lnflammatory response in gastrointestinal cancers: Overview of six transmembrane epithelial antigens of the prostate in pathophysiology and clinical implications
Ze-Xuan Fang,Wen-Jia Chen,Zheng Wu,Yan-Yu Hou,Yang-Zheng Lan,Hua-Tao Wu,Jing Liu
Abstract Chronic inflammation is known to increase the risk of gastrointestinal cancers (GICs),the common solid tumors worldwide.Precancerous lesions,such as chronic atrophic inflammation and ulcers,are related to inflammatory responses in vivo and likely to occur in hyperplasia and tumorigenesis.Unfortunately,due to the lack of effective therapeutic targets,the prognosis of patients with GICs is still unsatisfactory.Interestingly,it is found that six transmembrane epithelial antigens of the prostate (STEAPs),a group of metal reductases,are significantly associated with the progression of malignancies,playing a crucial role in systemic metabolic homeostasis and inflammatory responses.The structure and functions of STEAPs suggest that they are closely related to intracellular oxidative stress,responding to inflammatory reactions.Under the imbalance status of abnormal oxidative stress,STEAP members are involved in cell transformation and the development of GICs by inhibiting or activating inflammatory process.This review focuses on STEAPs in GICs along with exploring their potential molecular regulatory mechanisms,with an aim to provide a theoretical basis for diagnosis and treatment strategies for patients suffering from these types of cancers.
Key Words: Six transmembrane epithelial antigens of the prostate;Gastrointestinal cancer;Inflammation
lNTRODUCTlON
Gastrointestinal cancers (GICs),such as colorectal cancer (CRC),gastric cancer (GC),and hepatocellular carcinoma (HCC),are one of the leading causes of cancer-related death worldwide,with many cases and wide lesions.Among them,CRC is not only the fourth most common malignancy but also the third main cause of cancer-related death in the United States[1].GC is the fifth most common cancer and the third leading cause of cancer-related death worldwide[2],while HCC accounts for 90% of liver cancer with 850000 new cases each year[3].Although effective colonoscopy and upper endoscopy screening can detect precancerous polyps and precancerous lesions in the gastrointestinal tract,many patients have an advanced stage at their first diagnosis and a poor prognosis with current treatment methods[4].
The gastrointestinal tract is exposed to diverse foods and/or drugs daily,which may be related to various degrees of inflammatory response and kinds of diseases.Chronic inflammation is a well-established risk factor for GICs,which is also the molecular and pathophysiological basis of gastritis,inflammatory bowel disease (IBD),and upper and lower GICs[5].Chronic inflammation initiates tumorigenesis,and mechanisms by which tumor-induced and treatment-related inflammatory processes interact with cancer cells support that inflammatory responses may be closely related to the oncogenesis and/or development of GICs[6].Inflammatory features involved in the development of CRCs include inflammasome activation and noncanonical nuclear factor-kappaB (NF-κB) pathway activation mediating the production of proinflammatory cytokines,both of which can be activated by changes in the mutant environment or stimulation by microorganisms such as the gut microbiota[7,8].And smoking,alcohol consumption,various infections,susceptibility gene mutations,and epigenetic changes are associated with the occurrence of GICs[9-11].Due to the high incidence rates and poor prognosis associated with GICs globally,they represent a significant public health challenge[12].
Recently,the six transmembrane epithelial antigen of the prostate (STEAP) family,a group of key metal oxidoreductases,has been associated with the overexpression of a range of proinflammatory cytokines[13],which are considered promising therapeutic targets for various cancers,especially prostate cancer,due to their role in regulating proinflammatory cytokines[14-16].However,Gomesetal[17] also found that the localization of STEAPs on the cell membrane and their differential expression in normal tissues and gastric,colorectal,and liver cancers make them promising potential targets for the treatment of GICs.Therefore,this review aims to explore the role of STEAPs in inflammatory responses in GICs and provide a new strategy for the prevention and early intervention of GICs.
STRUCTURAL CHARACTERlSTlCS OF STEAP FAMlLY MEMBERS
The STEAP family is composed of four structurally similar members,namely,STEAP1,STEAP2,STEAP3,and STEAP4[17],as cell-surface transmembrane proteins with six potential transmembrane regions,and intracellular amino and carboxyl termini[18].It is shown that,unlike STEAP1,the C-terminal and conserved N-terminal domains of STEAP2-4 proteins are similar to the structures of yeast FRE metalloreductase and homologous to the paleontological and bacteriological F420H2:NADP+oxidoreductase (FNO) binding protein domains,respectively[19].Normally,STEAPs perform physiological functions as oxidoreductases,involved in the uptake and reduction of iron and copper[19,20] (Figure 1).
