A β-Carotene Ketolase Gene NfcrtO from Subaerial Cyanobacteria Confers Drought Tolerance in Rice
Gao Ningning, Ye Shuifeng, Zhang Yu, Zhou Liguo, Ma Xiaosong, Yu Hanxi1, , Li Tianfei, Han Jing, Liu Zaochang1, , Luo Lijun1,
Research Paper
A β-Carotene Ketolase Genefrom Subaerial Cyanobacteria Confers Drought Tolerance in Rice
Gao Ningning1, 2, #, Ye Shuifeng3, #, Zhang Yu2, 4, Zhou Liguo2, Ma Xiaosong2, Yu Hanxi1, 2, Li Tianfei2, Han Jing2, Liu Zaochang1, 2, Luo Lijun1, 2
(College of Plant Science and Technology, Huazhong Agricultural University, Wuhan 430070, China; Shanghai Agrobiological Gene Center, Shanghai 201106, China; College of Life Sciences, Shangrao Normal University, Shangrao 334001, China; Institute for Agri-food Standards and Testing Technology, Shanghai Academy of Agricultural Sciences, Shanghai 201403, China; These authors contributed equally to this work)
is a terrestrial cyanobacterium that can resist many types of stressors, including drought, ultraviolet radiation, and extreme temperatures. In this study, we identified the drought tolerance gene, which encodes a β-carotene ketolase, through screening the transcriptome of.under water loss stress. Prokaryotic expression ofunder 0.6 mol/L sorbitol or under 0.3 mol/L NaCl stress significantly increased the growth rate of. Whenwas heterologously expressed in rice, the seedling height and root length of-overexpressing rice plants were significantly higher than those of the wild type (WT) plants grown on ½ Murashige and Skoog solid medium with 120 mmol/L mannitol at the seedling stage. Transcriptome analysis revealed thatwas involved in osmotic stress, antioxidant, and other stress-related pathways. Additionally, the survival rate of the-overexpression lines was significantly higher than that of the WT line under both hydroponic stress (24% PEG and 100 mmol/L H2O2) and soil drought treatment at the seedling stage. Physiological traits, including the activity levels of superoxide dismutase, peroxidase, catalase, total antioxidant capacity, and the contents of proline, trehalose, and soluble sugar, were significantly improved in the-overexpression lines relative to those in the WT line under 20% PEG treatment. Furthermore, when water was withheld at the booting stage, the grain yield per plant of-overexpression lines was significantly higher than that of the WT line. Yeast two-hybrid analysis identified interactions between NfcrtO and Dna J protein, E3 ubiquitin-protein ligase, and pyrophosphate-energized vacuolar membrane proton pump. Thus, heterologous expression ofin rice could significantly improve the tolerance of rice to osmotic stress, potentially facilitating the development of new rice varieties.
antioxidant enzyme; β-carotene ketolase; drought resistance;; osmotic stress; rice; transcriptome analysis
The terrestrial prokaryotic cyanobacteria, which thrive in arid and semi-arid desert grasslands, possess the critical ability to tolerate adversity and undergo rapid dehydrate and rehydrate (Scherer et al, 1984; Gao, 1998; Xu et al, 2021).can be consumed as food (Takenaka et al, 1998), however, its growth is hindered by the harsh environment (Sand-Jensen, 2014). Due to overexploitation by humans and severe ecological damage, there has been a sharp decrease in the available amount of., leading to its classification as a Class I of protected national resource. Additionally,.is of great importance to preserve its harsh habitats (Gao et al, 2016).Currently, most studies on.focus on artificial culture efficiency, photosynthesis, and oxidative stress. A comparative transcriptome analysis has revealed that photosynthesis contributes to the drought tolerance of., as evidenced by the performance of its light-harvesting complex (Bar Eyal et al, 2017; Wang et al, 2018). In addition, more and more researchers are now turning their attention to its stress tolerance, including resistance to high and low temperatures (Gao et al, 2021), ultraviolet (UV) radiation, and water deficiency. Some researchers have also investigated the algal filament structure of.. Extracellular polysaccharides (Cui et al, 2017; Liu et al, 2017; Wu et al, 2021), scytonemin (Gao, 2017), mycosporine-like amino acids (Sinha et al, 2001; Zhang et al, 2021), small molecular metabolites (Gao et al, 2019), and membrane lipids (Wang M et al, 2022) all play important roles in resistance against dehydration, UV-B stress, and other factors.can also withstand salt stress (Hagemann, 2011; Yuan et al, 2022). Protein expression analysis of.has been employed to elucidate its mechanisms of dehydration, rehydration, and UV-B resistance (Liang et al, 2012; Wang et al, 2019; Li et al, 2022; Wang L X et al, 2022; Peng et al, 2023). The genome of.has been released (Shang et al, 2019), enabling experiments to explore its stress-resistance mechanisms.
Many reviews have summarized the mechanisms by which organisms cope with stress (Zhu, 2016; Zhang et al, 2018, 2020; Gong et al, 2020; Zhang et al, 2022). Rice has been extensively studied as a model monocotyledonous plant, and is crucial for improving its yield, quality, and stress resistance as a globally critical food crop. Given the deteriorating ecological environment, breeding resistant rice varieties, especially those with drought tolerance, has become essential. Rice production requires more water input than most major crops, such as wheat and maize (Luo, 2010, 2019; Latif et al, 2023).Drought resistance is a complex trait that can be classified into three aspects: drought avoidance, drought tolerance, and drought recovery (Luo, 2010). These aspects are often interconnected, with drought tolerance becoming the primary concern as drought intensifies. Dehydration tolerance refers to a plant’s capacity to maintain its function under low leaf water status. This includes the active accumulation of osmotic adjustment substances in plant cells, thus enhancing osmotic adjustment capacity to maintain high turgor. It also involves improving the removal of harmful substances accumulated in plants and antioxidation among other responses. The evaluation of drought resistance involves assessing several physiological traits, including the activity levels of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), total antioxidant capacity (T-AOC), and the contents of proline, trehalose, and other substances. Scientists have cloned many important genes and clarified their functions and regulation mechanisms, such as(Hu et al, 2006),(Xiang et al, 2008),(Chen et al, 2013), and(Zhu and Xiong, 2013). One key objective is to identify abiotic stress-related gene resources in terrestrial macroscopic cyanobacteria for genetic engineering of crops (Ye and Gao, 2015).
Carotenoids are natural tetraterpenoid pigments found in photosynthetic organisms. As antioxidants, carotenoids are involved in protecting cells from reactive radicals, preventing lipid peroxidation, and maintaining the stability and functionality of the photosynthetic apparatus (Gong and Bassi, 2016). Cyanobacteria, which are photoautotrophic bacteria, hold promise as potential cell factories for the productionof secondary metabolites, including carotenoid pigments (Xue and He, 2015). The major carotenoids in cyanobacteria are β-carotene, hydroxycarotenoids (zeaxanthin and nostoxanthin), and ketocarotenoids (echinenone and canthaxanthin) (Wada et al, 2013). Cyclization reactions occur on lycopene, giving rise to the formation of α- and β-carotene. β-carotene is further modified by β-carotene ketolase (crtO or crtW) to generate canthaxanthin via echinenone. The crtO protein (slr0088) fromis the first reported case of a new type of β-carotene ketolase, which is homologous to bacterial phytoene dehydrogenases (Fernández-González et al, 1997). The mutantexhibits greater sensitivity to H2O2treatment than the wild type (WT) strain R1, indicating the importance of crtO in the antioxidant activity of carotenoids in(Sun et al, 2009).
In this study, we identified a candidate gene,(a β-carotene ketolase gene), through transcriptome analysis of.under water loss stress.encodes a protein that participates in the carotenoid metabolic pathway. Carotenoids in cyanobacteria can resist UV-B radiation (Llewellyn et al, 2020) and enablethe terrestrial cyanobacteria.to adapt physiologically to desiccation (Yang et al, 2019). Thegene in.encodes a proteininvolved in the biosynthesis of echinenone and canthaxanthin. Overexpression ofin thesp. PCC 7120 strain has been demonstrated to improve the biosynthesis of these pigments under both normal and osmotic stress conditions(Gao et al, 2020). Furthermore, it was observed that the transgenic strain gains desiccation tolerance. In this study, we utilized heterologous overexpression ofin rice to investigate phenotypic differences in rice at various growth and developmental stages and to examine its effect on rice, particularly its impact on rice drought stress tolerance, thereby providing a new potential resource for genetic engineering of rice.
RESULTS
Isolation and analysis of NfcrtO gene
We identified several genes through transcriptome analysis of.under water loss stress (available in NCBI, BioProject ID: PRJNA876383). Among these genes, we discovered one named, which was determined to encode a protein involved in the carotenoid metabolism pathway.was observed to enhance water-holding capacity based on the reads per kilobase per million mapped reads (RPKM) data from the transcriptome analysis (Fig. 1-A). Notably, at water loss levels of 10%, 30%, 50%, 70%, and 90%, the fold change (FC) in relative gene expression was more than 2 relative to normal conditions, with the FC being particularly significant at water loss levels of 30% (13.6 FC) and 50% (12.0 FC).
