitrogen availability for supporting an accelerated synthesis of nucleotides and proteins (Heinrichet al.,2012). Considering the higher NPQ, maturation time and lower spore release for the thalli under LTML and LTHL treatments, it seems that the energy exist conversion between the growth and reproduction. Moreover, compared to low light intensity, we found a higher NPQ, lowerFv'/Fm', shorter mature time in the high light intensity,which might be caused by the enhancement oxidation state of the plastoquinone (PQ) pool. The decreasedFv'/Fm' could enhance oxidation state of the PQ pool could promote the spore formation ofU. prolifera(Wanget al., 2016). Additionally, the previous studies found that higher temperatures usually promote the reproduction and spores release ofUlvaspecies (Gaoet al., 2018; Jianget al.,2020b), but they do not affect or even inhibit spores release ofU. proliferain this study. That may be caused by the difference ofUlvaspecies and experiment condition.In detail,U. proliferaused in this study was grown in the laboratory from seed to adult, which is different from the other twoUlvaspecies (U. rigidaandU. intestinalis)which were collected from intertidal areas. Moreover, the reproduction rate could be reduced by higher temperature under nitrogen enrichment condition (Jianget al., 2020b),the experiment condition for this study was nitrogen enrichment, that’s may be the reason why spore release inhibited by higher temperature (25℃ and 30℃). Although we do not get enough information based on the current results in this study, in-depth omics studies have been done in order to verify the supposition.
Similar to the promote effects of photoperiod on the growth ofU. prolifera(Liet al., 2018), the maturation time and spore release number were affected by the photoperiod in this study. Previous studies showed that the day length plays a crucial role in the reproduction of seaweed (Hiraoka and Enomoto, 1998; Forbordet al., 2012).In our experiment, compared to the 12:12 light/dark cycle,the short photoperiod reduced the spore release number,especially for the that at 25℃, but theFv'/Fm' and light utilization efficiency (α) were increased, indicating the rate of photosynthesis was increased to use the light of the shorter illumination period most efficiently (Baerenfalleret al., 2015).
We also found that polar growth appeared in the mature thalli under LTLL treatment with 14:10 light/dark cycle. This phenomenon was also observed inU. fenestrate(Kalita and Titlyanov, 2011). The reason for this maybe that the increase in photoperiod resulted in a shortened dark time, and insufficient time required for division and differentiation, coupled with low light intensity,resulting in a phenomenon like polar growth. This behavior ofUlvaalso explains why the green tide can form,and why it quickly disappears in the later period.
5 Conclusions
In this study, we showed that the spore maturation and release was affected by the photoperiod, temperature and light intensity, and the optimum condition for the spore maturation and release was 12:12 light/dark cycle, 25℃,400 μmol m−2s−1. Under low temperature and long photoperiod conditions, although the spore release number was decreased, the spore maturation was promoted and polar growth appeared, showing the higher environmental adaptability. This study is the first attempt to the elucidate coincidence effects of photoperiod, temperature and light intensity on the reproduction ofUlva. The regulation mechanism of the environmental factors on spore release and maturation needs further investigation.
Acknowledgements
This study was supported by the Natural Science Foundation of Zhejiang Province (No. LY23D060003), the Key Program of Science and Technology Innovation in Ningbo (2021Z114, 2023Z118), and was also sponsored by K. C. Wong Magna Fund in Ningbo University.
 Journal of Ocean University of China2024年1期
Journal of Ocean University of China2024年1期
- Journal of Ocean University of China的其它文章
- Using Natural Radionuclides to Trace Sources of Suspended Particles in the Lower Reaches of the Yellow River
- Eutrophication of Jiangsu Coastal Water and Its Role in the Formation of Green Tide
- Evaluation of the Shallow Gas Hydrate Production Based on the Radial Drilling-Heat Injection-Back Fill Method
- Microstructure Characterization of Bubbles in Gassy Soil Based on the Fractal Theory
- Morphological and Sulfur-Isotopic Characteristics of Pyrites in the Deep Sediments from Xisha Trough, South China Sea
- Deformation Characteristics of Hydrate-Bearing Sediments