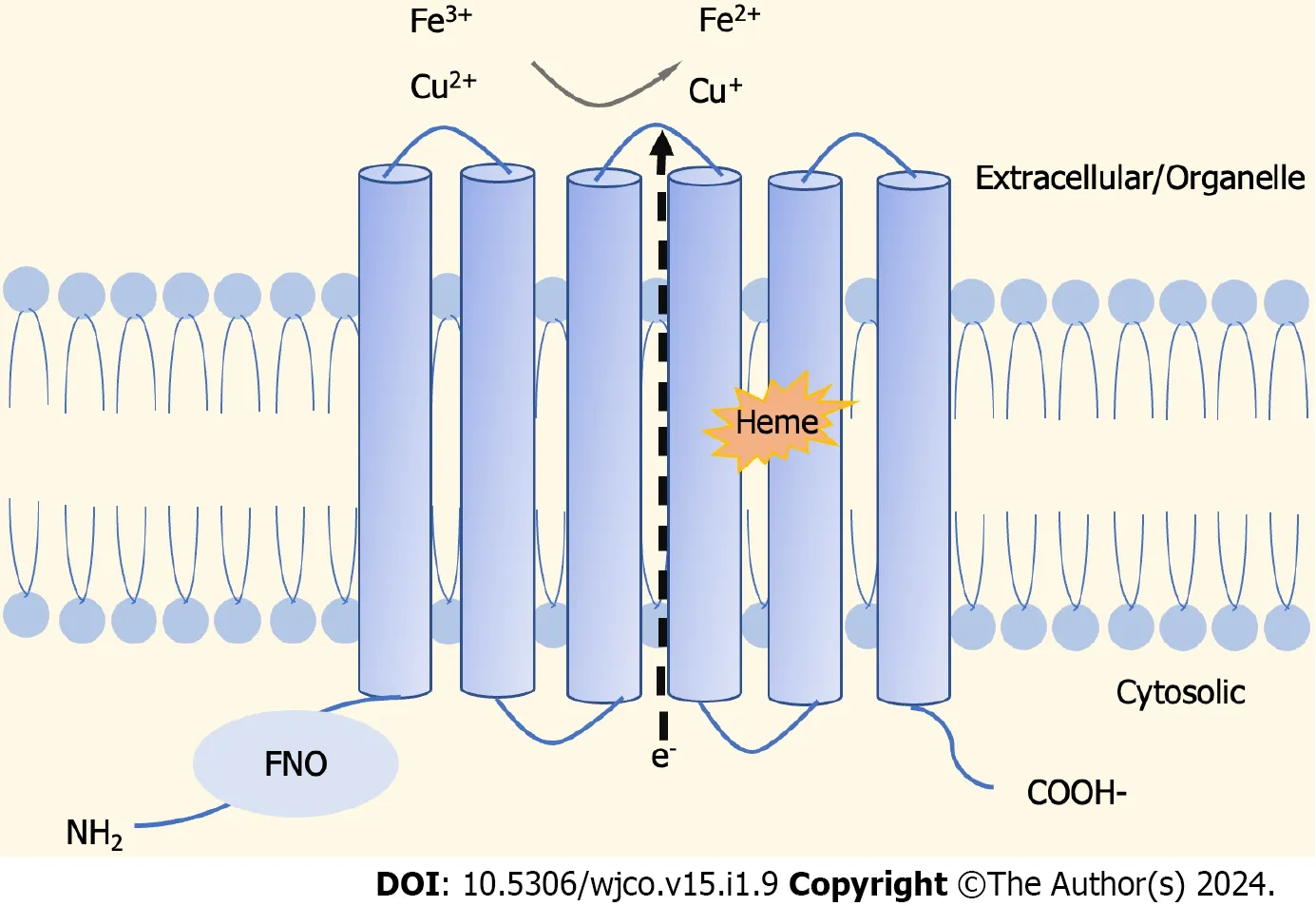
Figure 1 Schematic representation of protein structure of six transmembrane epithelial antigens of the prostate. Six transmembrane epithelial antigens of the prostate (STEAPs) are similar in structure,with six transmembrane domains,an intracellular C-terminus,and an intracellular N-terminus containing the intracellular heme group,while STEAP1 Lacks FNO-like domain to perform intrinsic metal reductase activity.STEAP2-4 are involved in cell proliferation,tumor progression,and intercellular communication through their metalloreductase activity.FNO: F420H2:NADP+oxidoreductase.
STEAP1 is the first reported STEAP family member with a molecular weight of 39.9 kDa (NP_036581.1) and an intramembrane heme binding site[18,21].Although STEAP1 is widely expressed and co-localizes with transferrin (Tf) and Tf receptor 1 (TfR1),unlike other members,STEAP1 does not independently promote iron or copper reduction or uptake.Although it lacks the FNO-like reductase domain,which is thought to be essential for metal oxidoreductase activity,it is suggested that STEAP1 may play a role in iron or copper metabolism,which may be due to its interaction with the NAPDH-binding FNO domain of STEAP2 or STEAP4[19,22,23].STEAP1B is a newly discovered member that has an extremely high (88%) identity with STEAP1.What distinguishes it from other proteins is that it only has four potential transmembrane domains without NADPH oxidoreductase domain or heme binding site.Hence,it is not expected to have oxidoreductase activity based on previous studies[15,24].
Besides STEAP1,STEAP2-4 are also composed of six transmembrane α-helices and intracellular hydrophilic N-and Cterminal domains,with the N-terminus containing the intracellular NADPH-binding FNO domain and the C-terminus containing bis-heme with the FRE domain.Invitrostudies have found that STEAP2-4 perform Fe3+and Cu2+reductase activities and increase intracellular iron and copper uptake[20].
STEAP2 is known as STAMP1,which is composed of 490 amino acid residues.It is found that STEAP2 is highly expressed in the androgen-reactive prostate cancer cell line LNCaP,but not in androgen receptor-negative prostate cancer cell lines PC3 and DU145[25,26].However,the expression of STEAP2 is not regulated by androgen receptors,but requires the presence of an intact androgen receptor[25].In addition,the characteristics of STEAP2 expression and localization in human microvascular endothelial cells suggest that STEAP2 has a potential role in iron transport across the blood-brain barrier[27],which is further supported by the co-localization of STEAP2 and Tf in primary hippocampal neurons[28].However,further research is needed to confirm this effect.
STEAP3 was first identified in prostate tissue and proposed as a candidate for prostate cancer immunotherapy,which is also known as tumor suppressor activating pathway 6[29].STEAP3 co-localizes with Tf,TfR1,and divalent metal transporter 1 (DMT1) to participate in iron-uptake mediated by Tf endosome in erythroid cells and is thus an important component of the Tf-TfR1 cycle[20,30].