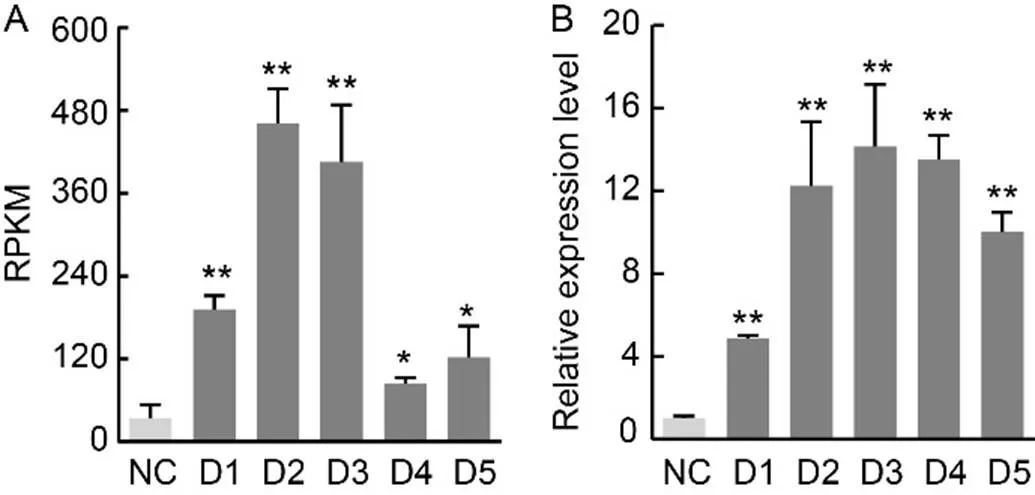
Fig. 1. Transcriptome andexpression profile analyses ofunder drought stress.
A, Reads per kilobase per million mapped reads (RPKM) data offrom a transcriptome analysis of.under drought stress.
B, Expression profile analysis ofunder drought stress.
NC, Normal conditions; D1–D5, RNA sequencing of.underwater loss levels of 10%, 30%, 50%, 70%, and 90%. Data are Mean ± SD (= 3). *,< 0.05; **,< 0.01, by the Student’s-test.
Similar results were obtained, suggesting thatcan enhance responses to various stresses, including water loss, sorbitol, and NaCl, based on the expression profile (Fig. 1-B, showing only water loss data). The relative expression levels ofunder different degrees of water loss (10%, 30%, 50%, 70%, and 90%) exhibited values of 4.8, 12.2, 14.1, 13.5, and 10.0 FC, respectively, when compared with normal conditions. These results indicated an association betweenand drought stress resistance.
NfcrtOconfers osmotic stress resistance in Escherichia coli
We clonedand transformed it into the prokaryotic expression vector pGEX-6P-1. In, we initiated a test for protein expression through isopropyl-β-d-1- thiogalactopyranosid (IPTG) induction. The induction of NfcrtO protein was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 2-A and -B). Under various IPTG induction conditions,was expressed at different times and concentrations, except for 1 h induction time. Furthermore, NfcrtO showed resistance to stress in, as indicated by the OD600data. IPTG was able to induce the expression of NfcrtO (Fig. 2-C and -D). However, when NfcrtO was exposed to sorbitol or NaCl, there was no discernible difference between the empty vector and the target gene vector (data not shown). Nevertheless, under conditions involving 0.6 mol/L sorbitol or 0.3 mol/L NaCl with 0.4 mmol/L IPTG induction, the OD600oftransformants was higher than that of the empty vector control (Fig. 2-E and -F). Therefore, NfcrtO was found to induce osmotic stress resistance in.
NfcrtO increases osmotic stress resistance in rice at the seedling stage
To investigate stress tolerance in rice, we heterologously expressed.-transgenic rice plants were confirmed using PCR with the hptprimer pair (Fig. S1-A). Furthermore, we tested the gene expression levels of-overexpression lines in the transgenic T1generation through semi-quantitative analysis with the clone primer (Fig. S1-B). Four transgenic lines (lines 1, 6, 14, and 17 in Fig. S1-B), referred to as overexpression 1 (OE1), OE2, OE3, and OE4, were selected for a series of experiments in rice.
At the seedling stage, there were no differences in plant height under normal conditions (Fig. 3-A and -C). However, the root lengths of most- overexpression (-OE) lines were longer than those of the WT under normal conditions (Fig. 3-A and -E). Additionally, the plant heights and root lengthsof the rice seedlings from the-OE lines exceededthose of the WT under 120 mmol/L mannitol treatment (Fig. 3-B, -D, and -F). These results indicated that-overexpression can promote rice resistance to osmotic stress at the seedling stage. To delve further into the mechanism, we sampled the stems from all lines for RNA sequencing. Simultaneously, we measured 12 carotenoid metabolites to quantify their levels.
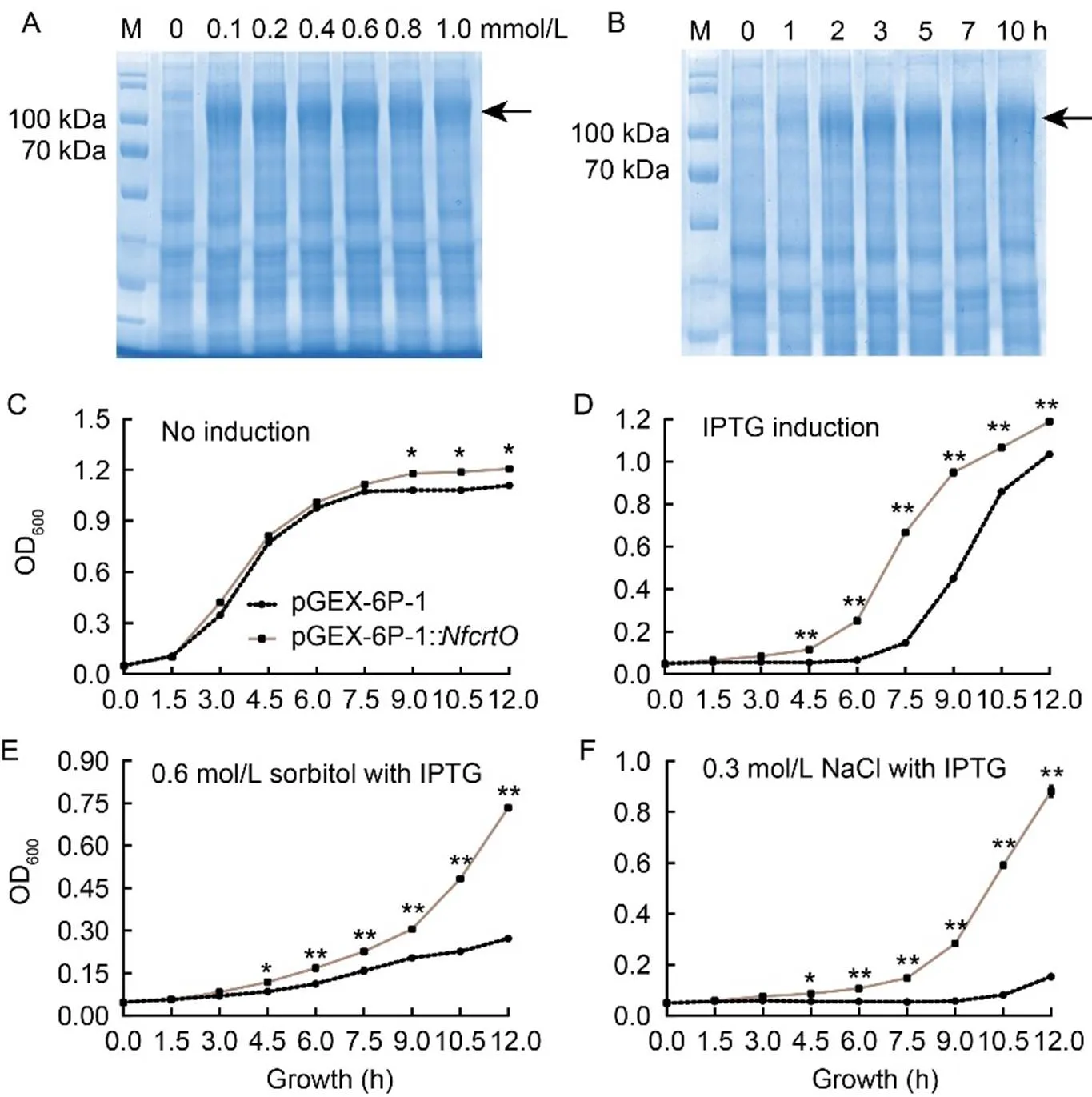
Fig. 2. Heterologous expression of NfcrtO incoli.
A and B, Protein expression of NfcrtO under different concentrations (A) and times (B) of isopropyl-β-d-1-thiogalactopyranosid (IPTG) induction. Arrow indicates expressed NfcrtO.
C‒F, Growth charts of NfcrtO-expressingunder normal conditions (C), 0.4 mmol/L IPTG induction (D), 0.6 mol/L sorbitol treatment and 0.4 mmol/L IPTG induction (E), and 0.3 mol/L NaCl treatment and 0.4 mmol/L IPTG induction (F). Data are Mean ± SD (= 3). *,< 0.05; **,< 0.01, by the Student’s-test.
To elucidate the molecular mechanism ofin rice under osmotic stress, we analyzed the transcriptomes of-OE and WT plants using high-throughput sequencing. We identified differentially expressed genes (DEGs) between the transgenic and WT plants. Specifically, we found 1 044 up-regulatedgenes (FC ≥ 2.0) and 1 074 down-regulated genes (FC ≤ 0.5) in the-OE transgenic plants compared with WT plants under the mannitol treatment (Fig. 4-A and -B; Table S1). The up-regulated genes enriched in the-OE plants primarily belonged to several biological process categories, including response to stress, plant hormone signal transduction, regulation of biosynthetic process, peroxidase activity, and oxidoreductase activity (Fig. 4-C and -D). The expression levels of several DEGs were confirmed by qRT-PCR (Fig. 4-E and -F). These results demonstrated that the transcription of these DEGs was affected by, implying that these DEGs might be target genes regulated by.
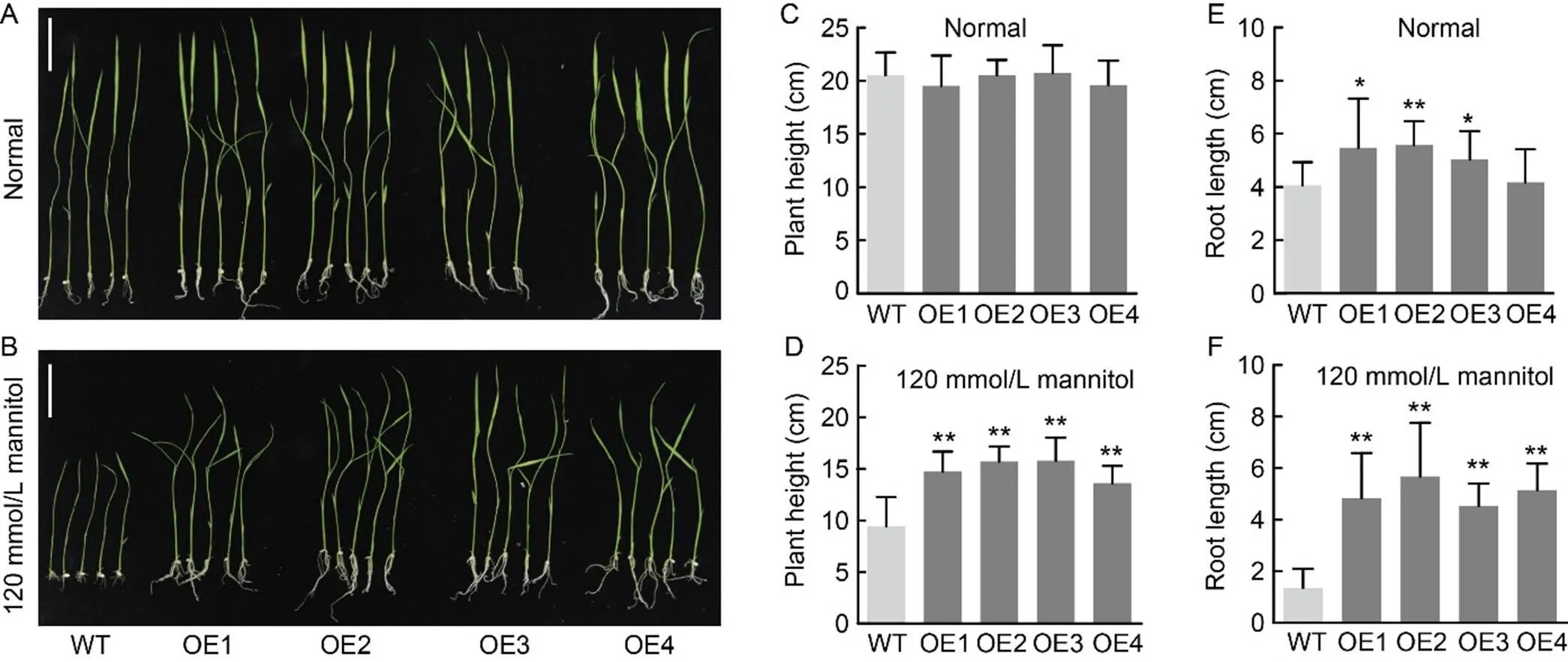
Fig. 3.-overexpressing rice seedlings on ½ Murashige and Skoog (MS) solid medium under mannitol treatment.
A and B, Phenotypes of-overexpression (OE1‒OE4) and wild type (WT) lines under normal (A) and 120 mmol/L mannitol (B) treatments on ½ MS solid medium. Scale bars, 5 cm.
C and D, Seedling plant heights of-overexpression and WT lines under normal (C) and 120 mmol/L mannitol (D) treatments on ½ MS solid medium.
E and F, Seedling root lengths of-overexpression and WT lines under normal (E) and 120 mmol/L mannitol (F) treatments on ½ MS solid medium.
Data are Mean ± SD (= 3). *,< 0.05; **,< 0.01, by the Student’s-test.
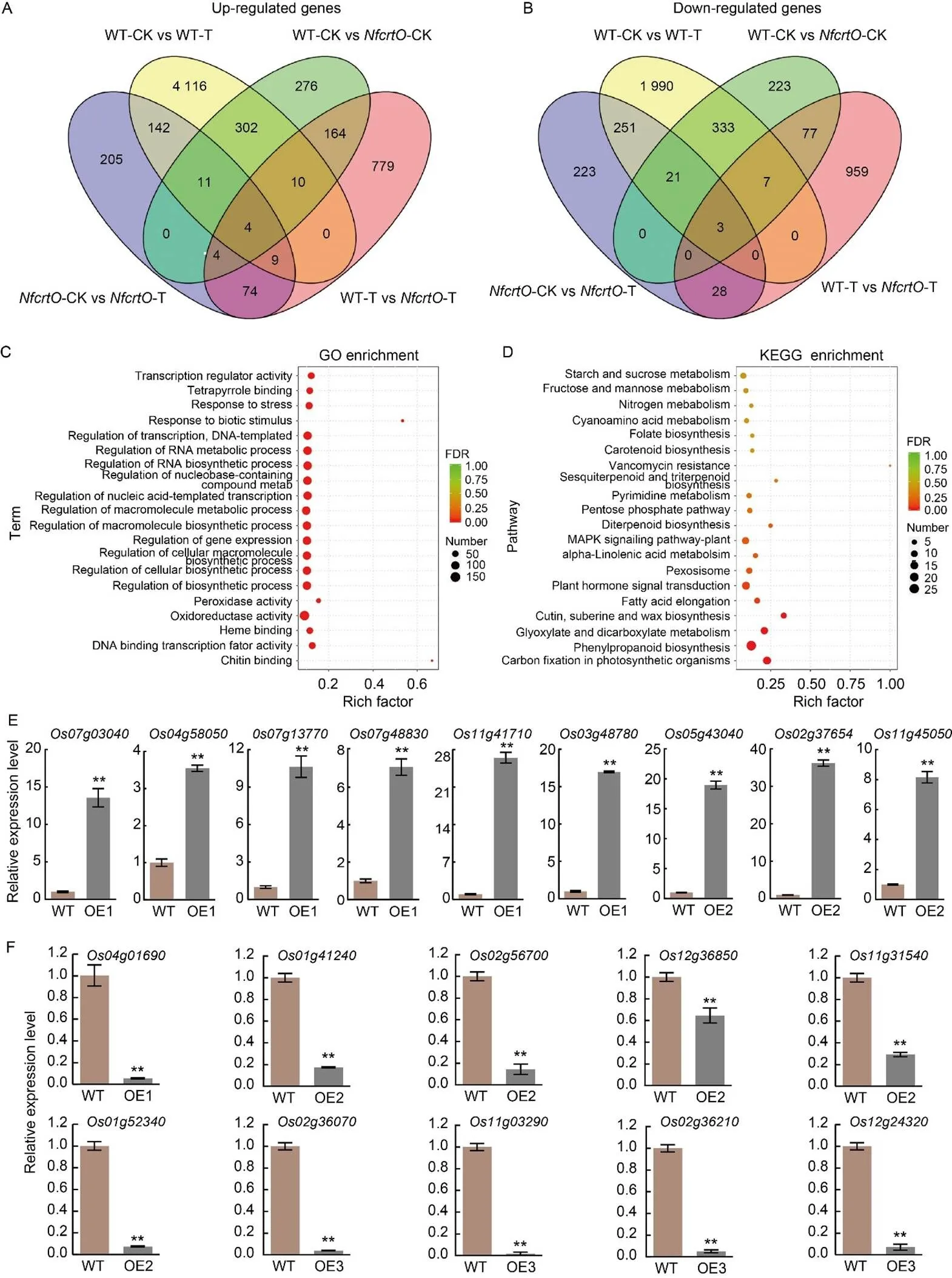
Fig. 4. RNA-sequencing of-overexpression seedlings in rice on ½ Murashige and Skoog (MS) solid medium under mannitol stress.
A and B, Up-regulated (A) and down-regulated (B) genes as determined by comparative transcriptome analysis between the wild type (WT) and overexpression () lines. WT-CK,-CK, WT-T, and-T represent WT and overexpression lines under normal conditions (CK) and mannitol stress treatment (T), respectively.
C, Enriched Gene Ontology (GO) terms in the comparative transcriptome analysis between the WT and-overexpression lines. FDR, False discovery rate.
D, Enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in the comparative transcriptome analysis between the WT and- overexpression lines.