STEAP4 is also named STAMP2 because of its sequence similarity to STAMP1.STEAP2 and STEAP4 are highly expressed in the Golgi complex,trans-Golgi network,and plasma membrane,and co-localizes with endosome antigen 1,which is involved in the secretion-endocytosis pathway[25,26,31].In addition to metal oxidoreductase activity,STEAP4 also plays a role in regulating inflammatory responses,fatty acid metabolism,and glucose metabolism[31-33].To date,the possible effects of STAMP2 on iron metabolism have not been reported,and more studies are needed to directly evaluate the possible role of STAMP2 in human iron metabolism (Figure 2).

Figure 2 Molecular structure diagram of six transmembrane epithelial antigens of the prostate 1-4. The six transmembrane epithelial antigens of the prostate (STEAP) family is composed of a group of cell surface transmembrane proteins with six potential transmembrane domains,one intracellular amino terminus,and a carboxyl terminus that exert physiological functions by acting as oxidoreductases.STEAP1-4 consist of 339,490,488,and 459 amino acid residues,respectively.Except STEAP1,the N-terminus of the STEAP2-4 proteins contains the F420H2:NADP+oxidoreductase binding protein domain.TM: Transmembrane domain;STEAP: Six transmembrane epithelial antigen of the prostate.
ROLE OF STEAPS lN lNFLAMMATORY RESPONSE lN PHYSlOLOGlCAL AND PATHOLOGlCAL PROCESSES
In mammals,iron and copper metabolism are related.Iron and copper,as important metal ionsinvivo,are absorbed in the small intestineviareduction-state-specific DMT1[34-36] and copper transporter 1[37],respectively.Iron and copper often alternate between two oxidation states and participate in the redox processinvivo.Additionally,iron and copper can be used as cofactors of several enzymes to participate in the transformation of substances[34,38,39].Iron and copper deficiencies are known to cause low-chromium microcell anemia in mammals,while excess iron or copper will lead to organ poisoning,particularly in the liver and brain.More importantly,since tumor cells have stronger proliferative ability than normal cells,their demand for iron and copper exceeds that of normal cells.The imbalance of iron and copper homeostasis is also closely related to cancer progression.
Under physiological conditions,STEAPs are a class of metalloproteinases that play an essential role in iron and copper homeostasis[19].Iron and its homeostasis are closely related to inflammatory responses and provide a major protective mechanism in human physiology.In the past decades,a series of studies confirmed that iron overload can aggravate inflammatory responses and susceptibility to infection.Persons with hereditary hemochromatosis and iron overload are more susceptible to pathogens,whereas iron deficiency confers relative resistance to infection[40-42].STEAPs are known to be a participant in iron-copper homeostasis,and their importance in protein functional activity,tissue expression patterns,and subcellular localization in cancer progression has been demonstrated.In addition,STEAPs have also been found to play a role in regulating cell proliferation and apoptosis,alleviating oxidative stress and mediating the Tf cycle[22,43].
Although inflammation is associated with an activated immune system (including immune cells and biological factors) under certain conditions,inflammation is a natural defense response,which is fundamentally different from the immune response[23].Inflammation is a defense mechanisminvivoto remove invading foreign bodies such as bacteria,parasites,and viruses.In the process of inflammatory reaction,excessive or uncontrolled production of inflammatory products will lead to host cell damage,and even lead to chronic inflammation,chronic disease,and tumor transformation[44,45].
STEAP proteins are involved in the regulation of various physiological cellular functions,and plays a potentially important role in various metabolic processes,such as iron uptake and conversion,inflammatory stress response,and glucose metabolism in cells[15,43].STEAPs have been suggested to play a role in iron metabolism in acute and chronic diseases associated with inflammation,as well as in the oncogenesis and development of malignancies.Liangetal[46] reported that STEAP1 and STEAP4 positively regulate the induction of proinflammatory,neutrophil-activated cytokines,such as chemokine (C-X-C motif) ligand (CXCL)1 and interleukin (IL)-8,in pustular skin disorders.More complexly,STEAP4 is found to be regulated by multiple cellular signaling pathways,revealing a positive association of STEAP1 and STEAP4 with theinvivoproinflammatory cytokines IL-1,IL-36,CXCL1,and CXCL8 in several neutrophil-driven diseases in humans.In addition,significant changes in genes related to iron biology were observed in patients with pustular skin disorders,suggesting that the inflammatory activity of STEAP has a causal relationship with its regulation of ion metabolism.Timmermansetal[47] found that STEAP2 plays a role in pathways involved in a chronic low-grade inflammatory disease state,namely,obesity,and lipid metabolism.Zhangetal[13] showed that STEAP3,the only member of this family that is highly expressed in macrophages that play a role in inflammatory immunity,regulates iron homeostasis during inflammatory stress through the translocation-associated membrane protein-dependent pathway.This study provides important insights into the function of STEAP3 as a coordinated regulator of iron homeostasis and inflammation.
In addition to its role as a metal oxidoreductase,STEAP4 is significantly overexpressed in low-grade inflammatory responses[48].In a study by Gordonetal[49],STEAP4 was shown to play a protective role in the face of inflammatory stress in models of metabolic disorders.It is in turn up-regulated by acute inflammation or islet-level cytokine exposure.Even in septic patients,the expression of STEAP4 is elevated in the early stage of sepsis,which can be used to predict the clinical outcome of these patients[50].These findings point to the complex regulation of STEAP4 that makes its protective role in inflammatory metabolic disorders.It is reported that iron and its homeostasis are closely related to the inflammatory response,which provides a major protective mechanism in human physiology,while iron overload worsens inflammation and infection susceptibility[13].
ROLE OF STEAPS lN lNFLAMMATORY RESPONSE lN GlCS
In recent years,a large amount of research data have revealed that inflammation is a key component of tumor progression.The oncogenesis of GICs,such as GC,CRC,and HCC,is related to infection and chronic inflammatory stimuli,and the tumor microenvironment,coordinated to a large extent by inflammatory cells,is to a large extent an indispensable participant in tumor formation,promoting tumor cell proliferation,survival,and migration[44].Clinical studies have revealed that about 15%-20% of cancer patients have an infection,chronic inflammation,or autoimmune disease in the same tissue or organ site before the cancer development[44,51].This suggests that the pre-cancerous inflammatory response is present before tumor formation.The strongest association between inflammation and malignancy is exemplified by CRC patients with IBD,including chronic ulcerative colitis and Crohn’s disease,predispositions to liver cancer in patients with hepatitis,and chronicHelicobacterpylori(H.pylori) infection as a major cause of GC[52].
Meanwhile,various environmental factors may induce and promote the development of cancer by inducing chronic inflammation,which may accompany tumor development and increase the risk of many different cancers such as liver,pancreatic,colon,and other malignancies[53,54].So,how does the inflammatory response induce and promote tumorigenesis? It is well known that one of the causes of cancer is the loss of tumor suppressor function,with the most common mutation being the tumor suppressor p53.In the tumor microenvironment,loss of p53 function leads to increased expression of NF-κB dependent inflammatory genes[55,56],which promotes CRC progression and metastasis[56-58].In addition,oncogene activation leading to excessive production of inflammatory cytokines and chemokines may be another mechanism by which chronic inflammation triggers cancer occurrence[59,60].Activation of the oncogene K-Ras leads to increased secretion of cytokines and chemokines of “aging-associated secretory phenotypes”[60].With increasing research on human symbiotic microbiomes,researchers have found that symbiotic microbiomes may be involved in the occurrence and development of many cancers,perhaps through microbial adhesion to cancer cells and translocation or long-distance release of microbial metabolites[61].
As mentioned above,the localization of STEAP proteins on the cell membrane,their differential expression in normal and cancer tissues,and their metal-oxidoreductase activity mechanism make them potential candidate targets for the biomarkers of a variety of cancers,as well as potential targets for the alleviation or treatment of these cancers[62,63].STEAP4 has been shown to play an important role in the inflammatory response and other physiological metabolic processes[31-33].Although the role of STEAP1,STEAP2,and STEAP3 in the inflammatory response is rarely reported,it is tempting to speculate that STEAP1-3 may also have similar functions.
GC
GC is one of the most common GICs in humans,and chronicH.pyloriinfection is one of the main risk factors for GC occurrence[64].The latency ofH.pylorileads to a variety of changes in the gastric mucosa,such as gastritis,atrophic gastritis,intestinal metaplasia,dysplasia,and eventually GC[65].Chronic infection withH.pyloriin the gastric mucosa can occur freely in mucus,by attaching to cells,or intracellularly,requiring iron for bacterial growth[66].In an irondeficient medium,H.pylorican bind and extract iron from hemoglobin,Tf,and lactoferrin to support its growth,and preferentially bind the iron-free forms of Tf and lactoferrin,limiting its ability to extract iron from normal serum[67].Hamedi Asletal[68] investigated the expression of genes involved in iron homeostasis and their role in the pathogenesis ofH.pyloriinfection.It is found that TfR and ferritin light chain were overexpressed in allH.pylori-positive tissues,while increased iron regulatory protein 2 expression was associated withH.pylori-positive chronic gastritis and intestinal metaplasia,confirming the role of iron acquisition-related genes inH.pyloriattachment into the gastric mucosa.On the other hand,the colonization ofH.pyloriinduces a substantial production of reactive oxygen species (ROS) and develops various strategies to quench the deleterious effects of ROS,resulting in persistent ROS production.However,excessive ROS will incur chronic inflammation and cellular damage,as the major risk factor for gastric carcinogenesis[69].These investigations indicate the potential role of STEAPs in inflammatory responses forH.pylori-related GC.
The role of STEAP1 in GC was first reported by Wuetal[70],who defined the landscape of translationally regulated gene products with differential expression between non-metastatic and metastatic GC cohorts.Interestingly,STEAP1 was identified as the most translationally upregulated gene product,required for cell proliferation,migration/invasion,tumorigenesis,and chemoresistance to docetaxel treatment[70].To explore the regulatory mechanism,the same research group focused on the potential regulators for STEAP1 expression[71].They found that the RNA-binding protein poly r(C) binding protein 1 and miR-3978 function as repressors of peritoneal metastasis of GC,partially by downregulating STEAP1,while phosphorylated eIF4E upregulates STEAP1 expression at the level of cap-dependent translation initiation to facilitate the peritoneal metastasis of GC[71].A similar result was found by Zhangetal[72],that STEAP1 performed an oncogenic role in the occurrence and metastasis of GCviaactivating the AKT/FoxO1 pathway and epithelialmesenchymal transition process.
Besides STEAP1,STEAP4 was also found to be highly expressed in GC tissues,which is associated with advanced clinical stage and poor prognosis of GC patients.Importantly,the expression of STEAP4 was found to be positively correlated with the infiltration levels of B cells,CD4+T cells,macrophages,neutrophils,and dendritic cells,indicating its contribution to the regulation of the tumor microenvironment[73].Although the current investigation of STEAPs in GCs is limited,the potential role of STEAPs involved in immune response in GC is emerging and needs further exploration.