E and F, Validation by qRT-PCR of up-regulated (E) and down-regulated (F) genes. Leaves of rice seedlings on ½ MS culture flasks with and without 120 mmol/L mannitol for 7 d were sampled for transcriptome sequencing analysis. RNA was used for qRT-PCR, andgene was used as the reference gene to normalize the target gene expression. OE1, OE2, and OE3 represent-overexpression lines. Data are Mean ± SD (= 3). **,< 0.01, by the Student’s-test.

Fig. 5.-overexpression lines of rice under polyethylene glycol (PEG) and H2O2stresses.
A and B, Phenotypes of rice-overexpression (OE1, OE3, and OE4) and wild type (WT) lines under 24% PEG (A) and 100 mmol/L H2O2(B) treatments. Scale bars, 5 cm.
C and D, Survival rates of-overexpression and WT lines under 24% PEG (C) and 100 mmol/L H2O2(D) treatments. Data are Mean ± SD (= 3). *,< 0.05; **,< 0.01, by the Student’s-test.
During our assessment of carotenoid content under stress (20 metabolites, Table S2-A), we identified one metabolite, capsanthin, which exhibited differential expression among-CK vs-T, WT-T vs-T, and WT-CK vs-CK comparisons (Fig. S2-A and -B). Capsanthin is involved in the biosynthesis of carotenoids and secondary metabolites (Fig. S2-C and Table S2-B).
NfcrtO-overexpression enhances osmotic and oxidation tolerance in transgenic rice seedlings
The-OE lines were treated with either NaCl or polyethylene glycol (PEG) to simulate osmotic stress or H2O2to simulate oxidation stress. There was no obvious phenotypic differences between the transgenic and WT seedlings before treatment (Figs. 5-A, -B, and S3-C). However, the leaves of WT plants wilted after 3 d with 24% PEG (Fig. 5-A), 4 d with 100 mmol/L H2O2(Figs. 5-B and S3-C), and 2 d with 150 mmol/L NaCl treatment (Fig. S3-A). After recovery for several days, the survival rates of-OE rice plants were significantly higher than those of WT plants among the three stress treatments (Figs. 5-C, -D, and S3-B).
Several stress-related physiological traits of- OE and WT plants were examined under 20% PEG treatment. T-AOC activity of-OE plants was significantly higher than that of WT plants before and after PEG osmotic stress for 2 and 3 d (Fig. 6-A). Other traits had no differences before PEG treatment. SOD and POD activities of-OE plants were significantly higher than those of WT plants under PEG osmotic stress for 3 d (Fig. 6-B and -C). CAT activity of-OE plants was significantly higher than that of WT plants under PEG osmotic stress for 2 d (Fig. 6-D). Soluble sugar content of-OE plants was significantly higher than that of WT plants under PEG osmotic stress for 2 and 3 d (Fig. 6-E). Trehalose content of-OE plants was significantly higher than that of WT plants under PEG osmotic stress for 2 d (Fig. 6-F). Proline content of-OE plants was significantly higher than that of WT plants under PEG osmotic stress for 3 d (Fig. 6-G). These results suggested that the activated expression ofimproves the oxidative and osmotic stress tolerance of the transgenic rice plants compared with the WT plants.

Fig. 6. Physiological indices of-overexpression lines of rice under 20% polyethylene glycol (PEG) treatment.
A, Total antioxidant capacity (T-AOC) activity. B, Superoxide dismutase (SOD) activity. C, Peroxidase (POD) activity. D, Catalase (CAT) activity. E, Soluble sugar content. F, Trehalose content. G, Proline content.
NC, Normal conditions; PEG(2) and PEG(3), PEG osmotic stress for 2 and 3 d, respectively; WT, Wild type; OE3 and OE4,-overexpression lines. Data are Mean ± SD (= 3). *,< 0.05; **,< 0.01, by the Student’s-test.
NfcrtO improves drought resistance at the seedling and booting stages in rice
To simulate the agricultural environment for rice, a water loss experiment was conducted on-OE lines at the seedling stage using plastic pots filled with soil. The rice plants were grown normally in pots until the three-leaf stage. Then, watering was stopped and leaves began to curl after 6 d. When the leaf blades curled severely and necrosis occurred, plants were rewatered (Fig. 7-A). After a recovery period of 20 d, the survival rate was assessed. The survival rate ranged from 15.7% to 28.6% for the OE lines, significantly higher than 9.7%–11.8% of the WT (Fig. 7-B). Altogether, these results indicated that-OE plants exhibit greater tolerance to drought stress at the seedling stage in rice.
To investigate resistance to drought stress at the reproductive stage, water was withheld at the booting stage (Fig. 8-A). TheOE lines showed slower leaf wilting compared with the WT lines. Monitoring the water loss of detached leaves revealed that the WT lines experienced faster water loss than the OE lines (Fig. S4-A), though the relative leaf water content did not differ among these lines (Fig. S4-B). We also measured chlorophyll content and photosynthetic rate under drought treatment. No differences were observed under normal conditions. The contents of chlorophyll b and total chlorophyll were significantly higher in the OE lines than in the WT lines under drought treatment, though the content of chlorophyll a did not differ among the OE and WT lines (Fig. S5-A to -F). The photosynthetic rate was significantly higher in OE plants than in the WT plants under drought conditions (Fig. S6-A and -B).
After recovery, the OE lines exhibited a better growth status than the WT lines (Fig. 8-A). Additionally, the seed-setting rate of OE lines was significantly higher than that of the WT lines (Fig. 8-B). Based on the results shown in Fig. 8, it can be inferred that the grain yield per plant of the OE plants was considerably higher than that of the WT plants. These findings implied that-overexpression can improve drought resistance by minimizing water loss during the reproductive stage.

Fig. 7. Rice seedlings of-overexpression lines at the seedling stage in soil under drought treatment.
A, Phenotypes of-overexpression rice seedlings (OE1, OE3, and OE4) in soil under drought stress. Scale bar, 5 cm.
B, Survival rates of-overexpression rice seedlings under drought stress.
WT, Wild type. Data are Mean ± SD (= 3). *,< 0.05; **,< 0.01, by the Student’s-test.
NfcrtO may interact with stress responsive genes in rice
The plasmids pGBKT7-and pGBKT7 were transformed into AH109 yeast cells, which were then plated onto SD/-Trp (SD-T) plates and incubated at 30 ºC for 3‒4 d. Six randomly selected spots were verified by PCR with pGBKT7 vector primers. All selected spots yielded transformed clones, and three clones were randomly selected and transferred to SD-T, SD/-Trp/-His (SD-TH), SD/-Trp/-His/-Ade (SD-THA), and SD-THA + X-α-gal plates, which were incubated at 30 ºC for 3–5 d. Additionally, pGBKT7 empty vector transformants were used as the negative control. The negative control pGBKT7 transformants could grow on SD-T plates but could not grow properly on SD-TH, SD-THA, and SD-THA + X-α-gal plates (Fig. S7-A). This result suggested that pGBKT7-has no self-activation.
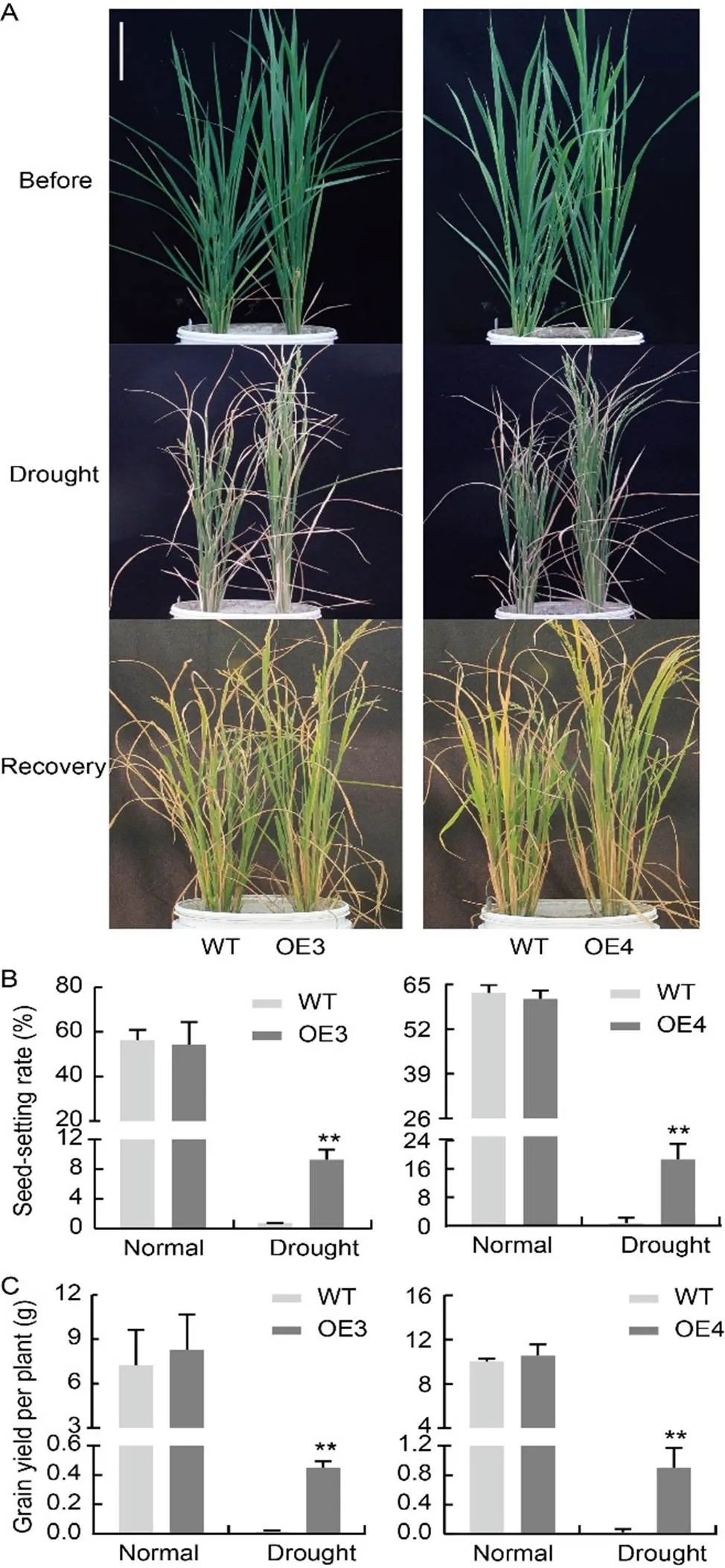
Fig. 8.-overexpression lines of rice at the booting stage under drought treatment.
A, Phenotypes of-overexpression lines (OE3 and OE4) at the booting stage under drought treatment. Scale bar, 20 cm.
B, Seed-setting rate of-overexpression lines.
C, Grain yield per plant of-overexpression lines.
WT, Wild type. Data are Mean ± SD (= 3). **,< 0.01, by the Student’s-test.
Positive yeast clones were obtained by screening with SD/-Trp/-Leu/-His (SD-TLH) screening plates. Colonies grew on the SD-TLH plates of the screening library, so 96 clones were picked from the plates for PCR validation. The verified results showed that the positive control could grow on SD/-Trp/-Leu (SD-TL), SD-TLH, SD/-Trp/-Leu/-His/-Ade (SD-TLHA), and SD-TLHA + X-α-gal plates, and the positive control formed blue colonies on SD-TLHA + X-α-gal plates. Meanwhile, the negative control could grow only on SD-TL plates, but could not grow on other plates. In total, 44 positive yeast clones were identified, with 44 positive clones able to grow normally on SD-TL plates, 44 positive clones able to grow on SD-TLH plates, 30 positive clones able to grow on SD-TLHA plates, only 26 positive clones able to grow on SD-TLHA + X-α-gal plates, and blue colonies appearing on SD-TLHA + X-α-gal plates (Fig. S7-B). Among these, we could find some genes that may interact with, such as Dna J protein, pyrophosphate- energized vacuolar membrane proton pump 1, and E3 ubiquitin-protein ligase (Table S3). However, these results require further validation.
DISCUSSION
NfcrtO serves as an osmotic stress tolerance gene
In this study,, a gene isolated from., was able to confer resistance to drought stress. Prokaryotic expression ofinindicated that it can induce resistance to 0.6 mol/L sorbitol and 0.3 mol/L NaCl stresses. Furthermore,was transformed into rice to explore its function. At the rice seedling stage, under both 120 mmol/L mannitol treatment on ½ MS medium, and PEG treatment in a water solution or cultivation in soil with drought treatment,-overexpression lines exhibited better growth than the WT lines. These results indicated thatcan provide stress resistance by enhancing osmotic adjustment.was also able to confer resistance to drought treatment and increase rice yield by improving the seed-setting rate at the booting stage.
Based on transcriptome analysis at the rice seedling stage,was observed to up-regulate genes related to stress response, plant hormone signal transduction, regulation of biosynthetic process, peroxidase activity, and oxidoreductase activity. The up-regulated gene() positively regulates panicle blast resistance in rice, possibly by modulating the accumulation of H2O2(Dong et al, 2021). Overexpression of two defense-related genes (and) in rice leads to significant resistance against sheath blight pathogen () without distressing any agronomically important traits (Karmakar et al, 2016). POD activity andoxidoreductase activity were enriched GO terms among up-regulated genes. Starch and sucrose metabolism, fructose and mannose metabolism, carotenoid biosynthesis, and peroxisome were enriched Enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways among up-regulated genes.
However, these results still leave several questions at least partially unanswered. How doesconfer resistance to drought? Is it dependent on the abscisic acid pathway or not? Is it instead dependent on stomatal aperture and/or conduction changes? Each of these questions needs to be explored further.
NfcrtO may interact with stress-responsive genes
A previously created-overexpressingspPCC 7120 strain with improved biosynthesis of echinenone and canthaxanthin under both normal and osmotic stress conditions exhibits increased desiccationtolerance (Gao et al, 2020). This study provided insights into the enhanced production of both carotenoid pigments by using a heterologousgene. Based on this information, further optimization of cultivation conditions could help achieve greater productivity. In our study, we delved deeper into the phenotype and underlying mechanism in rice. We identified interacting proteins in a yeast two-hybrid experiment, including Dna J protein, pyrophosphate-energized vacuolar membrane proton pump 1, and E3 ubiquitin-protein ligase.
Dna J-domain proteins inhave been identified in different organisms where they play crucial roles in the osmotic stress response (Tamadaddi et al, 2021, 2022).was identified to be the co-chaperone partner of, and their encoding genes share similar transcriptional responses to dehydration in desiccation-tolerant cyanobacteria (Xu et al, 2020).as an E3 ligase, has been shown to enhance drought resistance when over- expressed, resulting in higher leaf-related water content and lower leaf water loss rate. Conversely, RNAi and knock-out plants ofare more sensitive to drought (Chen et al, 2022). Plants need to regulate membrane-bound proton-pumping pyrophosphatase (V-PPase) pump activity to avoid hyperactivity and its negative feedback on cell viability under normal growthconditions. V-PPase proton pump functioning becomes increasingly important under salt stress for generating the pH gradient necessary for vacuolar proton-coupled Na+sequestration (Graus et al, 2018). Thus, additional molecular experiments should be conducted to confirm howinteracts with other genes.
Heterologous gene expression
Researchers have explored the evolutionary and adaptive mechanisms of cyanobacterial adaptations to their habitats, revealing a large number of genes that exhibit habitat-specific associations through comparative genomic analysis. Terrestrial cyanobacteria have expanded their gene families to cope with fluctuating environments. These genes are involved in the photosynthetic process, chemotaxis, nutrient transport, and osmotic stress, and they play a key role in cyanobacterial adaptation to specific environments (Chen et al, 2021). Therefore, further explorationof., a terrestrial cyanobacterium, is warranted to discover more stress- responsive genes for genetic engineering.
More and more genes can be heterologously expressedin model species to explore gene expression that confers stress resistance. Heterologous expression of three stress-responsive genes from.confers tolerance to abiotic stress in.(Ai et al, 2014a). The acidic water stress protein from terrestrial macroscopic cyanobacteria can confer resistance to osmotic stress in transgenicplants (Ai et al, 2014b). The genederived from the drought- adapted cyanobacterium., has been found to improve salt tolerance in transgenic6803 and(Cui et al, 2018). Overexpression offrom.has also been shown to enhance the drought tolerance of(Wang et al, 2020). Heterologous expression of fungalalleviates ammonium toxicity and suppresses photorespiration, thereby improving drought tolerance in rice (Yan et al, 2021).is taken as an important model plant for understanding responses to dehydration. The underlying genome architecture and response in relation to the hydration state of the plant and its role in the preservation of cellular integrity have significant implications for developing strategies to improve drought tolerance in crops (Xiao et al, 2015).
The concept of Green Super Rice, which can be grown with ‘less pesticide, less fertilizer, less irrigation, superior quality and high yield’, has been put forward. This concept includes objectives of reduced water use and drought resistance (Zhang, 2007). The blue revolution for food security was also proposed based on a case study involving water-saving and drought- resistant rice under carbon neutrality (Xia et al, 2022). Producing sufficient food with finite resources to feed the growing global population while having a smaller impact on the environment has always been a great challenge. The present work also provides possibilities for new horizons in genomic breeding technologies geared toward delivering green and nutritious crop varieties to further enhance the development of green agriculture and better nourish the world population (Yu et al, 2022).
METHODS
Material collection, treatment, and usage
samples were collected from the xeric steppe of Huhehaote, Inner Mongolia, China and stored in a dry and dark environment before use. To recover their activity, we simply soaked the samples in water, and then respiration, photosynthesis, and nitrogen fixation recovered (Gao, 1998).L.ssp.cv. Nipponbare was used to construct the overexpression lines of, which was obtained from the germplasm repository of our lab. Both the rice overexpression and WT lines were subjected to a series of experiments at the seedling and booting stages to explore the effect ofon stress resistance.
Identification of candidate gene NfcrtO
We treated.with drought stress to obtain samples under different water loss conditions (water loss levels of 10%, 30%, 50%, 70%, and 90%) and performed transcriptome sequencing. We identified a new gene from the dehydration transcriptome analysis (data unpublished) of.based on RPKM data, referred to as, which encodes a β-carotene ketolase. We also treated.samples with water loss (water loss levels of 10%, 30%, 50%, 70%, and 90%), high temperature (45 ºC), low temperature (4 ºC), sorbitol (0.4 mol/L), and NaCl (0.2 mol/L) to explore its gene expression profile. By qRT-PCR, we determined gene expression differences under various stress conditions.