CRC
CRC is the third most common cause of cancer death in the United States and other developed countries[1].It has been well-accepted that chronic inflammation is one of the recognized risk factors for the development of CRC,especially in colon cancer.The accumulation of immune cells and inflammatory factors in the intestinal mucosa constitute a complex chronic inflammatory environment and cause oxidative stress or DNA damage on the epithelial cells[74].In patients with IBD,the risk for CRC is increased significantly,which is strongly associated with chronic inflammation,and such CRC was named colitis-associated CRC[74].On the other hand,the gastrointestinal tract is the primary site to absorb copper,which is an essential micronutrient and critical enzyme cofactor for crucial copper-dependent enzymes.Elevated copper concentrations can cause multifaceted responses of pathogenic bacteria when invading the host[75],while in the fish model,Wangetal[76] found that copper exposure induced intestinal oxidative stress and inflammation,resulting in enrichment of potentially pathogenic bacteria and reduction of probiotic bacteria.Milleretal[77] found a significant difference in copper isotopic composition along with diverse bacterial populations,revealing a host-microbial interaction involved in the regulation of copper transport.
After being identified as a new target for preventative and/or therapeutic vaccine construction and immune monitoring in prostate cancer[78],STEAP1 was found to be highly expressed in CRC,predicting a poor overall survival in CRC patients[79].Mechanistically,Nakamuraetal[80] found that silencing STEAP1 suppressed CRC cell growth and increased ROS production,associated with decreased expression of antioxidant molecules regulated by the transcription factor nuclear erythroid 2-related factor.As an antigen present in various tumors,STEAP1 has the potential to stimulate cytotoxic T lymphocytes (CTLs) involved in antitumor immunotherapy.To explore the specific STEAP1 sequence capable of stimulating naïve HLA-A2-restricted CTLs,Rodebergetal[81] used MHC peptide binding algorithms to predict the potential sequences and verified their abilities to induce antigen-specific CTLs to kill peptide-pulsed HLA-A2 target cells.They provided strong evidence that STEAP1-292 peptide (MIAVFLPIV) is naturally processed by many types of tumors,including CRC,and recognized by CTLs,and the modified STEAP1-292.2L peptide (MLAVFLPIV) is more immunogenic to induce CTL recognition,serving as a potential antitumor peptide vaccine.Soon,Rodebergetal[82] reported another two peptides of STEAP1,which can be used for broad-spectrum-tumor immunotherapy.
As metalloreductases,STEAPs are involved in iron/copper homeostasis[21,83].Among the copper homeostasis-related genes,STEAP3 was found to be increased in CRC in oligonucleotide microarray analysis,related to copper accumulation[83].During the polarization of macrophages,the time-dependent change of intracellular Fe(II) during the inflammatory activation was consistent with the expression shifts of TfR,STEAP3,and Fe(II) exporter Slc40a1,indicating the role of Fe(II) in inflammatory-activated macrophages[84].Even in hypoferric conditions,STEAP3 overexpression increased iron storage,causing resistance to iron deprivation-induced apoptosis[85].In CRC cells,STEAP3 also facilitates exosomal trafficking to increase the secretion of exosomes[86],which are important interactors between tumor cells and their surroundings[87].Interestingly,hypoxia-induced antisense long non-coding RNA STEAP3-AS1 increased the expression of STEAP3 by competitively interacting with YTH domain-containing family protein 2 (YTHDF2) and leading to the disassociation of YTHDF2 with STEAP3 mRNA and upregulated STEAP3 mRNA stability in CRC.The enhanced STEAP3 expression increased intracellular Fe(II),which induced the phosphorylation and inactivation of glycogen synthase kinase 3β,releasing β-catenin translocated into the nucleus to activate the Wnt signal with promoted CRC progression[88].
Different from other STEAP family members,STEAP4 expression was found to be low in CRC tissues compared with normal tissues,which is positively correlated with immune infiltration and immune-related biomarkers[89].However,in colitis animal models and IBD patients,STEAP4 was also highly induced in a hypoxia-dependent manner,leading to a dysregulation in mitochondrial iron balance and enhanced ROS level.Using a colitis-associated colon cancer model,Xueetal[90] found that the mitochondrial iron dysregulation related to high STEAP4 level is a key mechanism by which inflammation impacts colon tumorigenesis,indicting STEAP4 as an important regulator of the inflammatory response.In the colitis-associated tumorigenesis model,the copper metabolism can also be mobilized by the pro-inflammatory cytokine IL-17,by inducing STEAP4-dependent cellular copper uptake,which is critical for colon tumor formation[91].
As mentioned above,STEAP1 and STEAP4 function as metalloreductases to regulate the iron/copper homeostasis during the oncogenesis and development of CRC related to inflammation.Although there are no reports of STEAP2 nor STEAP3 in CRC,the structural similarity of STEAP family proteins has prompted a further investigation of the potential role of STEAP2 or STEAP3 in CRC.
HCC
HCC is the most common type of primary liver cancer,listed as the third leading cause of cancer-related death worldwide[92].The increased incidence of primary liver cancer in several developed countries will likely continue for decades.Since primary liver cancer is mostly related to the infection with hepatitis B and C viruses (HBV and HCV),it is the first human cancer enormously amendable to prevention with HBV vaccines[93,94].Although serum copper concentration is not a specific diagnostic biomarker for liver disease,serum copper isotope ratio has been proven to be an assistant monitor for the diagnosis,prognosis,and follow-up of chronic liver diseases,as the imbalanced copper homeostasis exists in liver diseases[95,96].
Interestingly,STEAP1 was considered as a targeted tumor antigen with the cytotoxic potency of chemotherapeutic drugs for designing antibody-drug conjugates (ADCs).Boswelletal[97] constructed a humanized anti-STEAP1 antibodylinked ADC,and evaluated its pharmacokinetics,tissue distribution,and/or potential organ toxicity in rats,finding a general trend toward increased hepatic uptake and reduced levels in other highly vascularized organs.Another research group constructed a radio-labeled anti-STEAP1-conjugated probe for positron emission tomography detection,and the highest mean absorbed dose to the normal organ was found in the liver at 1.18 mGy/MBq[98].The above results indicate the ability of uptake for anti-STEAP1 ADCs in the liver,predicting the therapeutic potential for liver malignancies.Not surprisingly,the expression of STEAP1 was found to be high in liver tumors and associated with poor clinical outcomes,suggesting that STEAP1 is a druggable target in liver cancer[99].
Related to inflammatory responses,STEAP3 is a mediator and protector of hepatic ischemia-reperfusion injury through TAK1-dependent activation of the JNK/p38 pathways in hepatocytes[100].Interestingly,after HCV infection,STEAP3 was found to be downregulated in HCC and associated with the progression to cirrhosis and HCC,and it thus can be used as a potential monitoring biomarker for the development of HCC[101].The decreased expression of STEAP3 in HCC was also confirmed by Yietal[102],which is associated with the abnormal expression of ferroptosis-related genes.However,at the cellular level,Wangetal[101] found that nuclear STEAP3 was highly expressed in HCC,which was an independent prognostic factor for HCC patients.Mechanically,increased nuclear STEAP3 expression significantly promoted the stemness phenotype,cell cycle progression,and cellular proliferation of HCC cells,through RAC1-ERKSTAT3 and RAC1-JNK-STAT6 signaling axes,while STEAP3 also upregulated the expression and nuclear trafficking of epidermal growth factor receptor (EGFR) to promote EGFR-mediated STAT3 transcription activity in a positive feedback manner[101].As the matrix stiffness is a key factor impairing tumor immunity,Wangetal[103] analyzed the effect of stiffness in HCV-infected cirrhotic HCC,finding that stiffer matrix decreased STEAP3 in the invasive front region of HCC and the cirrhotic tissue,suppressing STEAP3-mediated immune infiltration of CD4+and CD8+T cells,macrophages,neutrophils,and dendritic cells,along with decreased ferroptosis.
As a plasma membrane metalloreductase,STEAP4 is controlled by inflammatory cytokines in the liver,such as IL-6,which significantly induced the transcription activity of STEAP4 through STAT3 and CCAAT/enhancer-binding protein alpha,playing a critical role in the response to nutritional and inflammatory stress[104].Hepatic STEAP4 decreases the stability of HBV X protein (HBx) by physically interacting with HBx,subsequentially suppressing HBx-mediated transcription of lipogenic and adipogenic genes and protecting hepatocytes from HBV gene expression[105].In a nonalcoholic fatty liver disease (NAFLD) animal model,recombinant fibroblast growth factor 21 treatment ameliorated hepatic steatosis and insulin resistance by increasing STEAP4-mediated hepatic iron overload and ferroportin expression,indicating STEAP4 as a suitable therapeutic intervention for NAFLD patients[106].However,in HCC tissues,genomewide DNA methylation analysis revealed significantly hypermethylated and downregulated STEAP4 compared to the non-tumor liver tissues,which may be associated with the development of HCC[107],while STEAP4 methylation in plasma DNA was not associated with HCC risk[108].Not surprisingly,Zhouetal[88] reported that the methylation level of the STEAP4 promoter was correlated with the downregulation of STEAP4,functioning as a tumor suppressor in HCC by inhibiting the PI3K/AKT/mTOR pathway.The reduced STEAP4 expression is significantly associated with tumor aggressiveness and poor prognosis in HCC patients,likely due to its link to various biological processes and induction of HCC immune evasion[109].
As mentioned above,STEAPs,through iron/copper metabolism or different cytokines,participate in the inflammatory process of the gastrointestinal tract,and then induce GIC occurrence and promote GIC development accordingly (Table 1).