Total RNA was extracted using TRNzol reagent (DP424, TIANGEN, Dalian, China), and cDNA was synthesized using EasyScript®One-Step gDNA Removal and cDNA Synthesis SuperMix (AE311, TransGen, Beijing, China). Then, qRT-PCR was performed in a 96-well plate with a Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) using the PerfectStart®Green qPCR SuperMix (AQ601, TransGen, Beijing, China) according to the manufacturer’s instructions. The reaction conditions were as follows: 94 ºC for 30 s, followed by 40 cycles at 94 ºC for 5 s and 60 ºC for 32 s. The gene(forward primer, 5′-CTGACACTGAGGGA CGAA-3′; reverse primer, 5′-TGGCAACTAAAAACGAGG-3′) was used as the reference gene to normalize the target gene expression, calculated using the relative quantification method (2-∆∆CT) (Livak and Schmittgen, 2001).
Prokaryotic expression of NfcrtO in E. coli
We cloned the geneand then transformed it into the GST-tag prokaryotic expression vector pGEX-6P-1, and the GST fusion expression constructs were introduced intoBL21. The expression of the fusion protein was induced by adding IPTG and incubated at 37 ºC. We set a series of different IPTG induction time points (0, 1, 2, 3, 5, 7, and 10 h) and concentrations (0, 0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 mmol/L) to identify the best induction method. The solubility of the protein was checked using SDS-PAGE.
expression by IPTG (0.4 mmol/L) and sorbitol (0.6 mol/L) or NaCl (0.3 mol/L) was carried out to test osmotic adjustment by OD600in. Four processing conditions were tested for approximately 12 h: only Luria-Bertani (LB) liquid medium (including ampicillin, the same as below), LB with IPTG, LB with sorbitol or NaCl, LB with IPTG and sorbitol, or LB with IPTG and NaCl. The transformedBL21 strains were cultured in 5 mL of LB liquid medium at 37 ºC until the OD600reached approximately 0.4–0.6. Then, the cultures were inoculated into 20 mL of LB liquid medium and further incubated until the OD600was measured. Initial cell concentrations were adjusted to be equivalent in each flask in these experiments. The velocity of the shaker was set at 200 r/min and the growth temperature was maintained at 37 ºC. The concentration ofcells at each time point was determined at least three times and was measured in terms of OD600. Finally, growth curves were plotted to characterize whether this gene was involved in stress tolerance or not.
NfcrtO overexpression lines on ½ MS solid medium with 120 mmol/L mannitol
Thegene was transformed into Nipponbare to investigate its effect in crops. The husked seeds of bothoverexpression and WT lines were sterilized with 75% ethanol for 1 min, followed by treatment with NaClO for 16–20 min in a shaker to further sterilize the samples. We poured off the disinfection reagent and washed the seeds with sterilized double-distilled water 5–6 times and then absorbed water from the seed surfaces with sterilized filter paper. The seeds were spread onto ½ MS solid medium in Petri dishes. After 2–3 d, the seeds with consistent germination were transferred to ½ MS culture flasks (120 mmol/L mannitol or not) for each line. Then, 7–10 d later, we measured the plant height and root length of rice seedlings.
Seedlings were sampled for transcriptome sequencing analysis.After the extraction and purification of RNA and the construction of libraries, next-generation sequencing was performed on these libraries using the Illumina sequencing platform with paired-end sequencing. We used SeqPrep to strip adaptors and/or merge paired reads that overlapped into single reads and used sickle to remove low-quality reads. The clean data were then aligned to the reference genome of rice using HISAT2 v2.1.0. FPKM values were then calculated to estimate the expression level of these genes. DESeq2 v1.6.3 was used to analyze the DEGs between two samples, and genes with< 0.05 and |log2(FC)| ≥ 1 were identified as DEGs (Wang et al, 2010). GO (http://geneontology.org/) enrichment of DEGs was determined by hypergeometric tests in which each-value was calculated and adjusted to a-value. GO terms with< 0.05 were considered significantly enriched.
Carotenoid contents were determined using MetWare (http:// www.metware.cn/) with the AB Sciex QTRAP 6500 Liquid chromatography-tandem mass spectrometry (LC-MS/MS) platform. Based on raw data, hierarchical cluster analysis (HCA) results of samples and metabolites were visualized as heatmaps with dendrograms. HCA was performed using the R package heatmap. For HCA, normalized signal intensities of metabolites (unit variance scaling) were visualized as a color spectrum. Significantly regulated metabolites between groups were determined based on absolute log2FC values. Identified metabolites were annotated using the KEGG compound database (http://www. kegg.jp/kegg/compound/), and annotated metabolites were then mapped according to the KEGG pathway database (http://www. kegg.jp/kegg/pathway.html). Pathways with significantly regulated metabolites were subjected to metabolite set enrichment analysis, and their significance was determined by-values from a hypergeometric test.
Stress treatment of NfcrtO-overexpression lines of rice at the seedling stage and physiological index determination
Seeds of-overexpression and WT lines were sterilized with a 2% sodium hypochlorite solution, soaked for 1 d, and germinated at 37 ºC for 2 d. The most uniformly germinated seeds were sown in a 96-well plate with the bottom removed for osmotic stress treatment. The seedlings were grown in a liquid culture solution in a growth chamber with a 16 h light (30 ºC)/8 h dark (26 ºC) photoperiod/temperature treatment. At approximately three weeks, the seedlings were transferred into liquid culture solution supplemented with 24% PEG6000, 100 mmol/L H2O2, or 150 mmol/L NaCl for stress treatment. After the WT plants wilted, they were transferred back to normal culture solution for about one week, and the number of surviving plants was recorded to calculate the survival rate. Subsequently, leaves from the plants treated with 20% PEG were sampled for physiological trait analysis. The analyzed physiological traits included the activity levels of SOD, POD, CAT, and T-AOC, and the contents of proline, trehalose, and soluble sugar in theoverexpression lines, after PEG treatment at the seedling stage. The data were obtainedusing a commercial kit from the Nanjing Jiancheng Bioengineering Institute, Jiangsu Province, China. All experimental procedures followed the corresponding instructions.
NfcrtO-overexpression lines under drought stress at the seedling and booting stages
Each small pot (diameter 12.5 cm, depth 16.5 cm) was filled with 1.8 kg of soil. Half of each pot was allocated for the overexpression line, and half for the WT line, with about 25 plants per line. Drought stress was induced around 21 d of growth. When there was a significant difference between the WT and overexpression lines (approximately 7–10 d later), the plants were rewatered and allowed to grow for about 14 d more. Subsequently, images were captured and survival rates were determined.
To simulate the effects of drought throughout the entire reproductive period in rice, we conducted an experiment where rice seedlings were grown in large circular pots (21.5 cm diameter at the mouth, 19.0 cm diameter at the bottom, and 21.0 cm deep). Each pot contained two rice seedlings, namely one WT plant and one-overexpression plant. Each overexpression line was planted in 6–8 pots as biological replicates. The experiment began at the panicle developmental stage, after the water supply was stopped. Once the WT plants showed severe wilting, all the plants were rewatered until harvest. At harvest, their agronomic and yield traits were measured.
Determination of leaf water content, photosynthesis, and chlorophyll content of rice at the booting stage
The rate of water loss was continuously monitored by measuring the fresh weight of isolated leaves. Rice leaf water retention capacity was determined by dissipating water from the isolated leaves to ascertain whether the drought-related phenotype was related to stomatal regulation. In the field or greenhouse, 3 to 6 fully expanded rice leaves of each line were quickly cut with scissors and placed in a plastic bag and then put in an icebox. The initial weight was recorded as0, and subsequently, the fresh weight was measured every hour astuntil it reached a constant weight. The water loss rate of excised leaves (%) is calculated as (0‒t) /0× 100%.
Rice leaves exhibit significant differences in water content under abiotic stress conditions. The ability of rice to retain water under adverse conditions was determined by assessing differences in relative leaf water content. It was observed that higher relative water content corresponded to better resistance to water loss in rice. In the field or greenhouse, 3 to 6 fully expanded rice leaves of each line were quickly cut with scissors, placed in a plastic bag, and then stored in an icebox. The fresh weight was recorded as1. The leaves were transferred to a 50 mL centrifuge tube and soaked in water for 2 h. After drying the leaf surface, they were quickly weighed and recorded as2. The leaves were then placed in a drying oven at 60 ºC for 10‒15 h until a constant weight was received. The dry weight was measured and recorded as3. The relative leaf water content (%) is calculated as (1‒3) / (2‒3) × 100%.
The MIC-100 photosynthetic rate instrument (Masa, Kyoto, Japan) was used to record the measurements of the- overexpression and WT lines under drought conditions at the booting stage. At least three duplicates were used for each line. For chlorophyll content analysis, fresh rice leaves were sampled, wiped dry, and had their midribs removed. The leaves were then cut into pieces, mixed, and weighed into 2-mL centrifuge tubes using an analytical balance. Each sample was divided into triplicates for three technical replicates. Subsequently,2 mL of extraction buffer (ethanol:acetone:ddH2O = 4.5:4.5:1.0) was added to each tube, sealed with parafilm and then extracted at 4 ºC for 12 h in darkness. The absorbance values of the chlorophyll extracts were measured using the DU640 UV spectrophotometer (Backman, Indianapolis, USA) at wavelengths of 645 and 663 nm, with the extraction buffer as a blank control. The chlorophyll content was calculated according to the method of Arnon (1949).