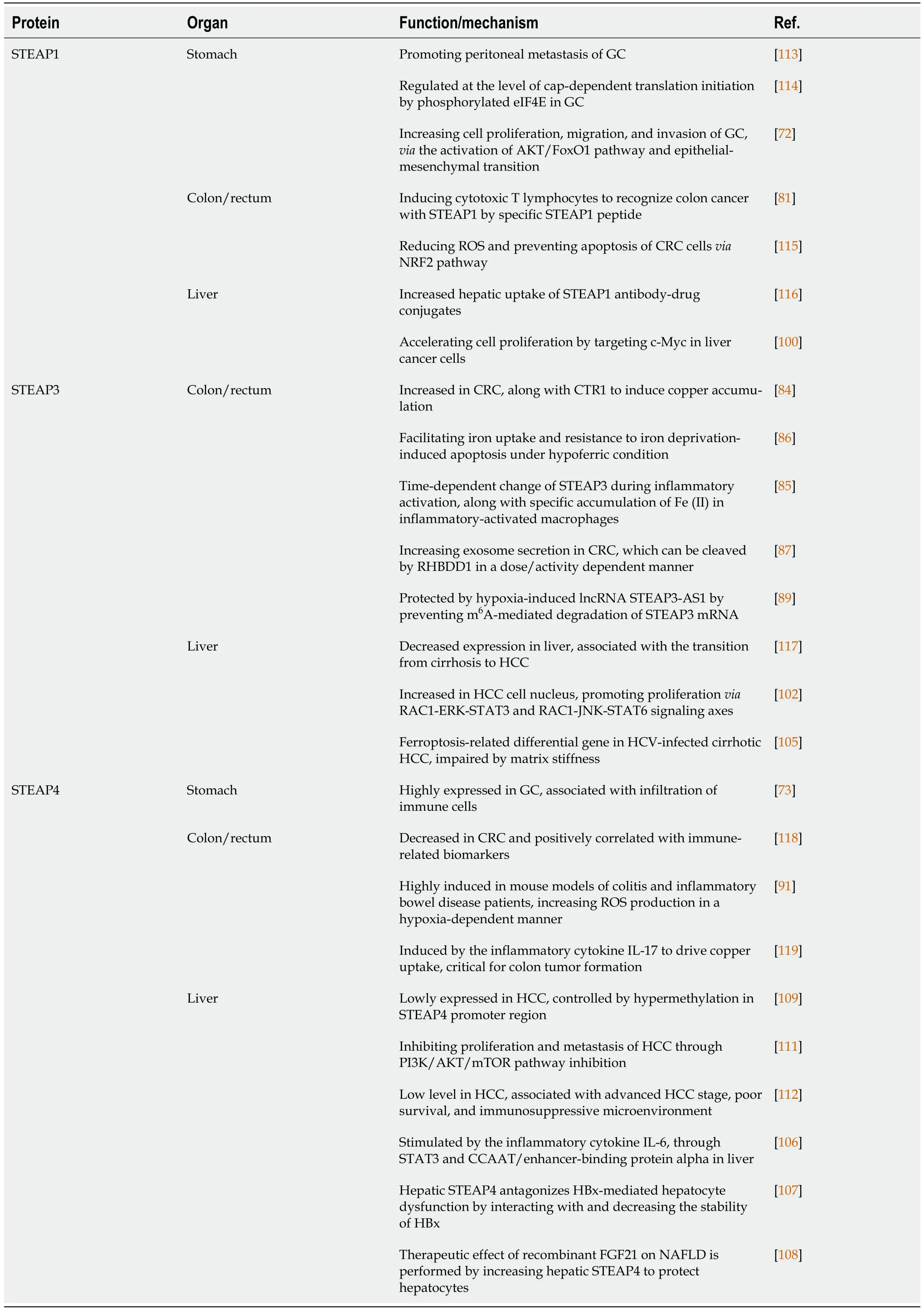
Table 1 Functions/mechanisms of six transmembrane epithelial antigens of the prostate involved in gastrointestinal cancers
CLlNlCAL lMPLlCATlON OF STEAPS lN GlCS
The strict maintenance of a specific microbial consortium in the gastrointestinal tract is critical for health,while gut microbiota alteration and dysbiosis will cause inflammation and pathogenic intestinal conditions[110].The connection between inflammation and tumorigenesis has been well-established for decades based on genetic,pharmacological,and epidemiological evidence.Even obstructive sleep apnea-induced hypertension is found to be associated with gut dysbiosis,which may serve as the trigger for gut and neuroinflammation,and preventing or reversing gut dysbiosis may reduce neuroinflammation and hypertension accordingly[111].Therefore,monitoring microbiota alteration or inflammation in the gastrointestinal tract is a research hotspot for the diagnosis or treatment of gastrointestinal inflammationrelated diseases,including GICs.
Gopalakrishnanetal[112] implemented a miniaturized smart capsule to monitor inflammatory lesions throughout the gastrointestinal tract by detecting ROS level,a biomarker of inflammation,which provided a new milestone of smart ingestible electronics for improving the diagnosis and treatment of digestive disease.The exosomes derived from human placental mesenchymal stem cells used in the myocardial infarction model,notably modulated gut microbial community,increased the gut microbiota metabolites short chain fatty acids (SCFAs),and decreased lipopolysaccharide[113].By sorting and sequencing of immunoglobulin (Ig) A-coated microbiota to define immune-reactive microbiota,Limaetal[114] identified that transferable IgA-coatedOdoribactersplanchnicusin responders to fecal microbiota transplantation for patients with ulcerative colitis increases mucosal regulatory T cells,and induces the production of IL-10 and SCFAs,resulting in the resolution of colitis.Such investigation provided potential strategies or vectors for the treatment of the gastrointestinal tract,as well as inflammation-related GICs.
As a common oncogene in diverse malignancies,STEAP1 was considered a promising candidate therapeutic target,with abundant expression in malignancies[17,18].Importantly,STEAP1 is also found to be a transporter,participating in intercellular communication[115,116].Since the first prostate cancer-specific immunotherapy was licensed in 2010,immunotherapy represents a promising approach to harness the host’s immune system with an anti-tumor effect[117,118].89Zr-DFO-MSTP2109A,a radiolabeled antibody targeting STEAP1,was well tolerated and showed good visualization in the study,thus establishing its potential role as a potential biomarker for STEAP1 directed therapy and confirming its diagnostic value[119].Given STEAP1’s mechanism in cancers,therapeutic strategies targeting STEAP1,such as monoclonal antibodies (mAbs),DNA vaccines,and ADCs,have been developed.Challita-Eidetal[115] identified STEAP1 mediating the transfer of small molecules between adjacent cells and first generated two mAbs that bind to STEAP1 epitopes at the cell surface,which significantly inhibited STEAP-1-induced intercellular communication in a dose-dependent manner.Soon,an anti-STEAP4 mAb that binds to the extracellular domain of STEAP4 was also shown to cause insulin resistance in adipocytes by disrupting cellular mitochondrial function,in addition to inducing apoptosis and inhibiting preadipocyte proliferation and glucose uptake without affecting human preadipocyte differentiation[120],while anti-STEAP1 based ADCs performed exciting anti-tumor function by regulating the immune response[97,121].