Yeast library screening
PGBKT7-and pGBKT7 were introduced into AH109 yeast cells, which were then plated on SD-T plates and incubated at 30 ºC for 3‒4 d. Six randomly chosen clones were examined by PCR using pGBKT7 vector primers to ensure they were all positive clones. Three clones were selected at random and cultivated at 30 ºC for 3‒5 d on SD-T, SD-TH, SD-THA, and SD-THA + X-α-gal plates. PGBKT7 was served as the negative control.
The library plasmid pGADT7 rice cDNA was added to the AH109 yeast strain, which harbored the pGBKT7-bait plasmid. Colonies were screened by culturing on SD-TLH plates. Positive clones were amplified from yeast cells for DNA sequencing and compared with the sequences in the GenBank database for BLAST analysis to identify the genes of the positive clones screened from the SD-TLH plate. Positive clones grown on the SD-TLH plates were diluted with sterile water, plated onto SD-TL, SD-TLH, SD-TLHA, and SD-TLHA + X-α-gal plates, and cultured at 30 ºC for 3–4 d.
ACKNOWLEDGEMENTS
This study was supported by the National Key Research and Development Program of China (Grant No. 2018YFE0106200), the Science and Technology Research Project of Jiangxi Provincial Department of Education, China (Grant No. K4100131), and the Science and Technology Research Project of Shangrao, Jiangxi Province, China (Grant No. K4000019).
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
Fig. S1. Positive detection and expression level of- overexpression rice lines.
Fig. S2. Measurement of saponified carotenoid metabolites of-overexpression rice lines on ½ Murashige and Skoog medium.
Fig. S3.-overexpression lines in rice under 150 mmol/L NaCl and 100 mmol/LM H2O2treatments at the seedling stage.
Fig. S4. Determination of water loss rate of excised leaves and relative leaf water content of-overexpression lines of rice at the booting stage.
Fig. S5. Chlorophyll content of-overexpression and wild type lines of rice at the booting stage under drought stress.
Fig. S6. Photosynthetic rate of-overexpression and wild type lines of rice at the booting stage under drought stress.
Fig. S7. Yeast two-hybrid screening of NfcrtO.
Table S1. Differentially expressed genes of-overexpression and wild type lines of rice under 120 mmol/L mannitol based on transcriptome analysis.
Table S2. Category and content of saponified carotenoid metabolites of-overexpression lines of rice at the seedling stage on ½ Murashige and Skoog medium under 120 mmol/L mannitol treatment.
Table S3. Genes that potentially interact with NfcrtO based on yeast two-hybrid screening.
Ai Y F, Yang Y W, Gao X, Qiu B S. 2014a. Heterologous expression of three stress-responsive genes fromconfers tolerance to abiotic stresses in., 26: 123–129.
Ai Y F, Yang Y W, Qiu B S, Gao X. 2014b. Unique WSPA protein from terrestrial macroscopic cyanobacteria can confer resistance to osmotic stress in transgenic plants.,30(9): 2361–2369.
Arnon D I. 1949. Copper enzymes in isolated chloroplasts. polyphenoloxidase in., 24(1): 1–15.
Bar Eyal L, Ranjbar Choubeh R, Cohen E, Eisenberg I, Tamburu C, Dorogi M, Ünnep R, Appavou M S, Nevo R, Raviv U, Reich Z, Garab G, van Amerongen H, Paltiel Y, Keren N. 2017. Changes in aggregation states of light-harvesting complexes as a mechanism for modulating energy transfer in desert crust cyanobacteria., 114(35): 9481–9486.
Chen L J, Wuriyanghan H, Zhang Y Q, Duan K X, Chen H W, Li Q T, Lu X, He S J, Ma B, Zhang W K, Lin Q, Chen S Y, Zhang J S. 2013. An S-domain receptor-like kinase, OsSIK2, confers abiotic stress tolerance and delays dark-induced leaf senescence in rice., 163(4): 1752–1765.
Chen M Y, Teng W K, Zhao L, Hu C X, Zhou Y K, Han B P, Song L R, Shu W S. 2021. Comparative genomics reveals insights into cyanobacterial evolution and habitat adaptation., 15(1): 211–227.
Chen S J, Xu K, Kong D Y, Wu L Y, Chen Q, Ma X S, Ma S Q, Li T F, Xie Q, Liu H Y, Luo L J. 2022. Ubiquitin ligase OsRINGzf1 regulates drought resistance by controlling the turnover of OsPIP2;1., 20(9): 1743–1755.
Cui L J, Xu H Y, Zhu Z X, Gao X. 2017. The effects of the exopolysaccharide and growth rate on the morphogenesis of the terrestrial filamentous., 6(9): 1329–1335.
Cui L J, Liu Y H, Yang Y W, Ye S F, Luo H Y, Qiu B S, Gao X. 2018. The drnf1 gene from the drought-adaptedimproved salt tolerance in transgenicandplant., 9(9): 441.
Dong J F, Zhou L, Feng A Q, Zhang S H, Fu H, Chen L, Zhao J L, Yang T F, Yang W, Ma Y M, Wang J, Zhu X Y, Liu Q, Liu B. 2021. The,andpositively regulate panicle blast resistance in rice., 14(1): 51.
Fernández-González B, Sandmann G, Vioque A. 1997. A new type of asymmetrically acting β-carotene ketolase is required for the synthesis of echinenone in thesp. PCC 6803., 272(15): 9728–9733.
Gao K S. 1998. Chinese studies on the edible blue-green,: A review., 10(1): 37–49.
Gao X. 2017. Scytonemin plays a potential role in stabilizing the exopolysaccharidic matrix in terrestrial cyanobacteria., 73(2): 255–258.
Gao X, Xu H Y, Ye S F, Liang W Y. 2016. A proposal on the restoration offor sustainable improvement in the ecology of arid steppes in China., 3(2): 14.
Gao X, Liu B, Ji B Y. 2019. Profiling of small molecular metabolites induring periodic desiccation., 17(5): 298.
Gao X, Xu H Y, Zhu Z X, She Y, Ye S F. 2020. Improved production of echinenone and canthaxanthin in transgenicsp. PCC 7120 overexpressing a heterologousgene from., 236: 126455.
Gao X, Zhu Z X, Xu H Y, Liu L T, An J, Ji B Y, Ye S F. 2021. Cold adaptation in drylands: Transcriptomic insights into cold-stressedand characterization of a hypothetical gene with cold and nitrogen stress tolerance., 23(2): 713–727.
Gong M Y, Bassi A. 2016. Carotenoids from microalgae: A review of recent developments., 34(8): 1396–1412.
Gong Z Z, Xiong L M, Shi H Z, Yang S H, Herrera-Estrella L R, Xu G H, Chao D Y, Li J R, Wang P Y, Qin F, Li J J, Ding Y L, Shi Y T, Wang Y, Yang Y Q, Guo Y, Zhu J K. 2020. Plant abiotic stress response and nutrient use efficiency., 63(5): 635–674.
Graus D, Konrad K R, Bemm F, Patir Nebioglu M G, Lorey C, Duscha K, Güthoff T, Herrmann J, Ferjani A, Cuin T A, RoelfsemaM R G, Schumacher K, Neuhaus H E, Marten I, Hedrich R. 2018. High V-PPase activity is beneficial under high salt loads, but detrimental without salinity., 219(4): 1421–1432.
Hagemann M. 2011. Molecular biology of cyanobacterial salt acclimation., 35(1): 87–123.
Hu H H, Dai M Q, Yao J L, Xiao B Z, Li X H, Zhang Q F, Xiong L Z. 2006. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice., 103(35): 12987–12992.
Karmakar S, Ali Molla K, Chanda P K, Sarkar S N, Datta S K, Datta K. 2016. Green tissue-specific co-expression ofandgenes in rice for enhanced resistance against sheath blight., 243(1): 115–130.
Latif A, Sun Y, Pu C X, Ali N. 2023. Rice curled its leaves either adaxially or abaxially to combat drought stress., 30(5): 405–416.
Li X X, Ding M M, Wang M, Yang S J, Ma X R, Hu J H, Song F, Wang L X, Liang W Y. 2022. Proteome profiling reveals changes in energy metabolism, transport and antioxidation during drought stress in., 22(1): 162.
Liang W Y, Zhou Y W, Wang L X, You X R, Zhang Y P, Cheng C L, Chen W. 2012. Ultrastructural, physiological and proteomic analysis ofin response to dehydration and rehydration., 75(18): 5604–5627.
Liu W, Cui L J, Xu H Y, Zhu Z X, Gao X. 2017. Flexibility-rigidity coordination of the dense exopolysaccharide matrix in terrestrial cyanobacteria acclimated to periodic desiccation., 83(22): e01619-17.
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCTMethod., 25(4): 402–408.
Llewellyn C A, Airs R L, Farnham G, Greig C. 2020. Synthesis, regulation and degradation of carotenoids under low level UV-B radiation in the filamentous cyanobacteriumPCC 6912., 11: 163.
Luo L J. 2010. Breeding for water-saving and drought-resistance rice (WDR) in China., 61(13): 3509–3517.
Luo L J, Mei H W, Yu X Q, Xia H, Chen L, Liu H Y, Zhang A N, Xu K, Wei H B, Liu G L, Wang F M, Liu Y, Ma X S, Lou Q J, Feng F J, Zhou L G, Chen S J, Yan M, Liu Z C, Bi J G, Li T F, Li M S. 2019. Water-saving and drought-resistance rice: From the concept to practice and theory., 39: 145.
Peng Z, Huwanixi A, Wan C H. 2023. Identification of novel smORFs and microprotein acting in response to rehydration of., 23(12): e2200473.
Sand-Jensen K. 2014. Ecophysiology of gelatinouscolonies: Unprecedented slow growth and survival in resource-poor and harsh environments., 114(1): 17–33.
Scherer S, Ernst A, Chen T W, Böger P. 1984. Rewetting of drought-resistant blue-green algae: Time course of water uptake and reappearance of respiration, photosynthesis, and nitrogen fixation., 62(3): 418–423.
Shang J L, Chen M, Hou S W, Li T, Yang Y W, Li Q, Jiang H B, Dai G Z, Zhang Z C, Hess W R, Qiu B S. 2019. Genomic and transcriptomic insights into the survival of the subaerial cyanobacteriumin arid and exposed habitats., 21(2): 845–863.
Sinha R P, Klisch M, Walter Helbling E, Häder D P. 2001. Induction of mycosporine-like amino acids (MAAs) in cyanobacteria by solar ultraviolet-B radiation., 60(2/3): 129–135.
Sun Z T, Shen S C, Tian B, Wang H, Xu Z J, Wang L Y, Hua Y J. 2009. Functional analysis of γ-carotene ketolase involved in the carotenoid biosynthesis of., 301(1): 21–27.
Takenaka H, Yamaguchi Y, Sakaki S, Watarai K, Tanaka N, Hori M, Seki H, Tsuchida M, Yamada A, Nishimori T, Morinaga T. 1998. Safety evaluation of(Nostocales, Cyanophyceae) as a potential food., 36(12): 1073–1077.
Tamadaddi C, Sagar V, Verma A K, Afsal F, Sahi C. 2021. Expansion of the evolutionarily conserved network of J-domain proteins in themitochondrial import complex., 105(4/5): 385–403.
Tamadaddi C, Verma A K, Zambare V, Vairagkar A, Diwan D, Sahi C. 2022. J-like protein family of: The enigmatic cousins of J-domain proteins., 41(6): 1343–1355.
Wada N, Sakamoto T, Matsugo S. 2013. Multiple roles of photosyntheticand sunscreen pigments in cyanobacteria focusing on the oxidative stress., 3(2): 463–483.
Wang B, Yang L, Zhang Y Q, Chen S L, Gao X, Wan C H. 2019. Investigation of the dynamical expression ofproteome in response to rehydration., 192: 160–168.
Wang L K, Feng Z X, Wang X, Wang X W, Zhang X G. 2010. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data., 26(1): 136–138.
Wang L X, Lei X T, Yang J, Wang S P, Liu Y, Liang W Y. 2018. Comparative transcriptome analysis reveals that photosynthesis contributes to drought tolerance of(Nostocales, Cyanobacteria)., 57(1): 113–120.
Wang L X, Wang S P, Li X X, Wang M, Zhang Z, Shi J, Xu T T, Liang W Y. 2020. Overexpression ofgene fromimproves the drought tolerance of., 132: 127–131.
Wang L X, Li X X, Wang M, Ma X R, Song F, Hu J H, Liang W L, Liang W Y. 2022. Carbon metabolism and the ROS scavenging system participate in’s adaptive response to dehydration conditions through protein acetylation., 21(2): 482–493.
Wang M, Zhu Q, Li X X, Hu J H, Song F, Liang W L, Ma X R, Wang L X, Liang W Y. 2022. Effect of drought stress on degradation and remodeling of membrane lipids in., 11(12): 1798.
Wu S J, Yu K Q, Li L, Wang L X, Liang W Y. 2021. Enhancement of exopolysaccharides production and reactive oxygen species level ofin response to dehydration., 28(26): 34300–34308.
Xia H, Zhang X X, Liu Y, Bi J G, Ma X S, Zhang A N, Liu H Y, Chen L, Zhou S, Gao H, Xu K, Wei H B, Liu G L, Wang F M, Zhao H Y, Luo X X, Hou D P, Lou Q J, Feng F J, Zhou L G, Chen S J, Yan M, Li T F, Li M S, Wang L, Liu Z C, Yu X Q, Mei H W, Luo L J. 2022. Blue revolution for food security under carbon neutrality: A case from the water-saving and drought- resistance rice., 15(9): 1401–1404.
Xiang Y, Tang N, Du H, Ye H Y, Xiong L Z. 2008. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice., 148(4): 1938–1952.
Xiao L H, Yang G, Zhang L C, Yang X H, Zhao S, Ji Z Z, Zhou Q, Hu M, Wang Y, Chen M, Xu Y, Jin H J, Xiao X, Hu G P, Bao F, Hu Y, Wan P, Li L G, Deng X, Kuang T Y, Xiang C B, Zhu J K, Oliver M J, He Y K. 2015. The resurrection genome of: A blueprint for survival of dehydration., 112(18): 5833–5837.
Xu H F, Dai G Z, Ye D M, Shang J L, Song W Y, Shi H Z, Qiu B S. 2020. Dehydration-induced DnaK2 chaperone is involved in PSII repair of a desiccation-tolerant cyanobacterium., 182(4): 1991–2005.
Xu H F, Raanan H, Dai G Z, Oren N, Berkowicz S, Murik O, Kaplan A, Qiu B S. 2021. Reading and surviving the harsh conditions in desert biological soil crust: The cyanobacterial viewpoint., 45(6): fuab036.
Xue Y, He Q F. 2015. Cyanobacteria as cell factories to produce plant secondary metabolites., 3: 57.
Yan L, Gong Y Y, Luo Q, Dai G X, Teng Z N, He Y, Wu X X, Liu C, Tang D Y, Ye N H, Deng G F, Lin J Z, Liu X M. 2021. Heterologous expression of fungalalleviates ammonium toxicity and suppresses photorespiration, thereby improving drought tolerance in rice., 305: 110769.
Yang Y W, Yin Y C, Li Z K, Huang D, Shang J L, Chen M, Qiu B S. 2019. Orange and red carotenoid proteins are involved in the adaptation of the terrestrial cyanobacteriumto desiccation., 140(1): 103–113.
Ye S F, Gao X. 2015. Excavating abiotic stress-related gene resources of terrestrial macroscopic cyanobacteria for crop geneticengineering: Dawn and challenge., 6(6): 313–315.
Yu S B, Ali J, Zhou S C, Ren G J, Xie H A, Xu J L, Yu X Q, Zhou F S, Peng S B, Ma L Y, Yuan D Y, Li Z F, Chen D Z, Zheng R F, Zhao Z G, Chu C C, You A Q, Wei Y, Zhu S S, Gu Q Y, He G C, Li S G, Liu G F, Liu C H, Zhang C P, Xiao J H, Luo L J, Li Z K, Zhang Q F. 2022. From Green Super Rice to green agriculture: Reaping the promise of functional genomics research., 15(1): 9–26.
Yuan X L, An J, Zheng T, Liu W J. 2022. Exogenous melatonin improves salt tolerance mainly by regulating the antioxidant system in cyanobacterium., 10: e14479.
Zhang H, Li Y Y, Zhu J K. 2018. Developing naturally stress- resistant crops for a sustainable agriculture., 4(12): 989–996.
Zhang H, Zhao Y, Zhu J K. 2020. Thriving under stress: How plants balance growth and the stress response., 55(5): 529–543.
Zhang H M, Zhu J H, Gong Z Z, Zhu J K. 2022. Abiotic stress responses in plants., 23(2): 104–119.
Zhang Q F. 2007. Strategies for developing green super rice., 104(42): 16402–16409.
Zhang Z C, Wang K, Hao F H, Shang J L, Tang H R, Qiu B S. 2021. New types of ATP-grasp ligase are associated with the novel pathway for complicated mycosporine-like amino acid production in desiccation-tolerant cyanobacteria., 23(11): 6420–6432.
Zhu J K. 2016. Abiotic stress signaling and responses in plants., 167(2): 313–324.
Zhu X Y, Xiong L Z. 2013. Putative megaenzyme DWA1 plays essential roles in drought resistance by regulating stress-induced wax deposition in rice., 110(44): 17790–17795.
12 June 2023;
10 October 2023
Luo Lijun (lijun@sagc.org.cn); Liu Zaochang (lzc@sagc.org.cn)
Copyright © 2024, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
https://doi.org/10.1016/j.rsci.2023.10.002
(Managing Editor: Wu Yawen)
- Rice Science的其它文章
- Rice Variety Classification Based on Optimized Near-Infrared Spectral Classification Model
- Effects of Nitrogen-Regulating Gene AreA on Growth,Pathogenicity,and Fumonisin Synthesis of Fusarium proliferatum
- Rice Husk at a Glance:From Agro-Industrial to Modern Applications
- Smart Farming for Sustainable Rice Production:An Insight into Application,Challenge, and Future Prospect
- OsbZIP01 Affects Plant Growth and Development by Regulating OsSD1 in Rice
- Potential Secretory Transporters and Biosynthetic Precursors of Biological Nitrification Inhibitor 1,9-Decanediol in Rice as Revealed by Transcriptome and Metabolome Analyses