One of the goals of current tumor immunotherapy research is to design and validate multi-epitope/multi-antigen vaccines that can induce multi-specific anti-tumor responses and reduce the risk of selection of antigen loss escape variantsinvivo[122,123].The multivalent vaccine should be composed of a variety of epitopes of widely expressed tumor antigens for the purpose of wide application.Recently,many of the same tumor antigens expressed in most human tumors have been described,such as survivin[124],EphA2[125],pan-MAGE-A HLA-A* 0201-restricted epitopes,and Hsp70[126,127].STEAP1 protein was found to be overexpressed in prostate cancer,pancreatic cancer,CRC,HCC,breast cancer,bladder cancer,ovarian cancer,acute lymphoblastic leukemia,and Ewing sarcoma[18].This wide expression pattern strongly suggests the utility of this tumor antigen in broad-spectrum antitumor immunotherapy.It has been demonstrated that STEAP1 is a tumor antigen target of CD8+T cells by identifying two HLA-A* 0201-restricted antigen peptides,STEAP86-94 and STEAP262-270[82].
STEAP: Six transmembrane epithelial antigen of the prostate;GICs: Gastrointestinal cancers;GC: Gastric cancer;eIF4E: Eukaryotic initiation factor 4E;ROS: Reactive oxygen species;CRC: Colorectal cancer;NRF2: Nuclear erythroid 2-related factor;CTR1: Copper transporter 1;RHBDD1: Rhomboid domain containing 1;HCV: Hepatitis C virus;IL-17: Interleukin-17;HCC: Hepatocellular carcinoma;STAT3: Signal transducer and activator of transcription 3;PI3K: Phosphoinositide 3-kinase;mTOR: Mammalian target of rapamycin;JNK: c-Jun N-terminal kinase;ERK: Extracellular signal-regulated kinase;IL-6: Interleukin-6;HBx: Hepatitis B virus x protein;FGF21: Fibroblast growth factor 21;NAFLD: Non-alcoholic fatty liver disease.
In addition,immunotherapy has proved to be an effective treatment for a variety of cancers,especially for patients with tumors with overexpressed antigens that can be recognized by immune T/B cells.The use of STEAP peptides to induce helper T cells in the context of multiple major histocompatibility complex class II alleles have been studied for T cell immunotherapy against STEAP-expressing renal cell carcinoma and bladder cancer[128].These studies confirm that targeting STEAP family proteins in a variety of solid tumors is an attractive and promising effective approach.Although current therapeutic strategies targeting STEAPs have not been applied in clinical practice,their molecular transport mechanism and involvement in cancer progression make them promising targets for the treatment of patients with GICs.
CONCLUSlON
STEAP family members share similar structural features and function as metal oxidoreductases involved in a variety of cellular processes,such as copper/iron uptake,response to inflammation,fatty acid and glucose metabolism,and oxidative stress regulation.STEAPs are irregularly expressed in different cancers,which are involved in the proliferation,migration,invasion,and metastasis of cancer cells,and play a role in promoting or suppressing cancer.In addition,the inflammatory response may be caused by necrosis of rapidly growing tumor cells due to hypoxia and lack of nutrients.ROS and reactive nitrogen species produced by inflammatory cells can cause oxidative DNA damage in gastrointestinal cells,leading to the activation of oncogenes and/or inactivation of tumor suppressor genes,as well as various epigenetic changes that are conducive to the progression of GICs.Thus,molecules that affect cell survival or the subsequent inflammatory response are likely to have an impact on the course of GIC development.In conclusion,based on the increasing use of STEAPs as cancer therapeutic targets,inflammatory therapeutic strategies in GICs will be more considered in the future,most likely including STEAP family proteins.
FOOTNOTES
Author contributions:Liu J and Fang ZX designed this study;Fang ZX,Chen WJ,and Wu Z searched the publications;Fang ZX,Chen WJ,Wu Z,Hou YY,Lan YZ,Wu HT,and Liu J interpreted the results,constructed the structure of the review,and prepared the tables;Fang ZX prepared the draft of the manuscript;Fang ZX and Chen WJ prepared the figures;Liu J revised the manuscript critically;and all authors have read and approved the final manuscript.
Supported bythe National Natural Science Foundation of China,No.82 273457;the Natural Science Foundation of Guangdong Province,No.2021A1515012180,2023A1515012762 and No.2019A1515 010962;Special Grant for Key Area Programs of Guangdong Department of Education,No.2021ZDZX2040;Science and Technology Special Project of Guangdong Province,No.210715216902829.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Ze-Xuan Fang 0000-0002-6100-9012;Wen-Jia Chen 0000-0001-7157-3242;Zheng Wu 0000-0002-1393-7586;Yan-Yu Hou 0000-0002-4249-8770;Yang-Zheng Lan 0009-0000-4241-228X;Hua-Tao Wu 0000-0002-1640-6094;Jing Liu 0000-0002-7483-4572.
S-Editor:Wang JJ
L-Editor:Wang TQ
P-Editor:Zhang XD
 World Journal of Clinical Oncology2024年1期
World Journal of Clinical Oncology2024年1期
- World Journal of Clinical Oncology的其它文章
- Uveal melanoma: Recent advances in immunotherapy
- Scinderin promotes glioma cell migration and invasion via remodeling actin cytoskeleton
- Prognostic and immunological roles of heat shock protein A4 in lung adenocarcinoma
- ldentification of the key genes and mechanisms associated with transcatheter arterial chemoembolisation refractoriness in hepatocellular carcinoma
- Predicting colorectal cancer prognosis based on long noncoding RNAs of disulfidptosis genes
- Gene signatures to therapeutics: Assessing the potential of ivermectin against t(4;14) multiple myeloma
